Abstract
The bone marrow niche is composed of cells from hematopoietic and mesenchymal origin. Both require energy to power differentiation and these processes are intimately connected to systemic metabolic homeostasis. Glycolysis is the preferred substrate for mesenchymal stromal cells in the niche, although fatty acid oxidation and glutaminolysis are important during stage specific differentiation. Autophagy and lipophagy, in part triggered by adenosine monophosphate-activated protein kinase (AMPK), may also play an important but temporal specific role in osteoblast differentiation. Enhanced marrow adiposity is caused by clinical factors that are genetically, environmentally, and hormonally mediated. These determinants mediate a switch from the osteogenic to the adipogenic lineage. Preliminary evidence supports an important role for fuel utilization in those cell fate decisions. Although both the origin and function of the marrow adipocyte remains to be determined, and in some genetic mouse models high marrow adiposity may co-exist with greater bone mass, in humans changes in marrow adiposity are closely linked to adverse changes in skeletal metabolism. This supports an intimate relationship between bone and fat in the marrow. Future studies will likely shed more light on the relationship of cellular as well as whole body metabolism on the ultimate fate of bone marrow stromal cells.
Keywords: Bone marrow niche, Mesenchymal stem cell, Osteoblast, Adipocyte, Bone marrow adiposity, Energy utilization
I. Introduction
The bone marrow is composed of distinct cell types that exist at several developmental levels. Hematopoietic elements are the most prevalent and include erythrocytes, neutrophils, lymphocytes, and platelets. Hematopoietic stem cells (HSCs) are also found in even more abundance, primarily in or near niches adjacent to the endosteal bone surface or capillaries [1]. These HSCs are multi-potent with short and long term regenerative capacity to differentiate into the myeloid, lymphoid and erythroid lineages. Recently, attention has turned to cells within the marrow that originate from a mesenchymal source; these include a wide array of developmentally distinct cells including adipocytes, osteoblasts, and stromal fibroblasts. Mesenchymal stromal cells (MSCs) are also multi-potent and within the marrow can become mature adipocytes or osteoblasts [2]. These cells differ from HSCs in several important ways, including their origin, their ability to adhere to plastic in vitro and potentially their use of substrates for differentiation [3].
The bone marrow niche is an anatomical and functional unit that integrates endocrine, autocrine and paracrine signals for whole body homeostasis and provides a unique home for the stem cell [4]. Therefore it is not surprising that substrate availability plays an important role in cell fate decisions. Cells in the niche also influence their neighbors; for example adult mesenchymal cells, and in particular the osteoblast, have a vital role in maintaining the bone marrow niche for hematopoiesis [5]. The role of the adipocyte in hematopoiesis is more controversial but it is thought that these cells prevent active hematopoiesis at least during times of repopulation of the marrow after HSC transplantation [6].
The bone marrow niche has tremendous energy requirements. Over 2 million erythrocytes leave the marrow every second just to sustain the hemoglobin carrying capacity for oxygen, and this is only one of many hematopoietic cell types that are differentiating and then exiting the niche. During an inflammatory response anywhere in the body or activation of the sympathetic nervous system, neutrophils and some hematopoietic progenitors enter the circulation in large amounts [7]. These processes require adenosine triphosphate (ATP) and those molecules are principally derived from either glycolysis or oxidative phosphorylation in each cell. Moreover, the niche has to nourish progenitors and this requires not only nutrients but also support for their metabolism to generate ATP for subsequent use by the cell. During acute infectious episodes, hematopoietic cells may require an additional energy burst, probably through oxidation of fatty acids, to function properly. The bone marrow niche is uniquely suited anatomically to provide that support because of its rich vasculature, and, in the endosteal niche, abundant neural innervation [8]. There are also areas of hypoxia, which are attractive regions for HSC homing, particularly for quiescent cells [9]. Hypoxia is a potent stimulus for hypoxia-inducible factor 1-alpha (HIF1a), which can then induce a differentiation program for both pre-osteoblasts and HSCs [10]. HIF1a also serves to stimulate vascular endothelial growth factor (VEGF) that can in turn enhance vascular development and promote glycolysis through up-regulation of several key genes including glucose transporter 1 (GLUT1) [11].
In this review, we will discuss the energy needs of bone marrow MSCs particularly as it relates to lineage allocation between osteoblasts and adipocytes. We will then support the premise that those cell fate decisions have major clinical implications.
II. Bioenergetics of mesenchymal stem cells, osteoblast and adipocyte
Each of the cells in the bone marrow niche has its own specific nutrient requirement in order to survive, particularly in a hypoxic environment and these, in turn, are linked to ATP demands. In general, the more differentiated the cell, the greater the energy needs. However, within that context, there are major differences between mature osteoblasts and adipocytes. First, when considering survival and maintenance of MSC ‘stemness’ in the relative hypoxia of the marrow, there is a need for metabolic adaptation. Stemness allows a stable pool of progenitors that can be called on in numerous circumstances, particularly injury and inflammatory states [12]. Hypoxia induces the stabilization of HIF1a, a major transcription factor for stem cells and progenitors as well as multiple downstream target genes, particularly VEGFa [13]. Metabolic reprogramming of quiescent cells is necessary to prevent differentiation and this occurs through a shift from oxidative phosphorylation to glycolysis. Importantly, glycolysis, although less efficient in generating ATP than mitochondrial oxidation, reduces oxidative stress and reactive oxygen species (ROS) generation, key elements that drive stem cell differentiation.
Although glycolysis is the major driver of ATP generation in MSCs, entrance into a specific differentiation program, either adipogenic or osteogenic, requires distinct metabolic requirements that are very context specific [14]. For adipocytic differentiation, several studies have suggested that mitochondrial oxidation of fatty acids and the generation of ROS are essential to achieve full maturation. The process of glucose entry and fatty acid oxidation through the Krebs Cycle generates more molecules of ATP per mol of glucose (36:1) than glycolysis (2:1), but it comes at a cost as mitochondrial respiration leads to the generation of ROS from the electron transport chain (ETC). ROS (e.g. H2O2, superoxides) can further suppress mitochondrial respiration and promote an adipogenic program that is associated with more insulin resistance and less lipolysis [15, 16]. Excess ROS in adipocytes may also cause mitochondrial DNA damage or further changes to complex I in the ETC leading to metabolic dysfunction [17]. But, some ROS is generated during normal adipocyte differentiation; much less is produced during early stages of osteoblast maturation.
During osteoblastic differentiation, glycolysis is the predominant ATP generator, even though it is less efficient per mol of glucose [14]. Glycolysis can occur rapidly and can happen in both hypoxic and normoxic states (i.e. Warburg effect). Two key proteins, Glut1, the principal glucose transporter and lactate dehydrogenase (LDH), the enzyme that converts pyruvate to lactate are essential during glycolysis. Karsenty and colleagues showed that in osteoblasts, glucose uptake through the Glut 1 transporter inhibits AMPK, which in turn prevents ubiquitination of runt-related transcription factor 2 (Runx2) [11]. In a feed forward system, Runx2 begins the differentiation program in osteoblasts and increases Glut1 expression. Furthermore, pre-osteoblasts differentiate under the influence of various ligands, particularly the Wnts and insulin-like growth factors (IGFs). Long and colleagues demonstrated that glycolysis is a major feature of Wnt3a induced osteoblast differentiation [18]. More recently the Long group reported that HIF1a, which is important for ‘stemness’, is also a critical transcriptional regulator of glycolysis, triggered in part, by relative hypoxia in the bone marrow niche [19]. Remarkably, much older ex vivo studies from Neumann and colleagues demonstrated that parathyroid hormone (PTH) produces lactic acid in calvarial osteoblasts supporting the tenet that osteoblasts utilize glycolysis to generate lactate and fuel collagen synthesis and mineralization [20]. Guntur et al also showed that glycolysis was essential for terminal differentiation of osteoblasts and that oxidative phosphorylation was more important early in the differentiation scheme [21]. Thus there appears to be a distinct metabolic program that features a transient phase of oxidative phosphorylation following glycolysis and that is then switched off as glycolysis re-emerges as a predominant driver of ATP. Indeed, recent studies suggest that both oxidative phosphorylation and glycolysis are occurring in differentiating cells, and that it is the relative change in one versus the other that predominates. Too much oxidative phosphorylation or glycolysis can inhibit the other, so there clearly must be a fine balance, which is very context specific and is driven by immediate ATP demands.
The transient phase of mitochondrial respiration is very time sensitive and may occur in vitro between days three and nine of osteoblastic differentiation in vitro. During this time period, AMPK is activated and this may induce lipophagy as well as oxidative phosphorylation [22]. Other studies have shown that metformin, which up-regulates AMPK can enhance differentiation but only during specific time periods. In a similar vein, Karner et al. demonstrated that glutaminolysis is essential for osteoblast differentiation through the Wnt signaling system [23]. Hence, there are at least three substrates for ATP generation and differentiation of MSCs: glutamine- which enters the Krebs Cycle via alpha ketoglutarate, glucose- which through glycolysis can generate lactate as well as ribose nucleotides via the pentose phosphate shunt, and fatty acids- which are metabolized via acetyl CoA in the mitochondria. Generation of acetyl coenzyme A (acetyl CoA) can also lead to enhanced nuclear acetylation that in turn can impact transcriptional processes unifying the processes of protein production and energy utilization [24]. Finally autophagy cannot be overlooked as a fueling mechanism for the cell particularly during times of stress or nutrient deficiency. AMPK stimulates autophagy as well as glycolysis and inhibits mammalian target of rapamycin (mTOR) and overall protein synthesis [25]. This would also lead to increased fatty acid entry into the mitochondria for ATP generation as lipophagy is also stimulated. The recent finding from McGhee Lawrence that a small percent (~15%) of Runx2 positive cells in a mouse model of HDAC3 null mice with a phenotype of accelerated aging, had large lipid droplets that stained positive for perilipin, raises a provocative question about fuel utilization and the plasticity of marrow progenitors [26]. If indeed those cells represent a hybrid (i.e. an ‘osteo-adipocyte’) in which osteoblasts exhibit impaired lipid utilization, it implies that switching between adult cell phenotypes can occur in response to nutrient sensing or environmental stressors.
In summary, the bone marrow niche has significant energy requirements that are tissue and temporal specific. Allocation into the osteoblast or adipocyte lineage is dictated by multiple transcription factors, which in turn must be governed by specific metabolic programs. On the other hand, changes in energy availability are certain to alter cell fate decisions and these in turn can impact metabolic homeostasis. Taken together, the determination of the identity of marrow adipocytes as osteoblasts in disguise, or a novel fat cell, is a critical question because of the intimate relationship between adipogenesis and skeletal remodeling.
III. A Window into Cell Fate Decisions in the Marrow: Clinical Scenarios of Marrow Adiposity
At birth, the human bone marrow consists entirely of hematopoietic marrow, but during skeletal growth hematopoietic marrow is gradually replaced by adipose marrow tissue. This conversion proceeds from the peripheral to the central skeleton and by the age of 25 when peak bone mass is obtained, the conversion is almost complete in the femora whereas in the vertebrae 30–40% of the marrow consists of adipose tissue. During ageing, the conversion of hematopoietic to adipose marrow in the vertebrae continues, but now, in contrast, is accompanied by a decrease in bone mass.
In humans, marrow adiposity can be measured histologically by quantitating the amount of bone marrow fat in bone biopsies. Because of the invasiveness of the bone biopsy procedure, non-invasive imaging methods have been developed and magnetic resonance imaging (MRI) is currently standard practice to evaluate bone marrow adiposity in humans. MRI separates the water from the fat signal in the bone marrow and this enables calculation of the fat fraction in the bone marrow. Studies in healthy adults have shown that the marrow adiposity of the lumbar spine increases approximately 7% per decade from 30% at age 30 to >60% at the age of 80 years [27–29]. Comparing men and women, marrow adiposity is 10% greater in men than in women until menopause, when in women marrow adiposity accumulation accelerates resulting in higher marrow adiposity in women than men after menopause [27, 28]. Bone mass in humans is mostly evaluated by dual energy x-ray scanning (DXA). This technique uses two X-ray beams to measure the bone mineral content and the bone area and then calculates an areal bone mineral density (BMD). This is compared to a reference population and yields a standard deviation T-score that is used to diagnose osteopenia (T-score between −1 and −2.5) and osteoporosis (T-score <−2.5). In addition, DXA can also provide an estimate of an overall fat mass index but not marrow fat. The relation between marrow adiposity and bone mass has been studied extensively in the healthy adult population and an inverse relation was shown consistently across age groups in women and men in both the lumbar spine and the femur [30–33].
Many diseases are associated with increased fracture risk such as osteoporosis, anorexia nervosa, obesity, diabetes, Cushing’s disease, HIV and lipodystrophy. Some of these are characterized by decreases in bone mass, whereas in others areal BMD is maintained. Growing interest in marrow adiposity as a contributing factor to fracture risk has spurred the investigation of marrow adiposity in these diseases. Postmenopausal osteoporosis is characterized by accelerated bone loss following menopause in women ultimately leading to osteoporotic fractures classically of the vertebrae, femoral neck and forearm [34]. In osteoporotic patients, marrow adiposity is 10% higher compared to healthy age-matched subjects [30, 35–38]. Osteopenic patients have 5% higher marrow adiposity compared to healthy age-matched subjects suggesting a direct relation between BMD and marrow adiposity. Furthermore increased marrow adiposity is an independent risk factor for fracture [30] and vertebral weakness as measured by endplate depression and vertebral wedging [39].
Anorexia nervosa is a psychiatric disease defined by restricted eating patterns and profound weight loss resulting in low body mass. Despite the profound loss of body fat (visceral and subcutaneous), anorectic women have much higher vertebral marrow fat than healthy age-matched controls. In addition, bone mineral density is lower in anorexia and fractures are frequently occurring. In patients recovered from anorexia, vertebral marrow fat and bone mineral density are again comparable to healthy controls [40, 41].
Confirming the different behavior of body fat and marrow fat in underweight subjects, in normal weight subjects there is no clear correlation between marrow fat and other fat depots [42] or with cardiovascular risk factors such as insulin sensitivity and lipid profile. In obese subjects on the contrary, high body fat is positively related to high marrow fat and obese subjects have significantly higher marrow fat than normal weight controls [43, 44]. This implies that the regulation of marrow fat versus visceral and subcutaneous fat is different and depends at least partly, on the weight of the individual. Overweight individuals with and without diabetes type 2 have the same amount of marrow adiposity, although in the diabetic individuals there is a clear correlation with glycaemic control; the higher the HbA1c, the higher the marrow fat [45]. In addition, diabetic subjects had lower unsaturated lipid fraction of the marrow fat than the healthy same-weight subjects. Diabetes type 1 patients generally maintain normal body weight and again, there was no difference in marrow fat amount between healthy and type 1 diabetic, weight-matched subjects [46]. Comparing the studies between type 1 and 2 diabetic patients, this again shows that overweight individuals have higher marrow fat that normal weight individuals, although the studies used slightly different methods to estimate the amount of marrow fat. Interestingly, in the normal-weight healthy versus type 1 diabetes study, there was a positive correlation between serum lipids and marrow adiposity but not with HbA1c.
Cushing’s syndrome is characterized by hypercortisolemia and accompanied by increased subcutaneous and visceral fat. So far, only one study investigated the amount of marrow fat in Cushing syndrome patients and did not find a difference with healthy controls, although this was in just seven patients [47]. HIV infection is also accompanied by metabolic derangements and increased risk of fracture. Two studies showed that HIV patients have lower marrow fat than age and BMI-matched controls. Lipodystrophy, a loss of body fat occurring in some HIV patients treated with anti-retroviral therapy, augmented the decrease in marrow fat [48, 49]. Lipodystrophy can also present as a congenital syndrome of partial or generalized loss of body fat caused by a genetic mutation leading to a defect in adipocyte formation or maintenance. These patients have absent or low marrow fat, depending on the mutation and the resulting defect in adipocyte development (reviewed in [50]). The patients with absent marrow fat present with more severe bone phenotypes and metabolic derangements, implying that a lack of marrow fat, comparable to an excess of marrow fat, can be associated with negative effects on bone and metabolic health as well. Furthermore, this shows that a state of low body fat can be accompanied by both low and high marrow fat, implying that the mechanism behind the loss of body fat determines the effect on the marrow fat and not just the amount of fat itself.
Finally, classically an important determinant of both bone and body fat mass, exercise is also linked to changes in bone marrow fat. Both loss of exercise due to spinal cord injury as well as high impact exercise can have an effect on marrow fat. Spinal cord injury is associated with an increase in marrow fat; this was previously investigated in 1988 using bone biopsies [51] and later confirmed using MRI [52]. In both studies, the gain in marrow fat was associated with a decrease in bone mass and bone quality. A recent study with competitive athletes in different sports showed that athletes in impact versus non-impact sports and nonathletic healthy controls have lower marrow adiposity in the tibia and this was accompanied by a higher cortical bone and bone strength [53].
In conclusion, the relations between bone, body fat, weight, metabolic (insulin resistance, insulin deficiency, hypercortisolemia) and hormonal status, exercise and marrow fat are complex and the mechanisms behind the observed effects are not completely clarified or understood yet. Clinical interventional studies in humans and animal studies could shed light on these issues.
Several intervention trials studying the effectiveness of osteoporosis treatment have reported effects on marrow fat. First, Syed et al treated osteoporotic postmenopausal women with estrogen in a placebo-controlled trial during one year and showed that estrogen treatment prevented the increase in marrow fat observed in the control group resulting in a difference in marrow fat between the groups as assessed by bone biopsies, in addition to increasing bone mineral density [54]. Limonard et al showed that the decrease in marrow fat by estrogen is already present after two weeks of treatment as assessed by MRI and is accompanied by a decrease in bone resorption and an increase in bone formation as assessed by bone markers [55]. Duque et al treated postmenopausal osteoporotic women with risedronate (bisphosphonate) in a placebo-controlled trial during three years and showed that risedronate decreased marrow fat compared to an increase in the control group as assessed by bone biopsies, again in addition to increasing bone mineral density [56]. Cohen et al treated premenopausal women with low bone mass or fragility fractures with teriparatide (parathyroid hormone) during 18–24 months in a pilot trial and showed that marrow fat fraction decreased after 12 months as assessed by bone biopsies, in addition to increases in bone parameters [57]. Recently, Yang et al treated osteopenic postmenopausal women with teriparatide in a placebo-controlled trial during 12 months and demonstrated a reduction in marrow fat fraction by almost 6% after 12 months as assessed by MRI [58].
No interventional or prospective studies have been reported in anorexia patients or caloric restriction in normal weight humans so far, but Schafer et al studied the effect of weight loss following gastric bypass surgery on marrow fat in overweight individuals [59]. Six months after gastric bypass, 5 out of 6 diabetic individuals could stop taking diabetes medication and normalized their HbA1c, body fat was markedly reduced in all subjects, and this was accompanied by a decrease in bone mineral density. Marrow fat though, decreased almost 8% in the diabetic patients, but did not change in the overweight non-diabetic subjects. However at baseline, the diabetic subjects had 10% higher marrow fat than the non-diabetic subjects, so the effect on the marrow fat seems to be related to the change in HbA1c and not the weight loss, as reported previously in the study by Baum et al. Bosy-Westphal showed a >10% decrease in bone marrow adiposity measured with MRI in the arms, legs and pelvis in overweight premenopausal women and men in a 12 week diet intervention study (mean weight loss 9%) and observed an increase in bone marrow adiposity with weight regain [60]. Cordes et al on the contrary did not find a change in vertebral bone marrow fat in obese women during a 4-week diet intervention (mean weight loss 7%) although other fat depots (liver, subcutaneous and visceral) did show a decrease [61].
On the contrary, rosiglitazone, an antidiabetic drug to treat diabetes type 2 (PPARg agonist), is associated with increased fracture rates and deterioration of bone strength. This negative effect on bone was hypothesized to result from an increase in marrow fat by increased adipocyte formation due to peroxisome proliferator-activated receptor gamma (PPARg) activation. However, Harslof et al surprisingly showed in a placebo-controlled trial in postmenopausal healthy women with rosiglitazone treatment during 14 weeks, that rosiglitazone decreased the marrow fat fraction as assessed by MRI in 6 out of 8 subjects in addition to decreasing bone mineral density [62].
Growth hormone is an important hormone during growth and skeletal development, coinciding with the conversion of hematopoietic to fatty marrow. Abdominal adiposity is associated with lower growth hormone secretion and BMD. Therefore Bredella et al treated premenopausal healthy, abdominally obese women with growth hormone in a placebo-controlled trial during 6 months [63]. Growth hormone treated women had an increase in marrow fat during the study period, whereas in the control group there was a decrease in marrow fat, resulting in a significant difference between the groups. In addition the bone formation markers osteocalcin and N-terminal propeptide of type 1 procollagen (P1NP) increased as well, indicating a positive effect on bone metabolism. The interpretation of this study remains difficult considering the unexpected decrease in marrow fat in the control group.
A number of intervention studies investigated the effect of exercise or bed rest on marrow fat. Trudel et al performed two intervention studies, one in women and one in men, comparing the effect of 60 days of bed rest, to bed rest in combination with exercise or vibration [64, 65]. Both studies showed that 60 days of bed rest increases marrow fat fraction by approximately 3% and this effect is still present one year post-intervention. In the first study in women, exercise during the bed rest period did not prevent the increase in marrow fat. In the second study in men however, exercise decreased the fat fraction during the first 30 days, but not after 60 days and vibration decreased the marrow fat at both time points. Daly et al studied the effect of 18 months of exercise in older men in a controlled trial and showed that 18 months of training decreased the marrow fat fraction together with an increase in BMD, independent of a decrease in body fat [66]. Casazza studied the effect of 3 times per week exercise in 5-year old children during a controlled trial of 10 weeks and although they did not observe any changes in bone parameters, marrow adiposity decreased in the exercise group whereas it increased in the control group as expected during this skeletal growth period [67].
These studies show that interventions targeting improvement in bone mineral density in osteoporosis such as estrogen, bisphosphonates, parathyroid hormone and exercise, generally result in a decrease in marrow fat. However, interventions with less well-described mechanisms and in different patient groups can have several, sometimes surprising, effects on marrow fat (table 1).
Table 1.
| Human Studies | ||||
|---|---|---|---|---|
| Bone Mass | BM fat | Body Fat | Fracture Risk | |
| Observational studies | ||||
| Growth | ↑ | ↑ | ↑ | — |
| Aging | ↓ | ↑ | ↑ | ↑ |
| Osteoporosis | ↓ | ↑ | — | ↑ |
| Anorexia nervosa | ↓ | ↑ | ↓ | ↑ |
| Obesity | ↑ | ↑ | ↑ | |
| Diabetes | ↑ | ↑ | ↑ | ↑ |
| HIV | ↓ | ↓ | ↓ | ↑ |
| Lipodystrophy | ↓ | ↓ | ↓ | ↑/↓ |
| Interventional studies | ||||
| TZD | ↓ | ↓ | — | ↑ |
| Exercise | ↑ | ↓ | ↓ | ↓ |
| Estrogen | ↑ | ↓ | ↓ | ↓ |
| Bisphosphonate | ↑ | ↓ | — | ↓ |
| PTH | ↑ | ↓ | — | ↓ |
| Bariatric surgery | ↓ | ↓/—* | ↓ | |
| Growth hormone | ↑ | ↑ | — | ? |
depending on HbA1c
IV. Mechanisms
Several possible mechanisms have been proposed to explain the reciprocal relation between bone and marrow fat, or more specifically, the osteoblast and adipocyte. The central mechanism concerns the differentiation of the mesenchymal stem cell into either the osteoblastic or the adipocytic lineage. This differentiation is controlled by the osteoblastic transcription factor, Runx2 and the adipogenic transcription factor PPARg [68, 69]. In vitro experiments with mouse MSCs have shown that the addition of estrogen in vitro increases cell proliferation, expression of osteoblastic markers, alkaline phosphatase (AP) activity and calcium content [70, 71]. Mouse MSCs overexpressing the estrogen receptor showed an increase in AP activity and a decrease in adipocyte formation accompanied by a decrease in PPARg expression [72]. Human MSCs cultured with estrogen increase proliferation whereas adipocyte differentiation is decreased [73, 74].
PPARg is an essential adipocytic transcription factor, member of the nuclear hormone receptor superfamily and activated by ligands such as fatty acids, peroxisome proliferators and thiazolidinediones. Upon activation, PPARγ heterodimerizes with the retinoid X receptor and binds to the PPAR responsive element (PPRE) on the promoters of target genes. PPARg has also been described to act by interfering with the activator protein 1 (AP-1), transforming growth factor beta (TGFβ) or Wnt signalling pathways. To elucidate the function of PPARg, genetic mouse models have been developed. Homozygous knockout of the PPARg gene is embryonically lethal because of placental dysfunction. Embryonic stem cells of these mice lack adipogenesis in vitro and spontaneously initiate osteogenesis, which can be reduced by reintroduction of the PPARg gene. Heterozygous PPARg +/− mice develop normally but investigation of the bones of these mice shows an increase in trabecular bone mass by 40% caused by an increase in number of osteoblasts, accompanied by a lower number of adipocytes in the bone marrow. Ageing of the mice decreases bone mass, but to a lesser extent in the heterozygous mice than in the wildtype. Ovariectomy in female mice shows no difference in bone loss in heterozygous and wildtype mice [75]. Male mice overexpressing the PPARg gene specifically in osteoblasts, show a decrease in bone volume but no difference in adipocytes. Female mice overexpressing PPARg in osteoblasts do not have a difference in bone phenotype compared to wildtype, but following ovariectomy, overexpressing mice have an exaggerated bone loss compared to the wildtype mice [76]. Therefore, loss of PPARg inhibits adipogenesis and favours osteogenesis whereas PPARg overexpression favours adipogenesis in estrogen-deplete states. Thus, one could hypothesize that estrogen might stimulate osteogenesis and inhibit adipogenesis in the bone marrow by decreasing PPARg signalling.
Other mechanisms are likely operative when considering the fate of adipocytes versus osteoblasts in the niche. For example, zinc finger protein 467 (Zfp467), and Zfp 423 are transcription factors that can direct progenitors into the adipogenic lineage. The former, Zfp467, is downregulated by PTH, and silencing this protein results in suppressed adipogenesis and enhanced osteogenesis. Sclerostin has been reported to stimulate adipogenesis in 3T3-L1 cells. The underlying mechanism may be related to antagonism of the Wnt/Lrp5 signaling pathway, resulting in a shift towards adipocyte development. Dickkopf-related protein 1 (DKK-1), another Wnt antagonist, has also been shown to favor adipogenesis in the marrow. Global overexpression of receptor activator of nuclear factor kappa-B ligand (RANKL), an essential factor for osteoclastogenesis, leads to significant marrow adiposity.
In addition, increase in bone marrow adiposity can result not only from hyperplasia (increase in cell number) but also from hypertrophy (increase in cell size) or an increase in lipid droplet size. The increasing use of MRI to assess bone marrow adiposity in humans has made it difficult to address this issue since MRI cannot discriminate between these processes. However, histomorphometry can provide information on the underlying mechanism and some data addressing this issue are available. Cohen et al have performed serial bone biopsies in a clinical study of teriparatide treatment of premenopausal women with idiopathic osteoporosis [57]. Histomorphometric analysis showed a decrease in adipocyte volume, but no change in adipocyte number or density. Syed et al treated postmenopausal women with estrogen and performed serial bone biopsies. Histomorphometry showed that in the placebo group the adipocyte volume, number and size increased whereas in the estrogen treated women the adipocyte volume decreased and the number and size did not change [54]. These results imply that all of the above mentioned processes (increase in number, size and lipid droplet size) can be present, although it remains uncertain which of these dominates and what circumstances determine the response.
V. Summary
The last decade has been characterized by important advances in our understanding of the marrow niche and particularly the functional aspects of MSCs. But, several features are still not well established. The origin of the marrow adipocyte, as noted, and their identity is still a matter of debate. Whether these cells arise from progenitor stromal cells, bone lining cells or reticular cells in the marrow is not known. Moreover, we still do not have a consistent marker of the marrow adipocyte progenitor despite extensive efforts using lineage tracing. It is not clear if marrow adipocytes sense fuel availability in a manner distinct from other adipocytes, and if they possess unique fatty acid transporters. But it is clear that marrow fat cells are intimately tied to nutrient status and in turn these cells have a profound effect on bone remodeling and ultimately, skeletal integrity. Future directions will undoubtedly define precisely how lineage allocation in the marrow is linked to metabolic homeostasis and this may ultimately lead to new therapeutic options for both obesity and osteoporosis.
Figure 1.
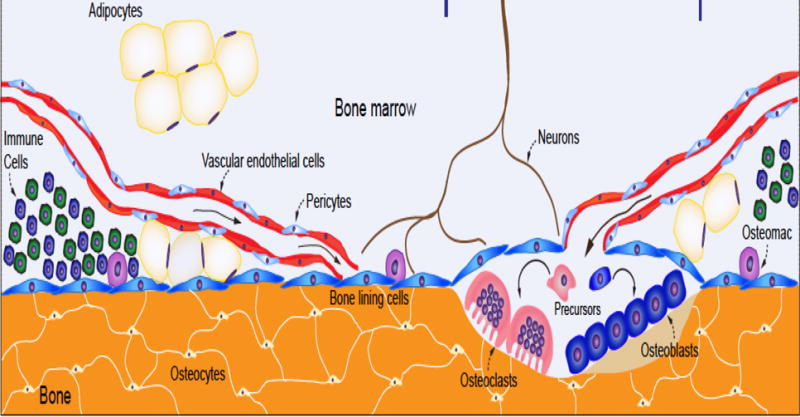
The bone marrow niche is a multicellular compartment of mesenchymal and hematopoietic progenitors that is highly vascularized. Blood supplies basic nutrients that are needed for each of the cell types shown. The marrow adipocytes are located on the endosteal surface of bone as well as throughout the marrow, and are increased in conditions such as calorie restriction and diabetes. It is conceivable that marrow adipocytes could arise from vascular endothelial cells, pericytes, bone lining cells or mesenchymal precursors. Notwithstanding their unclear origin, both osteoblast and adipocyte precursors require nutrients to fuel the needs of differentiation. This need links the marrow compartment with the peripheral sources of energy, particularly fat depots and the liver. Hence, both transcriptional and metabolic programming determine the fate of mesenchymal progenitors, which in turn, define the marrow adiposity phenotype.
Highlights.
The bone marrow niche contains hematopoietic and mesenchymal stem cells
Cell fate of the stem cells is influenced by substrate availability and utilization
Marrow adiposity can result from increases in adipocyte number, adipocyte size and lipid droplet size
Increased marrow adiposity is associated with skeletal deterioration
Acknowledgments
Funding
This work was supported by the National Institutes of Health [NIDDK: DK 092759-06] (CR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Both authors contributed equally to the conception and design of the review, the analysis and interpretation of the literature, drafting the article and approving the final version of the manuscript.
No conflicts of interest
References
- 1.Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116:375–85. doi: 10.1182/blood-2009-07-233437. [DOI] [PubMed] [Google Scholar]
- 2.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianco P. “Mesenchymal” stem cells. Annual review of cell and developmental biology. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 4.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuznetsov SA, Riminucci M, Ziran N, Tsutsui TW, Corsi A, Calvi L, et al. The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. The Journal of cell biology. 2004;167:1113–22. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 8.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, et al. The Essential Functions of Adipo-osteogenic Progenitors as the Hematopoietic Stem and Progenitor Cell Niche. Immunity. 2010;33:387–99. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, et al. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509–27. doi: 10.18632/oncotarget.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagegg M, Gaber T, Lohanatha FL, Hahne M, Strehl C, Fangradt M, et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS One. 2012;7:e46483. doi: 10.1371/journal.pone.0046483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, et al. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell. 2015;161:1576–91. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268–77. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- 13.Imanirad P, Dzierzak E. Hypoxia and HIFs in regulating the development of the hematopoietic system. Blood cells, molecules & diseases. 2013;51:256–63. doi: 10.1016/j.bcmd.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esen E, Long F. Aerobic glycolysis in osteoblasts. Curr Osteoporos Rep. 2014;12:433–8. doi: 10.1007/s11914-014-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Si Y, Shirihai OS, Si H, Schultz V, Corkey RF, et al. Respiration in adipocytes is inhibited by reactive oxygen species. Obesity (Silver Spring, Md) 2010;18:1493–502. doi: 10.1038/oby.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ushio-Fukai M, Rehman J. Redox and metabolic regulation of stem/progenitor cells and their niche. Antioxidants & redox signaling. 2014;21:1587–90. doi: 10.1089/ars.2014.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong X, Bi L, He S, Meng G, Wei B, Jia S, et al. FFAs-ROS-ERK/P38 pathway plays a key role in adipocyte lipotoxicity on osteoblasts in co-culture. Biochimie. 2014;101:123–31. doi: 10.1016/j.biochi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17:745–55. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, et al. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc Natl Acad Sci U S A. 2014;111:8673–8. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols FC, Neuman WF. Lactic acid production in mouse calvaria in vitro with and without parathyroid hormone stimulation: lack of acetazolamide effects. Bone. 1987;8:105–9. doi: 10.1016/8756-3282(87)90078-0. [DOI] [PubMed] [Google Scholar]
- 21.Guntur AR, Le PT, Farber CR, Rosen CJ. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology: The Endocrine Society. 2014 doi: 10.1210/en.2013-1974. p. en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi G, Rosen CJ, Clemmons DR. IGF-I and IGFBP-2 Stimulate AMPK Activation and Autophagy, Which Are Required for Osteoblast Differentiation. Endocrinology. 2016;157:268–81. doi: 10.1210/en.2015-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karner CM, Esen E, Okunade AL, Patterson BW, Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest. 2015;125:551–62. doi: 10.1172/JCI78470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–6. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 25.Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52:524–31. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 26.McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, et al. Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells. Journal of Bone and Mineral Research. 2016;31:116–28. doi: 10.1002/jbmr.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. Journal of Magnetic Resonance Imaging. 2001;13:263–8. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Griffith JF, Yeung DKW, Ma HT, Leung JCS, Kwok TCY, Leung PC. Bone marrow fat content in the elderly: A reversal of sex difference seen in younger subjects. Journal of Magnetic Resonance Imaging. 2012;36:225–30. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 29.Ergen FB, Gulal G, Yildiz AE, Celik A, Karakaya J, Aydingoz U. Fat fraction estimation of the vertebrae in females using the T2*-IDEAL technique in detection of reduced bone mineralization level: comparison with bone mineral densitometry. J Comput Assist Tomogr. 2014;38:320–4. doi: 10.1097/RCT.0b013e3182aa4d9d. [DOI] [PubMed] [Google Scholar]
- 30.Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217:527–38. doi: 10.1148/radiology.217.2.r00nv20527. [DOI] [PubMed] [Google Scholar]
- 31.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96:782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 33.Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab. 2010;95:2977–82. doi: 10.1210/jc.2009-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black DM, Rosen CJ. Postmenopausal Osteoporosis. The New England journal of medicine. 2016;374:2096–7. doi: 10.1056/NEJMc1602599. [DOI] [PubMed] [Google Scholar]
- 35.Yeung DKW, Griffith JF, Antonio GE, Lee FKH, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. Journal of Magnetic Resonance Imaging. 2005;22:279–85. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 36.Griffith JF, Yeung DKW, Antonio GE, Wong SYS, Kwok TCY, Woo J, et al. Vertebral Marrow Fat Content and Diffusion and Perfusion Indexes in Women with Varying Bone Density: MR Evaluation1. Radiology. 2006;241:831–8. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 37.Tang GY, Lv ZW, Tang RB, Liu Y, Peng YF, Li W, et al. Evaluation of MR spectroscopy and diffusion-weighted MRI in detecting bone marrow changes in postmenopausal women with osteoporosis. Clinical Radiology. 2010;65:377–81. doi: 10.1016/j.crad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Kuo D, Schafer AL, Porzig A, Link TM, Black D, et al. Quantification of vertebral bone marrow fat content using 3 tesla MR spectroscopy: Reproducibility, vertebral variation, and applications in osteoporosis. Journal of Magnetic Resonance Imaging. 2011;33:974–9. doi: 10.1002/jmri.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. Bone Marrow Fat and Bone Mineral Density on Proton MR Spectroscopy and Dual-Energy X-Ray Absorptiometry: Their Ratio as a New Indicator of Bone Weakening. American Journal of Roentgenology: American Roentgen Ray Society. 2004:1761–5. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- 40.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27:1864–71. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Iorgi N, Mittelman SD, Gilsanz V. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes. 2008;32:1854–60. doi: 10.1038/ijo.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring, Md) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48:748–54. doi: 10.1016/j.bone.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? Journal of magnetic resonance imaging: JMRI. 2012;35:117–24. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. Journal of diabetes and its complications. 2012;26:1–9. doi: 10.1016/j.jdiacomp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Mayo-Smith W, Rosenthal DI, Goodsitt MM, Klibanski A. Intravertebral fat measurement with quantitative CT in patients with Cushing disease and anorexia nervosa. Radiology. 1989;170:835–8. doi: 10.1148/radiology.170.3.2916039. [DOI] [PubMed] [Google Scholar]
- 48.Mulkern RV, Huang J, Vajapeyam S, Packard AB, Oshio K, Grinspoon S. Fat fractions and spectral T2 values in vertebral bone marrow in HIV- and non-HIV-infected men: A 1H spectroscopic imaging study. Magnetic Resonance in Medicine. 2004;52:552–8. doi: 10.1002/mrm.20205. [DOI] [PubMed] [Google Scholar]
- 49.Huang JS, Mulkern RV, Grinspoon S. Reduced intravertebral bone marrow fat in HIV-infected men. AIDS (London, England) 2002;16:1265–9. doi: 10.1097/00002030-200206140-00009. [DOI] [PubMed] [Google Scholar]
- 50.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Annals of the New York Academy of Sciences. 2014:n/a–n/a. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minaire P, Edouard C, Arlot M, Meunier PJ. Marrow changes in paraplegic patients. Calcif Tissue Int Calcified Tissue International: Springer-Verlag. 1984:338–40. doi: 10.1007/BF02405340. [DOI] [PubMed] [Google Scholar]
- 52.Gorgey AS, Poarch HJ, Adler RA, Khalil RE, Gater DR. Femoral Bone Marrow Adiposity and Cortical Bone Cross-Sectional Areas in Men With Motor Complete Spinal Cord Injury. PM&R. 2013;5:939–48. doi: 10.1016/j.pmrj.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Rantalainen T, Nikander R, Heinonen A, Cervinka T, Siev+ñnen H, Daly RM. Differential Effects of Exercise on Tibial Shaft Marrow Density in Young Female Athletes. Journal of Clinical Endocrinology & Metabolism. 2013;98:2037–44. doi: 10.1210/jc.2012-3748. [DOI] [PubMed] [Google Scholar]
- 54.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int Osteoporosis International: Springer-Verlag. 2008:1323–30. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limonard EJ, Veldhuis-Vlug AG, van Dussen L, Runge JH, Tanck MW, Endert E, et al. Short-Term Effect of Estrogen on Human Bone Marrow Fat. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2015;30:2058–66. doi: 10.1002/jbmr.2557. [DOI] [PubMed] [Google Scholar]
- 56.Duque G, Li W, Adams M, Xu S, Phipps R. Effects of risedronate on bone marrow adipocytes in postmenopausal women. Osteoporos Int Osteoporosis International: Springer-Verlag. 2011:1547–53. doi: 10.1007/s00198-010-1353-8. [DOI] [PubMed] [Google Scholar]
- 57.Cohen A, Stein EM, Recker RR, Lappe JM, Dempster DW, Zhou H, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98:1971–81. doi: 10.1210/jc.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Luo X, Xie X, Yan F, Chen G, Zhao W, et al. Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric. 2016;19:285–91. doi: 10.3109/13697137.2015.1126576. [DOI] [PubMed] [Google Scholar]
- 59.Schafer AL, Li X, Schwartz AV, Tufts LS, Wheeler AL, Grunfeld C, et al. Changes in vertebral bone marrow fat and bone mass after gastric bypass surgery: A pilot study. Bone. 2015;74:140–5. doi: 10.1016/j.bone.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bosy-Westphal A, Later W, Schautz B, Lagerpusch M, Goele K, Heller M, et al. Impact of intra- and extra-osseous soft tissue composition on changes in bone mineral density with weight loss and regain. Obesity (Silver Spring, Md) 2011;19:1503–10. doi: 10.1038/oby.2011.40. [DOI] [PubMed] [Google Scholar]
- 61.Cordes C, Dieckmeyer M, Ott B, Shen J, Ruschke S, Settles M, et al. MR-detected changes in liver fat, abdominal fat, and vertebral bone marrow fat after a four-week calorie restriction in obese women. Journal of Magnetic Resonance Imaging. 2015;42:1272–80. doi: 10.1002/jmri.24908. [DOI] [PubMed] [Google Scholar]
- 62.Harslof T, Wamberg L, Moller L, Stodkilde-Jorgensen H, Ringgaard S, Pedersen SB, et al. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab. 2011;96:1541–8. doi: 10.1210/jc.2010-2077. [DOI] [PubMed] [Google Scholar]
- 63.Bredella MA, Gerweck AV, Barber LA, Breggia A, Rosen CJ, Torriani M, et al. Effects of growth hormone administration for 6 months on bone turnover and bone marrow fat in obese premenopausal women. Bone. 2014;62:29–35. doi: 10.1016/j.bone.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trudel G, Payne M, M+ñdler B, Ramachandran N, Lecompte M, Wade C, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. Journal of Applied Physiology. 2009;107:540–8. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- 65.Trudel G, Coletta E, Cameron I, Belav+¢ DL, Lecompte M, Armbrecht G, et al. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. Journal of Applied Physiology. 2012;112:1824–31. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- 66.Daly R, Ebeling P, Kukuljan S, Harvey S, C N. Effects of Exercise on Appendicular Bone Marrow Fat and Its Relationship to Changes in Bone Density, Structure and Strength in Older Men. Journal of Bone and Mineral Research. 2009;24 [Google Scholar]
- 67.Casazza K, Hanks LJ, Hidalgo B, Hu HH, Affuso O. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: A pilot study. Bone. 2012;50:23–7. doi: 10.1016/j.bone.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan Y. PPARgamma in bone homeostasis. Trends Endocrinol Metab. 2010;21:722–8. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98 doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 70.Qu Q, Perala-Heape M, Kapanen A, Dahllund J, Salo J, Vaananen HK, et al. Estrogen enhances differentiation of osteoblasts in mouse bone marrow culture. Bone. 1998;22:201–9. doi: 10.1016/s8756-3282(97)00276-7. [DOI] [PubMed] [Google Scholar]
- 71.Zhou S, Zilberman Y, Wassermann K, Bain SD, Sadovsky Y, Gazit D. Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. Journal of cellular biochemistry Supplement. 2001;(Suppl 36):144–55. doi: 10.1002/jcb.1096. [DOI] [PubMed] [Google Scholar]
- 72.Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, et al. Estrogen Promotes Early Osteoblast Differentiation and Inhibits Adipocyte Differentiation in Mouse Bone Marrow Stromal Cell Lines that Express Estrogen Receptor (ER) +¦ or +¦. Endocrinology: The Endocrine Society. 2002:2349–56. doi: 10.1210/endo.143.6.8854. [DOI] [PubMed] [Google Scholar]
- 73.Zhao JW, Gao ZL, Mei H, Li YL, Wang Y. Differentiation of human mesenchymal stem cells: the potential mechanism for estrogen-induced preferential osteoblast versus adipocyte differentiation. Am J Med Sci. 2011;341:460–8. doi: 10.1097/MAJ.0b013e31820865d5. [DOI] [PubMed] [Google Scholar]
- 74.Hong L, Zhang G, Sultana H, Yu Y, Wei Z. The effects of 17-beta estradiol on enhancing proliferation of human bone marrow mesenchymal stromal cells in vitro. Stem Cells Dev. 2011;20:925–31. doi: 10.1089/scd.2010.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho SW, Yang JY, Her SJ, Choi HJ, Jung JY, Sun HJ, et al. Osteoblast-targeted overexpression of PPARgamma inhibited bone mass gain in male mice and accelerated ovariectomy-induced bone loss in female mice. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26:1939–52. doi: 10.1002/jbmr.366. [DOI] [PubMed] [Google Scholar]


