Abstract
We used electronic circular dichroism (CD) and UV resonance Raman (UVRR) spectroscopy at 204 nm excitation to examine the temperature dependence of conformational changes in cyclic and linear elastin peptides. We utilize CD spectroscopy to study global conformation changes in elastin peptides, while UVRR is utilized to probe the local conformation and hydrogen bonding of Val and Pro peptide bonds. Our results indicate that at 20 °C cyclic elastin predominantly populates distorted β-strand, β-type II and β-type III turn conformations. At 60 °C, the β-type II turn population increases while the distorted β-strand population decreases. Linear elastin predominantly adopts distorted β-strand and β-type III turn conformations with some β-type II turn population at 20 °C. Increasing temperature to 60 °C, results in a small increase in the turn population.
Keywords: Elastin, UV-Resonance Raman Spectroscopy, circular dichroism, inverse temperature transition, β-turns, distorted β-strand
INTRODUCTION
In recent years the unique elasticity and resilience of elastin based peptides has drawn interest from both the biophysics and material science communities.1–16 Elastin is an important structural peptide that enables elastic deformations of biological assemblies. Biological motion, for example, is enabled by elastin’s unique ability to repetitively and reversibly deform upon stress. We each benefit from the elastic properties of elastin, where, hopefully, our aorta reversibly contracts and expands 109 times during our lives. Biochemical, genetic and structural analysis of elastin fibers has demonstrated that the elastin found in skin, blood vessel walls and lung tissue is a composite of an amorphous component (elastin) and a microfibrillar component (fibrillin). Elastin mimetic peptide (VPGXG)n-where n is the number of repeat pentamers, is widely used as a model system for studying elastin’s remarkable viscoelastic properties.
Over the years, considerable effort have been expended to elucidate the mechanism of elastin’s unique elasticity.3–13,17–26 The elastic properties are thought to be associated with or a consequence of elastin’s unusual phase transition behavior, which closely resembles that of the thermal volume phase transition behavior of poly(N-isopropylacryamide) (PNIPAM).27–39 PNIPAM polymers contain both hydrophilic and hydrophobic regions whose exposure to the aqueous medium may change as the polymer collapses at high temperature. The transition temperature of PNIPAM polymers is determined by a delicate balance between amide-water hydrogen bonding, isopropyl group-water interaction, and van der Waals interactions between the hydrophobic groups.30
Elastin exists as a highly mobile expanded chain below a certain characteristic temperature that the elastin literature refers to as the “critical temperature, Tc”. Above Tc, the peptide chain adopts a compact conformation resulting in a significantly decreased radius of gyration and reduced chain mobility.24 The elastin literature calls this thermal behavior an “inverse temperature transition”.20,40 Just as for PNIPAM,29,30 the transition temperature of elastin peptides can be tuned by changing the hydrophobicity of the polymer. In the (VPGXG)n system we can substitute at the X residue.41
The remarkable viscoelastic properties of elastin peptides are thought to arise from weakening of peptide-water interactions at elevated temperatures which results in a hydrophobic collapse of the peptide15,24,40 The balance between enthalpy and entropy determines the critical temperature. Below Tc, the favorable enthalpic contribution from amide-water hydrogen bonds and/or charged side chain-water interactions compensate for the unfavorable entropy arising from solvation of hydrophobic groups.24 The relative importance of this enthalpy advantage is decreased at higher temperatures. Part of this loss results from increased thermal fluctuations which weaken peptide-water hydrogen bonds. The loss of water-peptide hydrogen bonding-derived stabilization, forces the extended peptide chains to contract to minimize the impact of the unfavorable entropy.24 Increased librational entropy42–45 of the released water molecules reduces the entropic cost of hydrophobic solvation and compensates for the loss of configuration entropy incurred upon elastin’s transition.3,4,22,23
Unfortunately, there are no high resolution NMR or X-ray structures of elastin because of the high mobility of the peptide’s backbone.40 This lack of detailed knowledge regarding conformational transitions in elastin has significantly impeded efforts for establishing a clear link between structure and function of elastin peptides. Based on spectroscopic studies, Venkatachalam and Urry23 proposed that that upon an increase in temperature, the highly mobile peptide chains adopt a rigid, well ordered β-spiral conformation. A β-spiral is composed of repetitive β-type II turns, which form a helix-like structure without inter-turn (i − i + 4) hydrogen bonds.17,22,23,46 This model is supported by NMR and Raman studies.47 These spectroscopic studies found that the collapsed state of the polymer resembles the structure of cyclic (VPGVG)3 peptide crystallized from a mixed water/D2O-methanol solvent.17,18,22,23
Below Tc, the cyclic peptide exists in a β-type II turn conformation which at high concentrations precipitates at elevated temperatures, presumably into an ordered β-spiral-like conformation.23 Reiersen et al40 used CD spectroscopy to demonstrate that the transition is independent of chain length. The smallest cooperative unit appears to be the pentamer (VPGXG), indicating that long range interactions do not play a significant role in elastin’s inverse transition. The authors demonstrate that small elastin peptides show a broad transition between 1 and 77 °C where the CD global minimum (200 nm) decreases with increasing temperature. The temperature dependent behavior of (VPGVG)-repeat elastin peptides appears to be pH independent.40
While Venkatachalam and Urry’s β-spiral model is consistent with some of the spectroscopic studies, it does not agree with studies of tissue derived, water-swollen elastin fibers, which indicate that elastin collapses to a random coil-like conformation.48–51 Recent molecular dynamics simulations by Li et al3,4 suggest that the collapsed state of elastin is best described as a “compact amorphous structure”. The MD study found that when starting from an idealized β-spiral conformation at low temperatures, the expanded peptide chain retained significant β-spiral population. At high temperatures, however, β-turns and β-strand-like conformations dominate. These results suggest that the collapsed state of elastin may be best described as a molten globule where the peptide chain locally adopts a short-range β-spiral-like conformation.3,4
In this work we used CD and UVRR to examine temperature-dependent conformation changes in cyclic and linear elastin. Our results indicate that the cyclic peptide predominantly populates distorted β-strand, β-type II and type III turn conformations. In contrast, linear elastin predominantly populates β-type III turn and distorted β-strand conformations, with a minor β-type II turn population, as well.
Experimental
The UV resonance Raman spectrometer has been described in detail elsewhere.52 Briefly, 2 mW of 204 nm UV light was generated by Raman-shifting the 355 nm 3rd harmonic output of an Nd:YAG laser (Coherent, Infinity) in H2 gas. These UV pulses were then focused at ~45 degrees from the normal to produce a sub-mm spot near the interior surface of a rotating fused silica NMR tube (Wilmad) filled with ~1 mL of the sample solution. A 135° backscattering geometry was used for collecting the Raman scattered light, which was then dispersed by a custom-made subtractive double monochromator onto a back thinned CCD camera (Princeton Instruments-Spec 10 System).52
The 15-residue long cyclic (VPGVG)3 and linear elastin GVG(VPGVG)2VP were obtained from the Pittsburgh Peptide Synthesis Facility (PPSF, >95% purity) and used at 1 mg/ml concentrations at pH 7. The cyclic peptide was prepared by cyclization of the linear variant (PPSF, >95% purity). Raman spectra (average of three five minute spectra) were normalized relative to the peak height of the 0.1 M perchlorate’s 932 cm−1 band. Three replicate measurements of separately prepared elastin solutions were recorded to ensure measurement reproducibility. Solution pH was adjusted by adding small aliquots of HCl or NaOH. Temperature dependent CD spectra (average of 10 spectral accumulations) of similarly prepared solutions (pH ~5) at 1 mg/ml were measured on a Jasco-715 spectropolarimeter using a 200 µm path length quartz cell. CD and Raman spectra of linear elastin measured at 20 °C subsequent to the elevated temperature measurements were similar to those measured prior to the temperature increase, indicating the absence of significant photo- or thermal degradation or irreversible aggregation. The CD spectrum of cyclic elastin shows a slight overall decrease in amplitude after heating. This change likely derives from temperature induced degassing/bubble formation on cell walls at high temperatures.
Results and Discussion
Cyclic Elastin
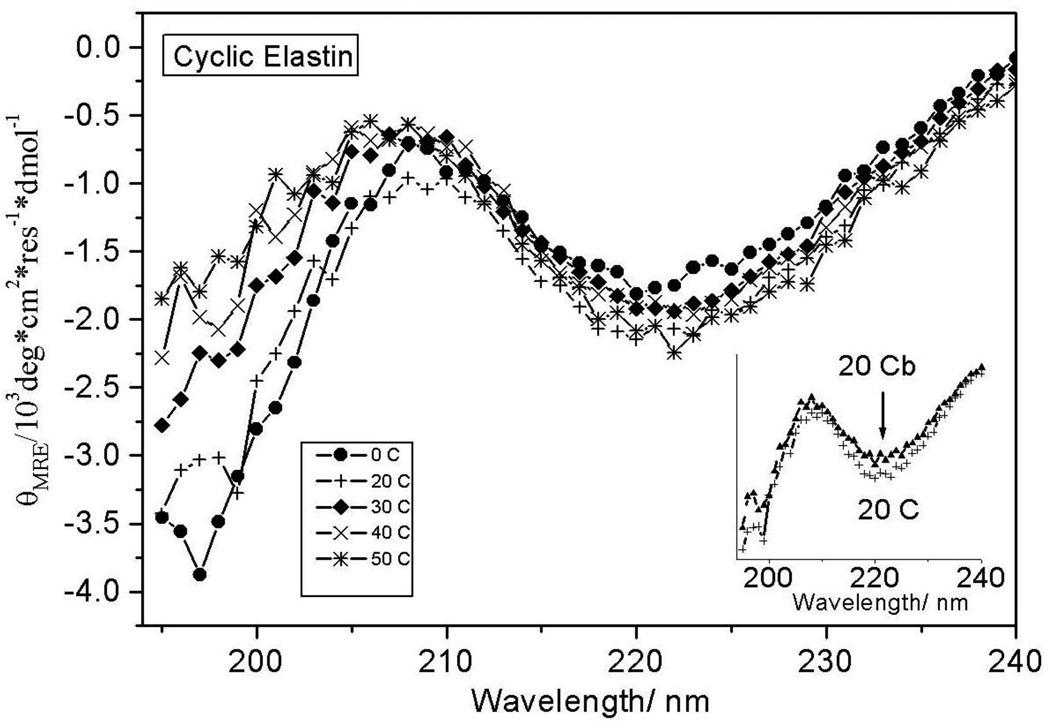
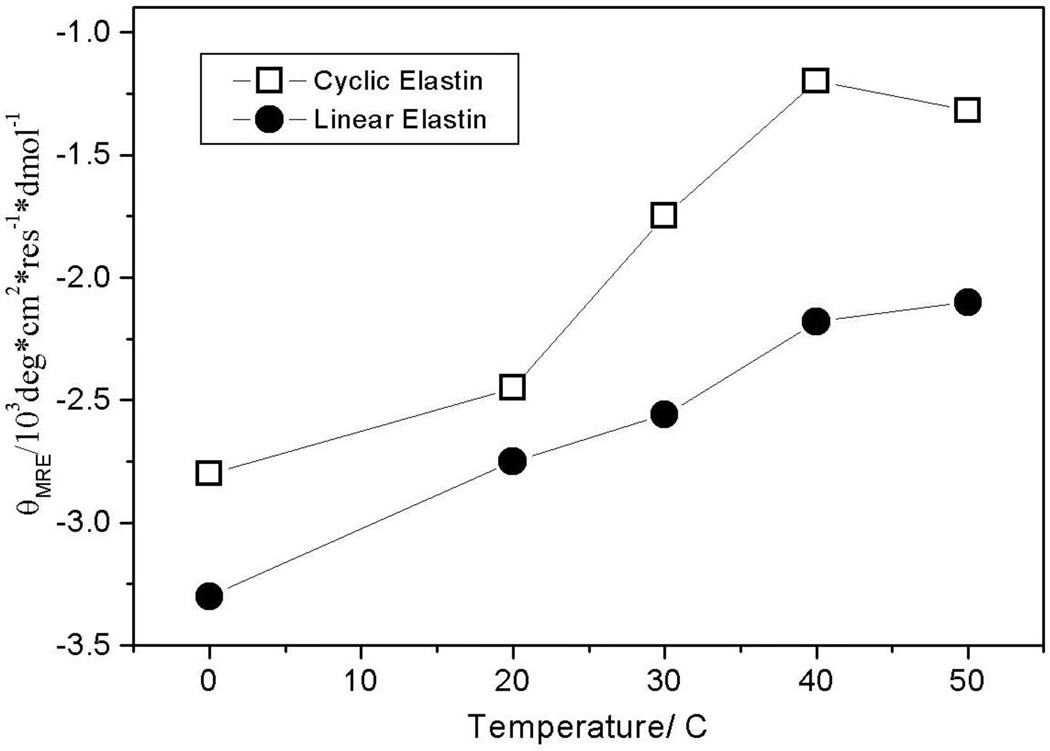
We examined the temperature dependence of cyclic elastin’s conformation by measuring the circular dichroism (CD) spectra between 0 and 50 °C (Fig 1). At 0 °C the cyclic elastin spectrum shows a local minimum at ~222 nm, a maximum at ~207 nm and a global minimum below 200 nm. Previous studies indicate that in elastin peptides the presence of a relatively weak minimum at ~222 nm is indicative of the presence of β-type I/III turn conformation, while the maximum at ~207 nm is indicative of a β-type II turn conformation.24,40,53 It should be noted that typically a minimum at 222 nm is associated with either an α-helix or 310-helix like conformation. However, in such cases the global minimum is located above 200 nm, typically around 207-205 nm.53–55 In cyclic elastin the global minimum is located below 200 nm, which indicates a significant random coil population, thus, excluding the possibility that the feature at 222 nm arises from either α-helix or 310 helix-like conformations. These results indicate the global conformation of cyclic elastin contains significant random coil and β type II and β-type III turn conformations. As the solution temperature is increased, the global minimum value becomes less negative (Fig 2). A decreased global minima is regarded as a hallmark of elastin’s inverse temperature transition.24,56,57 No significant changes are observed in either the 222 or 207 nm region (Fig 1).
Figure 1.
Temperature dependent CD spectra of cyclic elastin. The insert shows the 20 °C CD spectra of cyclic elastin during and subsequent to heating. The CD spectrum subsequent to heating (20 °Cb) shows a slight overall decrease in spectral intensity after heating. This change likely derives from temperature induced degassing/bubble formation on cell walls at high temperatures.
Figure 2.
Temperature dependent changes in the mean residue ellipticities of cyclic and linear elastin at 200 nm.
We further probed the temperature dependence of cyclic elastin’s backbone conformation by measuring the 204 nm excited UV resonance Raman spectra at 20 and 60 °C (Fig 3). There are only three unique types of peptide bonds in elastin, those involving Gly, Pro and Val. The Raman amide bands significantly differ for these peptide bonds such that they are easily differentiated.
Figure 3.
204 nm UV resonance Raman spectra of cyclic elastin measured at 20 and 60 °C normalized to the intensity of 932 cm−1 perchlorate band. The difference spectrum shows temperature dependent changes in the conformation-sensitive AmIII3 region, as well as intensity changes in the Cα-Hb. The AmII'p narrows at 60 °C suggesting the proline peptide bonds adopt a more well-defined conformation at higher temperatures. The trough in the AmII region is an artifact that derives from over-subtraction of the interfering O2 stretching vibration at 1560 cm−1 when the NMR tube contribution was subtracted from the measured spectra.
Proline is a tertiary amide where the pyrrolidine ring side chain loops back onto itself. Consequently, Pro peptide bond lacks the amide hydrogen (NH)58,59 and all the C-N stretching motion is concentrated in a single Raman band labeled AmII'p (amide II' of proline).60 The AmII'p band is thought to be sensitive to local conformation and/or hydrogen bonding of the Pro peptide bond.61–63
In secondary amino acids such as Val and Gly the amide hydrogen’s bending motion (NHb) couples with the C-Ns and CαHb motion, resulting in a complex spectrum that shows multiple bands, namely the AmII (C-Ns with some NHb at ~1550 cm−1), CαHb (1380 cm−1), and various AmIII bands (predominantly C-Ns and NHb, between ~1200 cm−1 and 1280 cm−1).
The Gly peptide bond spectrum spectroscopically differs from other non-prolyl peptide bonds due to its two low mass hydrogen atom side chains. The Gly peptide bonds show a single AmIII band at 1284 cm−1 (AmIIIG) in the amide III region.64,65 In contrast the Val peptide bonds show typical Raman spectra in the AmIII region comprising of the AmIII1, and AmIII2 bands along with the conformationally sensitive AmIII3 band. The conformation sensitivity of the AmIII3 band arises due to Ψ angle dependent coupling between NHb and CαHb motions.66 In extended PPII like conformations the NH and CαH hydrogens are cis allowing for a maximum coupling between NHb and CαHb motions. However, in α-helical conformations the NH and CαH hydrogens are trans which results in insignificant coupling.67 In Gly, this conformation sensitivity may be lost or decreased due to the presence of two Cα hydrogens (CαH2) which allows for some coupling with CαH for all accessible Ψ angles.
Thus, for elastin peptides we use the AmII'p and the Val AmIII3 bands to probe the conformation and/or hydrogen bonding state of Pro and Val peptide bonds. We utilize CD spectroscopy along with the UVRR information on the Val and Pro peptide bonds to infer Gly peptide bond conformation.
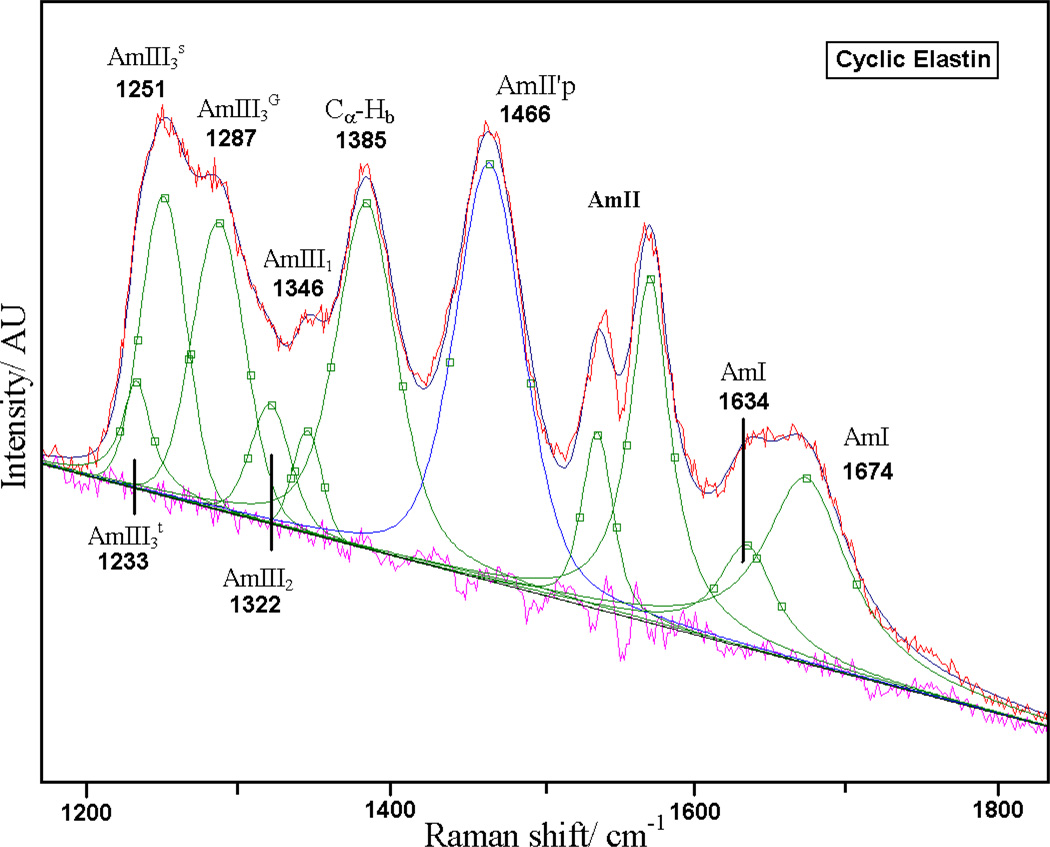
As shown in Fig 4, we deconvoluted the measured 204 nm excited UVRR spectrum into a sum of a minimum number of mixed Gaussian and Lorentzian bands by using the peak fitting routine in Grams (Galactic Industries Corporation, Grams version 5). The broad ~1660 cm−1 AmI band (predominantly C=Os) is composed of two overlapping bands centered at 1674 and 1635 cm−1. Thomas et. at. have reported that the amide I region of crystalline cyclic elastin suspended in its mother liquor shows two prominent AmI bands at ~1652 and 1676 cm−1. These authors suggest that both the 1652 and 1676 cm−1 AmI bands likely derive from β-type II turn conformations.47 However, the origin of the frequency difference between these two AmI bands was not discussed.
Figure 4.
Spectral deconvolution of 20 °C 204 nm UV resonance Raman spectra of cyclic elastin with mixed Gaussian and Lorentzian bands. The quality of the fit is evident from the flat residual displayed underneath.
The two underlying AmI components (1674 and 1635 cm−1) do not result from intrinsically different frequencies for the amide I bands of the Val, Gly and Pro peptide bonds. Polyproline peptides show AmI bands in the region between 1640 cm−1 and 1650 cm−1, while polyglycine peptides show AmI bands at ~1650 cm−1.65,68,69 The typical non-prolyl, non-glycyl peptide bonds in unfolded peptide conformations typically show a single broad amide I band at 1665 cm−1.70,71 This frequency spread would not typically result in two overlapping AmI bands at 1635 and 1674 cm−1.
The frequency differences observed for the amide I band either results from significantly different amide bond conformations or from different carbonyl hydrogen bonding. Lazarev et. al.68 reported that the IR absorption spectra of Z-(Gly1-Pro2-Gly3)4-OMe peptide in D2O shows a broad AmI band that appears to be composed of three different AmI components located at 1632 cm−1, 1650 cm−1 and 1669 cm−1. These authors attributed the high frequency 1669 cm−1 AmI component to the dehydrated/weakly hydrogen bonded carbonyl of Gly3.68 Recently Spiro and coworkers reported observing a similar high frequency UVRR AmI band in the PPII (1677 cm−1) and β-sheet (1670 cm−1) conformation of polyK peptide.72 Boury and coworkers73 suggest that a similar the high frequency AmI component in Gliadin and Globulin proteins derives from random coil and/or β-turn conformations.
Our CD results indicate that the cyclic elastin peptide predominantly adopts an extended β-strand like conformation with some β-turn character, discounting the possibility that the high frequency AmI component derives from β-sheet-like conformations. We therefore, conclude that the broad 1635 cm−1 AmI component of elastin likely derives from hydrogen bonded peptide bonds, whereas the 1674 cm−1 component of elastin derives from dehydrated or weakly hydrogen bonded peptide bonds.
The peptide bond dehydration may correlate with weak amide-amide hydrogen bonding in β–turn like conformations. X-ray studies of cyclic elastin peptide (VPGVG)3 indicate three β-type II turns. The β-turn spans the VPGV unit and includes a hydrogen bond between the two Val peptide bonds. The other Gly residue appears to serve as a bridge between adjacent turns.47,74 It is likely that in aqueous solutions, the weakly hydrogen bonded turn conformation is stabilized against strong peptide-water hydrogen bonding by the bulky β-branched side chains of Val which are known to restrict water access to the peptide backbone.75 A lack of significant change in elastin’s AmI region at high temperatures (Fig 2) suggests minimal changes in elastin’s hydrogen bonding state. Similar results were reported by Thomas et al. who did not observe any significant change in the amide spectra upon the phase transition.47 A lack of significant change in the AmI region at high temperatures suggests the inverse temperature transition of elastin does not significantly impact the hydrogen bonding state of the peptide backbone.
The AmII band at ~1558 cm−1 involves C-N stretching with some N-H bending.71 The trough in the AmII region (Fig 3) is an artifact from over-subtraction of the interfering molecular oxygen vibration at 1560 cm−1.
The AmII' band of Pro (AmII'p)60 located at 1466 cm−1 is predominantly a C-N stretch which is thought to be sensitive to local conformation and/or hydrogen bonding of Pro’s peptide bond. The AmII'p downshifts from 1466 to 1460 cm−1 and its band width (FWHM) appears to narrow from 50 cm−1 to 47 cm−1 as the solution temperature is increased from 20 to 60 °C (Fig 3). We recently demonstrated that the AmII'p band frequency is sensitive to the peptide backbone conformation of proline.61 The narrowing of the AmII'p band at high temperatures indicates a narrower conformational distribution of the proline peptide bond at 60 °C.
Takeuchi et al62, suggested that the AmII'p frequency depends on the hydrogen bonding state of Pro.62,76 If that were true then the observed changes in the AmII'p frequency would suggest weakening of amide-water hydrogen bonds at high temperature. However, we see little evidence from the AmI band for peptide bond dehydration.
The CαHb band is located at 1385 cm−1. The presence of the resonance enhanced CαHb band is correlated with the presence of non α-helical conformations.70,77 In elastin peptides the CαHb intensity derives only from the Val and Gly peptide bonds, although as discussed above, we expect that the impact of Val peptide bond conformational changes on the CαHb band intensity would be more than what would occur for the Gly peptide bond.
The Amide III region has recently been examined and reassigned.78–82 The AmIII1 band is located at 1346 cm−1, while a weak AmIII2 band is located at ~1322 cm−1. In cyclic elastin these bands derive only from Val peptide bonds. The 1287 cm−1 AmIII band likely derives from glycine peptide bonds. The broad AmIII3 band located at ~1246 cm−1 is composed of two overlapping bands at ~1251 and ~1233 cm−1 (Fig 3) which derive from the Val peptide bonds.
Recently, Mikhonin et al83 quantitatively demonstrated that the AmIII3 band frequency depends upon the ψ dihedral angle and the hydrogen bonding state of the amide bond.78,83 Furthermore, Mikhonin and Asher84 demonstrated that the resonance enhanced AmIII and CαHb bands each scatter independently i.e. there is negligible coupling between adjacent peptide bonds. The UVRR spectra in the AmIII and CαHb region can therefore be regarded as a linear sum of individual peptide bonds. Hence, the AmIII3 spectra represents the time-averaged conformation distribution of the peptide bonds.54,85
Using Mikhonin et al’s83 methodology for correlating the ψ dihedral angle with the AmIII3 band position, we find that the 1251 cm−1 band could originate from either a β-type III turn-like conformation (ψ ~ −35°) or a distorted β-strand-like conformation (ψ ~ +165°, see table 1). The presence of a strong CαHb band indicates the existence of significant non-α-helical conformations. As discussed above, the presence of a minimum at 222 nm along with a global minimum below 200 nm in the CD spectra (Fig 1) indicates that some fraction of cyclic elastin exists in a β-type III turn conformation with a significant content of random-coil like conformation. We therefore conclude that the 1251 cm−1 Raman band of cyclic elastin likely contains contributions from both the distorted β-strand and type-III turn like conformations. Similarly, Mikhonin et al’s data suggest the 1233 cm−1 AmIII3 band could originate from either anti-parallel β-sheet (ψ = +135°) or β-turn (ψ = 0°) conformations. Taking our cue from previous elastin studies23 we assign the 1233 cm−1 AmIII3 band to β-type II turn conformations of the Val peptide bonds.
Table 1.
Conformation Distribution of Linear and Cyclic Elastin
| Band Position (cm−1) | Ψ Angle (degree) | Conformation |
|---|---|---|
| Cyclic Elastin | ||
| 1251 | −35° | β-Type III turn |
| +165° | Distorted β-strand | |
| 1233 | 0° | β-Type II turn |
| Linear Elastin | ||
| 1250 | −35° | β-Type III turn |
| +165° | Distorted β-strand | |
| 1230 | 0° | β-Type II turn |
The spectra in the CαHb and AmIII3 region show some changes in band intensities and position as the temperature is increased from 20 to 60 °C. The frequency of CαHb band is temperature independent.54,78,86 The CαHb band region in the difference spectrum shows a small positive peak; decrease in the CαHb band intensity at high temperatures indicates a decrease in the extended state (distorted β-strand) population (Fig 3). The AmIII3 band appears to show increased band intensity at 60 °C, as indicated by a trough in the difference spectrum at ~1235 cm−1, which suggests an increased β-type II turn population.
Linear Elastin
At 20 °C, the 204 nm UVRR spectrum of linear elastin (Fig. 5A) resembles the 20 °C cyclic elastin spectrum—suggesting that the ensemble conformations of linear elastin’s Val and Pro peptide bonds are similar to their counterparts in cyclic elastin. The cyclic and linear elastin peptides have similar sequences, although the cyclic peptide has an extra peptide bond (P-G) due to cyclization of the linear variant.
Figure 5.
A) 204 nm UV resonance Raman spectra of linear and cyclic elastin at 20 °C and their difference spectra. The featureless difference spectrum indicates that the Val and Pro peptide bonds in cyclic and linear peptides have similar conformations. B) CD spectra of linear and cyclic elastin at 20 °C. Cyclic elastin shows a more prominent positive feature at ~207 nm, indicating a greater population of β-type II turns in the cyclic peptide.
The CD spectrum shows a smaller β-type II turn population for linear elastin than for cyclic peptide as indicated by a less prominent band maximum at ~207 nm (Fig 5B).12,24,40 A lack of significant difference in the UVRR spectra of linear elastin versus cyclic elastin suggests that the increased turn population is localized in conformational changes at the Gly peptide bonds. Previous studies suggested that linear’s elastin’s Gly residues are highly mobile.87
The CD spectra of linear elastin show little temperature dependence at 222 and 207 nm (Fig 6), whereas the global minimum below 200 nm becomes less negative with increasing temperature (Fig 2 and 6). The linear slope of the melting curve indicates a non-cooperative transition (Fig 2). The presence of a local minimum at ~222 nm indicates the presence of β-type III turn conformation, while a weak maximum at ~207 nm is indicative of some β-type II turn conformation.40 Presence of the global minimum below 200 nm indicates a significant random coil population.40,53
Figure 6.
Temperature dependent CD spectra of linear elastin. The insert shows the 20 °C CD spectra of cyclic elastin during and subsequent (20 °C) to heating.
The mean residual ellipticity (MRE) of linear elastin at 198 nm of ~ 4000 deg*cm2*residue−1 *dmol−1 at 0 °C is significantly smaller than the values reported by Reiersen et al40 for short elastin-like peptides. Although, the sequence of peptides in the two studies differs (our peptides have extra Val and Pro residues at the N-terminus) both Reiersen et al’s and our study show similar spectral features. The relatively small MRE indicates greater β-turn character40 for our peptide, which likely arises due to the presence of hydrophobic terminal Val and Pro residues. Our CD results indicate that the linear elastin peptide predominantly populates random coil and β-type III turn-like conformations along with a small population of β-type II turns.
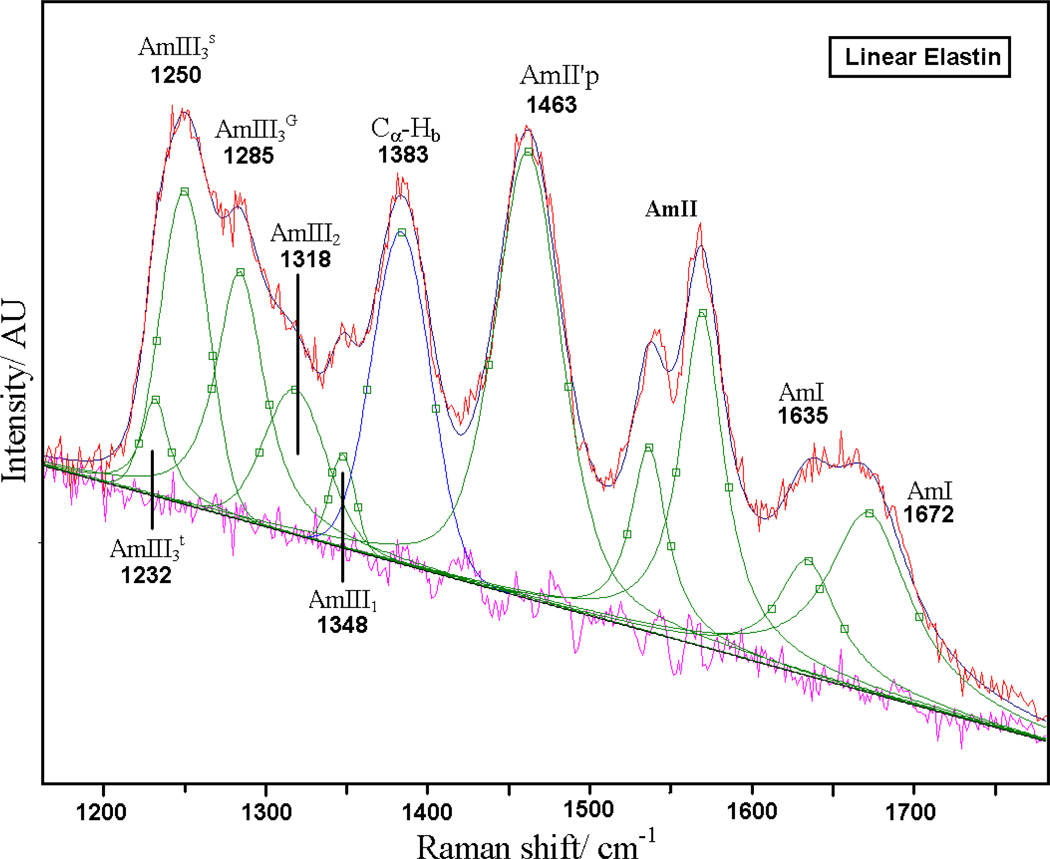
As shown in Fig. 7, spectral deconvolution of the UVRR spectrum of linear elastin at 20 °C reveals a similar AmI region as cyclic elastin’s with AmI bands at ~1635 cm−1 and 1672 cm−1. The trough in the AmII region is an artifact arising due to over-subtraction of the 1560 cm−1 molecular oxygen contribution. The AmII'p is located at 1463 cm−1, while the resonance-enhanced CαHb70,77 is located at 1383 cm−1. The AmIII1 band is located at 1348 cm−1 while a weak AmIII2 band is located at 1318 cm−1. The AmIIIG is located at ~1285 cm−1. The conformation-sensitive AmIII3 region (deriving from Val) shows a broad band at ~ 1247 cm−1 that is likely composed of two overlapping bands located at ~1250 and ~1232 cm−1 (Fig 7). According to Mikhonin et al83 the 1232 cm−1 band likely derives from β-type II turn like conformations (ψ ~ 0°), while the 1250 cm−1 band likely derives from distorted β-strand (ψ ~ +165°) and β-type III turn-like conformations (ψ ~ −35°, see table 1).
Figure 7.
Spectral deconvolution of 20 °C 204 nm UV resonance Raman spectra of linear elastin with Gaussian and Lorentzian bands. The quality of the fit is evident from the flat residual.
Recently, Ohgo et al88 utilized solid-state NMR techniques in conjunction with a statistical analysis of Brookhaven Protein Databank (PDB) to estimate the angular distribution of the two central Val peptide bonds (Val 14 and 16) in an isotope-labeled (VPGVG)6 lyophilized peptide. The authors found that Val-14 shows a bimodal distribution of (Φ, Ψ) values at: (−110 ± 20°, 130 ± 20°) and (−75 ± 15°, −15 ± 15°) which constitutes 70% and 30% of total population, respectively. Val-16 residue adopts the (Φ, Ψ) values of (−90 ± 15 °, 120 ± 15°). A bimodal distribution was also observed for the central Gly and Pro peptide bonds suggesting that elastin conformation is an ensemble of multiple conformations with some minor β-spiral contribution. Ohgo et al’s88 calculated Ψ angle values for Val are similar to our UVRR derived Ψ angles. The slight differences in Ψ angle values likely drive from increased peptide backbone mobility in our aqueous phase experiment. This suggests that that the aqueous conformations observed in our UVRR experiment also occur in the lyophilized solid-state samples of Ohgo et al.88
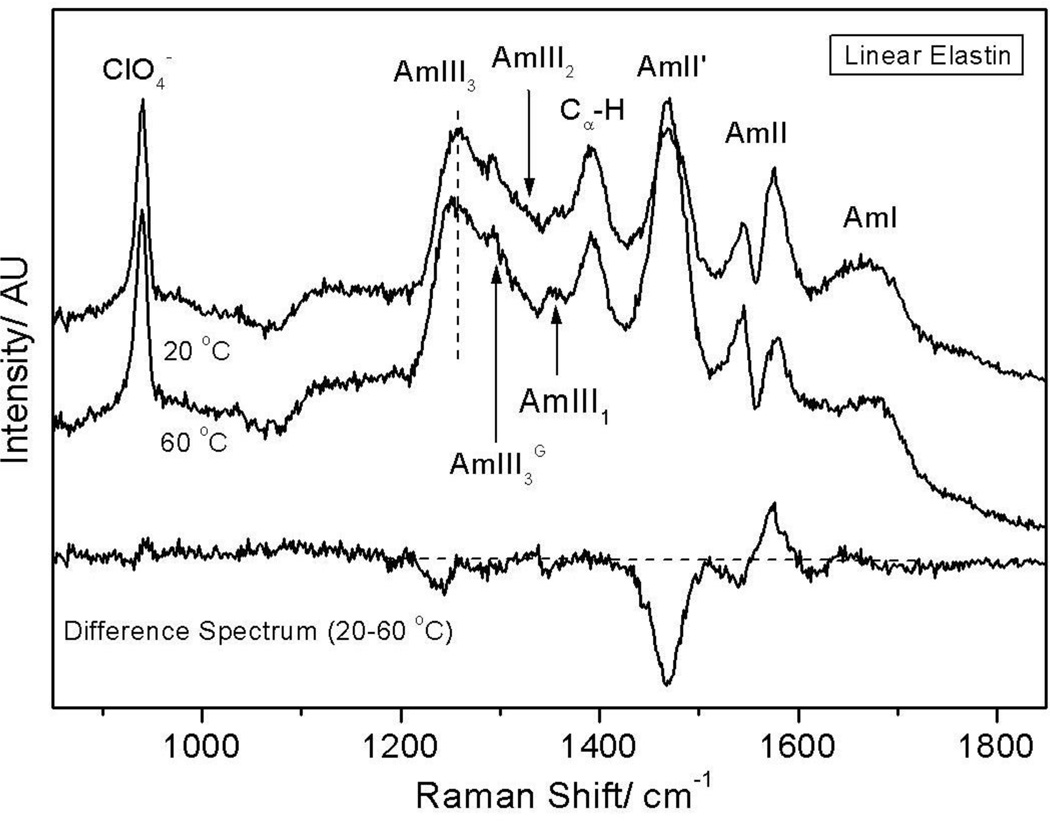
As the solution temperature is increased from 20 to 60 °C, the AmIII3 band shows a small increase in band intensity as indicated by a trough in the difference spectrum at ~1237 cm−1, suggesting an increased β-type II turn population (Fig 8). The AmII'p band narrows (ΔFWHM = 2 cm−1) as the solution temperature is increased from 20 to 60 °C, suggesting a narrower conformation distribution at 60 °C, although the narrowing in linear elastin ((ΔFWHM = 2 cm−1, Fig 8) is just a little less than in cyclic elastin (ΔFWHM = 3 cm−1, Fig. 2). Furthermore, unlike in cyclic elastin, the band position of the AmII'p band in linear elastin remains invariant with temperature. This suggests that unlike the prolines of cyclic elastin, linear elastin’s prolines do not undergo a significant conformation change with increasing temperature.
Figure 8.
204 nm UV resonance Raman spectra of linear elastin measured at 20 and 60 °C. The difference spectrum shows narrowing of the AmII' band width at 60 °C which is indicative of a transition to a relatively well-defined conformation. The trough in the AmII region is an artifact that derives from over-subtraction of the interfering O2 stretching vibration at 1560 cm−1 when the NMR tube contribution was subtracted from the measured spectra.
Conclusion
We utilize electronic circular dichroism (CD) and UV resonance Raman spectroscopy at 204 nm excitation to examine the temperature dependence of conformational changes in cyclic and linear elastin peptides. We use CD spectroscopy to examine global conformation changes in elastin peptides, while UVRR probes the local conformation and hydrogen bonding of Val and Pro peptide bonds. Our results indicate that the cyclic peptide predominantly populates distorted β-strand, β-type II and β-type III turn conformations at 20 °C. At 60 °C, the β-type II turn population increases somewhat while the distorted β-strand population decreases as indicated by changes in the AmIII3 and CαHb regions.
The ensemble population of linear elastin is predominantly β-type III turn and distorted β-strand with some β-type II turn population regardless of solution temperature. Increasing temperature results in a small increase in the β-type II turn population; furthermore, we observe narrowing of the AmII'p band with increasing temperature which suggests a narrowed conformation distribution at high temperatures.
Our CD spectra of linear elastin vs. cyclic elastin indicate a greater β-type II turn population for cyclic elastin. However, the UVRR of cyclic and linear elastin do not show any significant differences. This result suggest that the increased type II turn content of cyclic elastin is likely due to cyclization induced conformation changes at Gly peptide bonds.
Our results demonstrate that for both cyclic and linear elastin the global conformation changes are predominantly driven by conformation changes at the proline and glycine peptide bonds, whereas the Val peptide bonds show relatively little conformation changes with increasing temperature. However, the Val peptide bonds are probably not mere spectators; they likely play a pivotal role in the inverse temperature transition of elastin peptides.
We find that both the cyclic and linear elastin peptide show a broad AmI band which is likely composed of two overlapping bands at ~1674 and 1635 cm−1. The 1635 cm−1 component derives from hydrogen bonded peptide bonds, whereas the ~1674 cm−1 component likely derives from dehydrated/weakly hydrogen bonded peptide bonds that are likely engaged in weak intra-molecular hydrogen bonding as part of a local β-turn conformation.47 The weakly hydrogen bonded β-turn is likely protected against amide-water hydrogen bonds by the bulky β-branched side chains of Val, which are known to shield the peptide backbone from water molecules.75 The absence of some peptide-water hydrogen bonds in the extended conformation of elastin as compared to typical unfolded peptides such as the XAO peptide which predominantly adopts a PPII conformation,71,89–91 reduces the enthalpic advantage of the elastin’s extended state over its entropically unfavorable collapsed state and narrows the free energy gap between the extended and collapsed states such that a temperature increase induces the inverse transition.
A lack of significant change in the AmI region at high temperature suggests the inverse temperature transition of elastin does not significantly impact the hydrogen bonding state of the peptide backbone. Furthermore, small intensity changes in the AmIII and CαHb band region indicate that elastin’s phase transition is accompanied by only a small conformation change resulting in increased β-turn population. The increased β-turn content does not appear to impact the hydrogen bonding state of elastin. It is likely that the phase transition in elastin peptides is primarily driven by changes in conformation and/or hydration of the alkyl side chains.
Acknowledgments
The authors would like to thank Prof. D. W. Urry and Konstantin V. Pimenov for helpful discussions and NIH grant RO1 EB002053 for financial support.
REFERENCES
- 1.Alonso M, Reboto V, Guiscardo L, San Martin A, Rodriguez-Cabello JC. Macromolecules. 2000;33:9480–9482. [Google Scholar]
- 2.Urry DW, Parker TM. Journal of Muscle Research and Cell Motility. 2002;23:543–559. [PubMed] [Google Scholar]
- 3.Li B, Alonso DOV, Bennion BJ, Daggett V. Journal of the American Chemical Society. 2001;123:11991–11998. doi: 10.1021/ja010363e. [DOI] [PubMed] [Google Scholar]
- 4.Li B, Alonso DOV, Daggett V. Journal of Molecular Biology. 2001;305:581–592. doi: 10.1006/jmbi.2000.4306. [DOI] [PubMed] [Google Scholar]
- 5.Debelle L, Alix AJP, Wei SM, Jacob M-P. Eur J Biochem. 1998;258:533–539. doi: 10.1046/j.1432-1327.1998.2580533.x. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes RJ, Eyre DR. Biochemical and Biophysical Research Communications. 1999;261:635–640. doi: 10.1006/bbrc.1999.1092. [DOI] [PubMed] [Google Scholar]
- 7.Herrera B, Eisenberg G, Holberndt O, Desco MM, Rábano A, García-Barreno P, Del Cañizo JF. Cryobiology. 2000;41:43–50. doi: 10.1006/cryo.2000.2263. [DOI] [PubMed] [Google Scholar]
- 8.Kim B-S, Nikolovski J, Bonadio J, Smiley E, Mooney DJ. Experimental Cell Research. 1999;251:318–328. doi: 10.1006/excr.1999.4595. [DOI] [PubMed] [Google Scholar]
- 9.Lapis K, Tímár J. seminar in Cancer Biology. 2002;12:209–217. doi: 10.1016/S1044-579X(02)00024-X. [DOI] [PubMed] [Google Scholar]
- 10.Lindholt JS, Heickendorff L, Vammen S, Fasting H, Henneberg EW. Eur J Vasc Endovasc Surg. 2001;21:235–240. doi: 10.1053/ejvs.2001.1329. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura F, Suyama K. Archives of Biochemistry and Biophysics. 1996;325:167–173. doi: 10.1006/abbi.1996.0021. [DOI] [PubMed] [Google Scholar]
- 12.Reiersen H, Rees AR. Biochemical and Biophysical Research Communications. 2000;276:899–904. doi: 10.1006/bbrc.2000.3588. [DOI] [PubMed] [Google Scholar]
- 13.Robert L. seminar in Cancer Biology. 2002;12:157–163. doi: 10.1016/S1044-579X(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 14.Krukau A, Brovchenko I, Geiger A. Biomacromolecules. 2007;8:2196–2202. doi: 10.1021/bm070233j. [DOI] [PubMed] [Google Scholar]
- 15.Tamura T, Yamaoka T, Kunugi S, Panitch A, Tirrell DA. Biomacromolecules. 2000;1:552–555. doi: 10.1021/bm005606u. [DOI] [PubMed] [Google Scholar]
- 16.Bressan G, Prockop DJ. Biochemistry. 1977;16:1406–1412. doi: 10.1021/bi00626a026. [DOI] [PubMed] [Google Scholar]
- 17.Khaled MA, Prasad KU, Venkatachalam CM, Urry DW. Journal of the American Chemical Society. 1985;107:7139–7145. [Google Scholar]
- 18.Urry DW, Chang DK, Krishna NR, Huang DH, Trapane TL, Prasad KU. Biopolymers. 1989;28:819–833. doi: 10.1002/bip.360280404. [DOI] [PubMed] [Google Scholar]
- 19.Urry DW, Harris RD, Prasad KU. Journal of the American Chemical Society. 1988;110:3303–3305. [Google Scholar]
- 20.Urry DW, Haynes B, Zhang H, Harris RD, Prasad KU. Proc Natl Acad Sci USA. 1988;85:3407–3411. doi: 10.1073/pnas.85.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urry DW, Peng S, Parker T. Journal of the American Chemical Society. 1993;115:7509–7510. [Google Scholar]
- 22.Venkatachalam CM, Khaled MA, Sugano H, Urry DW. Journal of the American Chemical Society. 1981;103:2372–2379. [Google Scholar]
- 23.Venkatachalam CM, Urry DW. Macromolecules. 1981;14:1225–1229. [Google Scholar]
- 24.Urry DW. J Phys Chem B. 1997;101:11007–11028. [Google Scholar]
- 25.Urry DW, Peng S, Xu J, McPherson DT. Journal of the American Chemical Society. 1997;119:1161–1162. [Google Scholar]
- 26.Bressan G, Prockop DJ. Biochemisty. 1977;16:1406–1412. doi: 10.1021/bi00626a026. [DOI] [PubMed] [Google Scholar]
- 27.Debord SB, Lyon LA. J Phys Chem B. 2003;107:2927–2932. [Google Scholar]
- 28.Ding Y, Ye X, Zhang G. Macromolecules. 2005;38:904–908. [Google Scholar]
- 29.Reese CE, Mikhonin AV, Kamenjicki M, Tikhonov A, Asher SA. J Am Chem Soc. 2004;126:1493–1496. doi: 10.1021/ja037118a. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Gan D, Lyon LA, El-Sayed MA. J Am Chem Soc. 2001;123:11284–11289. doi: 10.1021/ja016610w. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Wu C. Macromolecules. 1999;32:4299–4301. [Google Scholar]
- 32.Hoare T, Pelton R. Langmuir. 2004;20:2123–2133. doi: 10.1021/la0351562. [DOI] [PubMed] [Google Scholar]
- 33.Hu T, You Y, Pan C, Wu C. J Phys Chem B. 2002;106:6659–6662. [Google Scholar]
- 34.Jones CD, Lyon LA. Macromolecules. 2003;36:1988–1993. [Google Scholar]
- 35.Kunugi S, Kameyama K, Tada T, Tanaka N, Shibayama M, Akashi M. Brazilian Journal of Medical and Biological Research. 2005;38:1233–1238. doi: 10.1590/s0100-879x2005000800011. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Bergbreiter DE. Chemical Industries (Dekker) 2003;89:545–550. [Google Scholar]
- 37.Zhu PW, Napper DH. Journal of Colloid and Interface Science. 1996;177:343–352. doi: 10.1006/jcis.1999.6222. [DOI] [PubMed] [Google Scholar]
- 38.Zhu PW, Napper DH. J Phys Chem B. 1997;101:3155–3160. [Google Scholar]
- 39.Zhu PW, Napper DH. Physical Review E: Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics. 2000;61:6866–6871. doi: 10.1103/physreve.61.6866. [DOI] [PubMed] [Google Scholar]
- 40.Reiersen H, Clarke AR, Rees AR. J Mol Biol. 1998;283:255–264. doi: 10.1006/jmbi.1998.2067. [DOI] [PubMed] [Google Scholar]
- 41.Luan CH, Parker TM, Gowda DC, Urry DW. Biopolymers. 1992;32:1251–1261. doi: 10.1002/bip.360320914. [DOI] [PubMed] [Google Scholar]
- 42.Frank HS. Journal of Chemcial Physics. 1945;13:478–492. [Google Scholar]
- 43.Searle MS, Williams DH. J Am Chem Soc. 1992;114:10690–10697. [Google Scholar]
- 44.Searle MS, Williams DH, Gerhard U. J Am Chem Soc. 1992;114:10697–10704. [Google Scholar]
- 45.Tanford C. The hydrophobic effect: formation of micelles and biological membranes. New York: Wiley; 1973. [Google Scholar]
- 46.Chang DK, Venkatachalam CM, Prasad KU, Urry DW. Journal of Biomolecular Structure & Dynamics. 1989;6:851–858. doi: 10.1080/07391102.1989.10506517. [DOI] [PubMed] [Google Scholar]
- 47.Thomas GJ, Jr, Prescott B, Urry DW. Biopolymers. 1987;26:921–934. doi: 10.1002/bip.360260611. [DOI] [PubMed] [Google Scholar]
- 48.Gosline JM. Biopolymers. 1978;17:697–707. doi: 10.1002/bip.1978.360170312. [DOI] [PubMed] [Google Scholar]
- 49.Gosline JM, French CJ. Biopolymers. 1979;18:2091–2103. doi: 10.1002/bip.1979.360180818. [DOI] [PubMed] [Google Scholar]
- 50.Gosline JM, Yew FF, Weis-Fogh T. Biopolymers. 1975;14:1811–1826. [Google Scholar]
- 51.Hoeve CAJ, Flory PJ. Biopolymers. 1974;13:677–686. doi: 10.1002/bip.1974.360130404. [DOI] [PubMed] [Google Scholar]
- 52.Bykov SB, Lednev IK, Ianoul A, Mikhonin AV, Asher SA. Appl Spectrosc. 2005;59:1541–1552. doi: 10.1366/000370205775142511. [DOI] [PubMed] [Google Scholar]
- 53.Manning MC, Woody RW. Biopolymers. 1991;31:569–586. doi: 10.1002/bip.360310511. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed Z, Asher SA. Biochemistry. 2006;45:9068–9073. doi: 10.1021/bi060858m. [DOI] [PubMed] [Google Scholar]
- 55.Biron Z, Khare S, Samson AO, Hayek Y, Naider f, Anglister J. Biochemistry. 2002;41:12687–12696. doi: 10.1021/bi026261y. [DOI] [PubMed] [Google Scholar]
- 56.Foster JA, Bruenger E, Rubin L, Imberman M, Kagan H, Mecham R, Franzblau C. Biopolymers. 1975;15:833–844. doi: 10.1002/bip.1976.360150503. [DOI] [PubMed] [Google Scholar]
- 57.Tamburro AM, Guantieri V, Daga-Gordini D, Abatangelo G. J Biol Chem. 1978;253:2893–2894. [PubMed] [Google Scholar]
- 58.Dorman DE, Torchia DA, Bovey FA. Macromolecules. 1973;6:80–82. doi: 10.1021/ma60031a013. [DOI] [PubMed] [Google Scholar]
- 59.Garrett RHG, Charles M. Biochemistry. Philadelphia, Pa: Saunders College Publishing; 1999. [Google Scholar]
- 60.Caswell DS, Spiro TG. J Am Chem Soc. 1987;109:2796–2800. [Google Scholar]
- 61.Ahmed Z, Myshakina NS, Asher SA. manuscript in publication. 2007 [Google Scholar]
- 62.Takeuchi H, Harada I. Journal of Raman Spectroscopy. 1990;21:509–515. [Google Scholar]
- 63.Jordan T, Mukerji I, Wang Y, Spiro TG. J Mol Struct. 1996;379:51–64. [Google Scholar]
- 64.Chen XG, Li P, Holtz JSW, Chi Z, Pajcini V, Asher SA, Kelly LA. J Am Chem Soc. 1996;118:9716–9726. [Google Scholar]
- 65.Pajcini V, Asher SA. J Am Chem Soc. 1999;121:10942–10954. [Google Scholar]
- 66.Asher SA, Ianoul A, Mix G, Boyden MN, Karnoup A, Diem M, Schweitzer-Stenner R. J Am Chem Soc. 2001;123:11775–11781. doi: 10.1021/ja0039738. [DOI] [PubMed] [Google Scholar]
- 67.Asher SA, Ianoul A, Mix G, Boyden MN, Karnoup A, Diem M, Schweitzer-Stenner R. J Am Chem Soc. 2001;123:11775–11781. doi: 10.1021/ja0039738. [DOI] [PubMed] [Google Scholar]
- 68.Lazarev YA, Grishkovsky BA, Khromova TB. Biopolymers. 1985;24:1449–1478. doi: 10.1002/bip.360240804. [DOI] [PubMed] [Google Scholar]
- 69.Li P, Chen XG, Shulin E, Asher SA. J Am Chem Soc. 1997;119:1116–1120. [Google Scholar]
- 70.Chi Z, Chen XG, Holtz JSW, Asher SA. Biochemistry. 1998;37:2854–2864. doi: 10.1021/bi971160z. [DOI] [PubMed] [Google Scholar]
- 71.Mikhonin AV, Ahmed Z, Ianoul A, Asher SA. J Phys Chem B. 2004;108:19020–19028. [Google Scholar]
- 72.JiJi RD, Balakrishnan G, Hu Y, Spiro TG. Biochemistry. 2006;45:34–41. doi: 10.1021/bi051507v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chourpa I, Ducel V, Richard J, Dubois P, Boury F. Biomacromolecules. 2006;7:2616–2623. doi: 10.1021/bm060131d. [DOI] [PubMed] [Google Scholar]
- 74.Cook WJ, Einspahr H, Trapane TL, Urry DW, Bugg CE. J Am Chem Soc. 1980;102:5502–5505. [Google Scholar]
- 75.Avbelj F, Baldwin RL. Proc Natl Acad Sci USA. 2003;100:5742–5747. doi: 10.1073/pnas.1031522100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jordon T, Mukerji I, Yang W, Spiro TG. J Biol Chem. 1996;379:51–64. [Google Scholar]
- 77.Wang Y, Purrello R, Jordan T, Spiro TG. J Am Chem Soc. 1991;113:6359–6368. [Google Scholar]
- 78.Mikhonin AV, Ahmed Z, Ianoul A, Asher SA. J Phys Chem B. 2004;108:19020–19028. [Google Scholar]
- 79.Lee S-H, Krimm S. Biopolymers. 1998;46:283–317. [Google Scholar]
- 80.Overman SA, Thomas GJ., Jr Biochemistry. 1995;34:5440–5451. doi: 10.1021/bi00016a015. [DOI] [PubMed] [Google Scholar]
- 81.Overman SA, Thomas GJ., Jr Biochemistry. 1998;37:5654–5665. doi: 10.1021/bi972339c. [DOI] [PubMed] [Google Scholar]
- 82.Overman SA, Thomas GJ., Jr J Raman Spectrosc. 1998;29:23–29. [Google Scholar]
- 83.Mikhonin AV, Bykov SV, Myshakina NS, Asher SA. J Phys Chem B. 2006;110:5509–5518. doi: 10.1021/jp054593h. [DOI] [PubMed] [Google Scholar]
- 84.Mikhonin AV, Asher SA. J Phys Chem B. 2005;109:3047–3052. doi: 10.1021/jp0460442. [DOI] [PubMed] [Google Scholar]
- 85.Ahmed Z, Illir AB, Mikhonin AV, Asher SA. J Am Chem soc. 2005;127:10943–10950. doi: 10.1021/ja050664e. [DOI] [PubMed] [Google Scholar]
- 86.Lednev IK, Karnoup AS, Sparrow MC, Asher SA. J Am Chem Soc. 1999;121:8074–8086. doi: 10.1021/ja003381p. [DOI] [PubMed] [Google Scholar]
- 87.Perry A, Stypa MP, Foster JA, Kumashiro KK. J Am Chem Soc. 2002;124:6832–6833. doi: 10.1021/ja017711x. [DOI] [PubMed] [Google Scholar]
- 88.Ohgo K, Ashida J, Kumashiro KK, Asakura T. Macromolecules. 2005;38:6038–6047. [Google Scholar]
- 89.Shi Z, Olson CA, Rose GD, Baldwin RL, Kallenbach NR. Proc Natl Acad Sci USA. 2002;99:9190–9195. doi: 10.1073/pnas.112193999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schweitzer-Stenner R, Eker F, Griebenow K, Cao X, Nafie LA. J Am Chem Soc. 2004;126:2768–2776. doi: 10.1021/ja039452c. [DOI] [PubMed] [Google Scholar]
- 91.Stapley BJ, Creamer TP. Protein Sci. 1999;8:587–595. doi: 10.1110/ps.8.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]