Abstract
We sought to determine clinical predictors of postpartum depression (PPD), including the role of medication, in a sample of women followed prospectively during and after pregnancy. Women with a history of mood disorder were recruited and evaluated during each trimester and 1 week, 1 month, and 3 months postpartum. DSM-IV criteria for a major depressive episode were assessed by a psychiatric interview at each time point. Sixty-three women with major depression and 30 women with bipolar disorder entered the study and 75.4 % met DSM-IV criteria for a MDE during pregnancy, postpartum, or both. We modeled depression in a given time period (second trimester, third trimester, or 1 month postpartum) as a function of medication use during the preceding period (first, second, or third trimester). The odds of being depressed for those who did not use medication in the previous period was approximately 2.8 times that of those who used medication (OR 2.79, 95 % CI 1.38–5.66, p=0.0048). Of 38 subjects who were psychiatrically well during the third trimester, 39.5 % (N=15) met the criteria for a MDE by 4 weeks postpartum. In women who developed PPD, there was a high rate of a family history of PPD (53.3 %) compared to women who did not develop PPD (11.8 %, p=0.02). While the use of psychiatric medications during pregnancy reduced the odds of being depressed overall, the use of psychiatric medications during pregnancy may not protect against PPD in women at high risk, particularly those with a family history of PPD.
Keywords: Postpartum depression, Women, pregnancy, Major Depression, Bipolar disorder
Introduction
Postpartum depression (PPD) occurs in approximately 10–15 % of women from the general population (Campbell and Cohn 1992; Yonkers et al. 2001; Dietz et al. 2007; O’hara 2009) and results in significant morbidity to both mother and child including potentially devastating consequences such as suicide and infanticide (Spinelli 2004). While the risk for suicide deaths and attempts is lower during and after pregnancy than in the general population of women, suicides account for up to 20 % of all postpartum deaths and represent one of the leading causes of peripartum mortality (Shadigian and Bauer 2005). PPD interferes with parenting behavior, including less adequate infant safety and healthy child development practices (Akman et al. 2006; Field 2011) such as the increased use of harsh discipline (Flynn et al. 2004). Likely due to changes in parenting behavior, exposure to postpartum depression is also associated with slower language development, more behavioral problems, and lower IQ in the child (Grace et al. 2003; Marcus 2009; Field 2011). Prevention and treatment of PPD therefore not only improves outcomes for the mother but the child as well.
In the literature, the term “PPD” has been broadly applied to a number of different clinical scenarios including a depressive episode that starts during pregnancy and continues postpartum, a depressive episode that begins in the immediate postpartum period (generally within the first 4 weeks), and a depressive episode that starts after the birth of a child but not necessarily in the immediate postpartum period—including time periods up to a year postpartum. According to the DSM-IV, the “postpartum” specifier for a major depressive episode (MDE) can only be used in the setting of a MDE that clearly began after delivery and began within the first 4 weeks postpartum. In support of this definition, a number of genetic studies (including our own) indicate that episodes that begin within the first 4 weeks postpartum have a genetic basis in both families with major depression (MDD) and BP (Forty et al. 2006; Murphy-Eberenz et al. 2006; Payne et al. 2008; Mahon et al. 2009). Further, there is evidence that women with a history of PPD are affectively susceptible to hormonal change: Bloch et al. (2000) demonstrated that 62% of women with a history of PPD developed mood symptoms in response to a blinded withdrawal of supraphysiological levels of estrogen and progesterone compared to none of controls. Thus, the development of mood symptoms in proximity to the hormonal changes associated with delivery became a DSM-IV requirement for the official definition of PPD. It is likely that women who develop a MDE after delivery in proximity to the hormonal change represent a more genetically homogenous group and the time period of 4 weeks postpartum is the critical time period during which if a woman is sensitive to times of hormonal change she will be at an elevated risk for PPD (Bloch et al. 2000; Forty et al. 2006; Murphy-Eberenz et al. 2006; Payne et al. 2008; Mahon et al. 2009).
PPD occurs at a much higher rate in women with a prior history of a mood disorder with estimates as high as 30–60 % (Bratfos and Hang 1966; Reich and Winokur 1970; Davidson and Robertson 1985; Kendell et al. 1987; Cox et al. 1993; Wisner et al. 2001, 2004; Payne et al. 2007; Viguera et al. 2011). The risk for PPD is increased in women with a history of MDD (Dean et al. 1989), Bipolar Disorder (BPAD) (Dean et al. 1989), or PPD following previous pregnancies (Cox et al. 1993; Wisner et al. 2001, 2004). These findings are corroborated by results from a cross-sectional study conducted in England suggesting a higher relative risk of PPD during subsequent pregnancies in women with a prior episode of PPD (Frank et al. 1987).
The available literature is conflicting as to whether or not antidepressant use during pregnancy decreases the risk of the development of a MDE either during or after pregnancy in women with mood disorders. Cohen et al. (2004, 2006) found in an observational study that the discontinuation of medication during pregnancy results in a high relapse rate (up to 70 %) during pregnancy. This study was conducted in a high-risk population. In contrast, in another observational study, Yonkers et al found in a community sample that the risk for onset of a MDE either during or after pregnancy was essentially the same whether or not antidepressants were continued or discontinued (Yonkers et al. 2011). Recently, Misri et al. (2013) found that women who continued antidepressant medications during pregnancy had significantly lower scores on the Hamilton Depression Scale during and after pregnancy as compared to women who declined medications. Further, scores on the Hamilton decreased over the course of the study in the women taking medication while scores increased in the women who declined. In another small study of 24 depressed women followed from mid-pregnancy to 2 months postpartum, 96 % were prescribed medication at some point during the course of the study and 2/3 showed improvement (Shivakumar et al. 2013). Notably, 1/3 of the sample continued to have significant depressive symptoms despite antidepressant treatment. Thus, whether or not medication use during pregnancy is warranted to prevent either antenatal or postpartum depression remains an open question.
As noted, a family history of PPD also increases the risk for PPD (Forty et al. 2006; Murphy-Eberenz et al. 2006; Payne et al. 2008). A limitation of the available genetics literature is the retrospective nature of these studies and little to no data regarding treatment during and after pregnancy. For example, it remains unclear exactly what the risk is for PPD in women who were clinically well during pregnancy and taking psychiatric medications. We therefore decided to follow women with a history of a mood disorder prospectively through pregnancy and the postpartum time period in order to determine the risk for women who were and were not depressed during pregnancy and who were and were not taking medications. We also examined our data for specific clinical risk factors associated with the development of PPD.
Methods
Participants
Pregnant women age 18 and older with a history of a mood disorder (MDD, BPI, BPII, or BPNOS) were recruited. Participants were recruited through the Johns Hopkins Women’s Mood Disorders Center, referrals from local psychiatrists and obstetricians, and through local advertisements and flyers. Participants could be in any trimester of pregnancy and were managed by their treating psychiatrist as clinically indicated, including the use of psychiatric medications. Exclusion criteria included current active suicidal ideation, medical instability, or active substance abuse or dependence during the last 90 days although patients with a past history of substance abuse were included. All but two subjects were recruited at Johns Hopkins. Two subjects were recruited and followed at the University of North Carolina through a collaborative project.
Procedures
Approval was obtained from the Johns Hopkins and the UNC Institutional Review Boards and written informed consent was obtained from all participants. Participants were evaluated by the research team during each trimester of pregnancy after study entry and then 1 week, 1 month, and 3 months postpartum. Each participant was interviewed with the Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID) at study entry. At each visit, the study psychiatrist determined if the participant met DSM-IV criteria for a MDE. Data collection included medication use, mood scales, measures of stress, clinical characteristics, personality traits and sleep quality, as well as blood samples. Previous psychiatric history was noted and subjects were interviewed about a history of premenstrual symptoms (PMS). Subjects were noted to have “Severe PMS” if they reported a significant impact on functioning based on the opinion of the interviewing psychiatrist. Lifetime Course of Illness was determined by the study psychiatrist based on clinical interview and was described as one of the following: remitting, chronic, frequent/brief episodes, or other.
We defined PPD as a MDE (based on DSM-IV criteria) that began within 4 weeks of delivery and did not begin during pregnancy. Thus, women who were depressed during pregnancy and continued to be depressed during the postpartum time period did not meet this definition of PPD. We also chose to examine the samples of women with MDD and BPAD together for two reasons: (1) sample size and (2) the available genetic data to date indicate that PPD as we have defined it demonstrates familiality in both forms of affective illness (Forty et al. 2006; Murphy-Eberenz et al. 2006; Payne et al. 2008). Further, the previously performed linkage and association study was performed in a mixed sample based on the hypothesis that the trigger for postpartum depressive illness may have a genetic basis for both forms of affective illness (Mahon et al. 2009).
Analysis
Clinical characteristics of the entire sample were summarized, calculating rates of diagnoses, course of illness, comorbidities, premenstrual symptoms, hospitalizations, race, marital status, education, and employment. We also calculated the rate of personal and family history of PPD, as well as family history of MDD or BP. Average age, gravidity, and the percentage who smoked during pregnancy were also calculated.
We then calculated the rate of medication use for the sample as a whole at each time point as well as for the following four groups of women: (a) those that were never depressed, (b) those that were depressed during pregnancy only, (c) those that were depressed during pregnancy and continued postpartum, and (d) those depressed postpartum only.
To assess the overall impact of medication use on depression, we modeled depression status (yes=depressed, no=not depressed) as a function of prior medication use (on meds vs. off meds) in the previous period using a generalized linear mixed model, with a binary response, logit link function, and a random intercept for subjects. This model included depression status at the second trimester, third trimester, and at 1 month after birth and medication status at first trimester, second trimester, and third trimester.
In order to examine the clinical characteristics of women who developed PPD (beginning after delivery), we identified 38 participants who were clinically well at the third trimester visit who also had postpartum data at the 1-month time point. We examined the relative proportion of several clinical characteristics in this smaller sample comparing women who developed PPD (N=15) to those that did not (N=23). We also calculated rates of medication use in these two samples. Fisher’s exact test was used to compare the two groups on clinical characteristics using a p value of 0.05 as the cutoff for significance. The p values were unadjusted for multiple comparisons.
Results
Ninety-three subjects were recruited: 63met criteria for MDD, 14 for BPI, 11 for BPII, and 5 for BPNOS (Table 1). The average age at study entry was 30.5 and most (66 %) were married. Thirty percent were from racial or ethnic minorities. This was a well-educated sample with 55 % obtaining a college or graduate degree. Approximately 50 % were working either part or full time and 50 % were not employed. The average number of previous pregnancies was 2.52 (SD 1.92) and 1/3 of the sample was primiparous.
Table 1.
Sample characteristics (N=93)
| Category | Percentage | |
|---|---|---|
| Mood disorder diagnosis | Major depressive disorder | 67.7 |
| N=93 | Bipolar affective disorder | 32.3 |
| -Type I | 15.1 | |
| -Type II | 11.8 | |
| -NOS | 5.4 | |
| Psychiatric comorbidities |
Total | 72.8 |
| History of substance abuse | 41.0 | |
| N=see Table 2 | Anxiety disorder | 53.1 |
| -Panic disorder | 25.9 | |
| -PTSD | 18.5 | |
| -GAD | 14.8 | |
| -OCD | 8.6 | |
| -SAD | 8.6 | |
| Age M (sd) | 30.5 (6.2) | |
| Race | White | 69.9 |
| N=93 | Black | 21.5 |
| Asian | 3.2 | |
| Hispanic | 1.1 | |
| Other | 4.3 | |
| Marital status | Married | 66.3 |
| N=92 | Cohabitating | 17.4 |
| Single | 15.2 | |
| Divorced | 1.1 | |
| Separated | 0.0 | |
| Widowed | 0.0 | |
| Education | Less than high school | 17.5 |
| N=91 | High school/GED | 14.3 |
| Associates | 12.1 | |
| Bachelor’s | 20.9 | |
| Graduate or professional | 35.2 | |
| Employment | Full time | 43.3 |
| N=90 | Part time | 8.9 |
| Unemployed | 47.8 | |
| Gravidity M (sd) | 2.52 (1.92) | |
| Smoking during pregnancy N=91 |
16.3 % |
PTSD posttraumatic stress disorder, GAD generalized anxiety disorder, OCD obsessive compulsive disorder, SAD social anxiety disorder
Table 2 describes the clinical characteristics of the total sample. A majority of the sample was described as having a remitting course of illness (57 %) while 25.8 % had a chronic presentation, and 11.8 % had frequent/brief episodes. On average, the participants had less than one lifetime hospitalization (range 0–20). Of the sample, 32.6 % had a prior hospitalization; 27.8 % had at least one family member that had a history of PPD, while 45.1 % described a personal history of PPD. A family history of mood disorder was also common with 40% of the sample reporting a family history of BP and 75.5 % reporting a family history of MDD. Comorbid conditions were common with 72.8 % of the sample with available data (n=81 completing the entire SCID) meeting the criteria for another psychiatric condition. Anxiety disorders were the most common comorbidity at 53.1 % but lifetime (past) substance abuse or dependence was also common at 41 %. Of those meeting criteria for past substance abuse or dependence, 71 % reported alcohol abuse or dependence.
Table 2.
Clinical characteristics of the entire sample
| Percent (N with available data) | |
|---|---|
| Remitting course | 57.0 % (93) |
| Chronic course | 25.8 % (93) |
| Frequent/brief course | 11.8 % (93) |
| Personal Hx PPD | 45.1 % (82) |
| Family Hx PPD | 27.8 % (72) |
| Family Hx MDD | 40.0 % (90) |
| Family Hx BP | 75.5 % (90) |
| Hospitalization | 32.6 % (92) |
| On meds T3 | 65.5 % (93) |
| Childhood abuse | 37.5 % (88) |
| Adult abuse | 1.1 % (88) |
| PMS | 57.0 % (86) |
| Significant PMS | 14.3 % (84) |
| Lifetime substance abuse | 41.0 % (83) |
| Lifetime any anxiety disorder | 53.1 % (81) |
Psychiatric medication use was widespread in this sample and increased over the course of pregnancy and the postpartum time period. We defined psychiatric medication use as reporting the use of an antidepressant, mood stabilizer, or antipsychotic medication (or more than one). Of the entire sample, 49.4 % were on medication during the first trimester. This increased to 62.7 % during the second trimester, 65.5 % during the third, and 75.7 % within the first month postpartum. Of the sample not taking medications during the first trimester, 31 % went back on medications at some point during pregnancy.
Despite medication use, 75.4 % of the sample met DSM-IV criteria for a major depressive episode either during pregnancy, postpartum, or both.
We had data on 68 subjects both during and after pregnancy. Seventeen participants remained well both during and after pregnancy. Seventeen subjects were depressed during pregnancy but recovered and were well postpartum. Twenty-three subjects were depressed both during pregnancy and postpartum. Eleven subjects were completely well during pregnancy but developed PPD.
We were particularly interested in the sample of women who were clinically well prior to delivery. Of 38 subjects who were well during the third trimester (including 10 who were depressed earlier in pregnancy), 15 or 39.5 % met the criteria for a MDE by 4 weeks postpartum and 23 remained well. Four women who had been depressed early in pregnancy developed PPD. Eighty percent of those that developed PPD had been on psychiatric medications (antidepressant or mood stabilizer) during the third trimester. Seventy-four percent of those that did not develop PPD were on psychiatric medications during the third trimester. Of the 38 subjects well in the third trimester, 13 had BPAD and 4 or 30.8 % of these developed PPD. None of the women with BPAD who developed PPD had BPI. In comparison, 44 % of the 25 women with MDD who were well in third trimester developed PPD. These rates of PPD were not statistically different between subjects with BPAD and MDD (Fisher’s exact test, p=0.5).
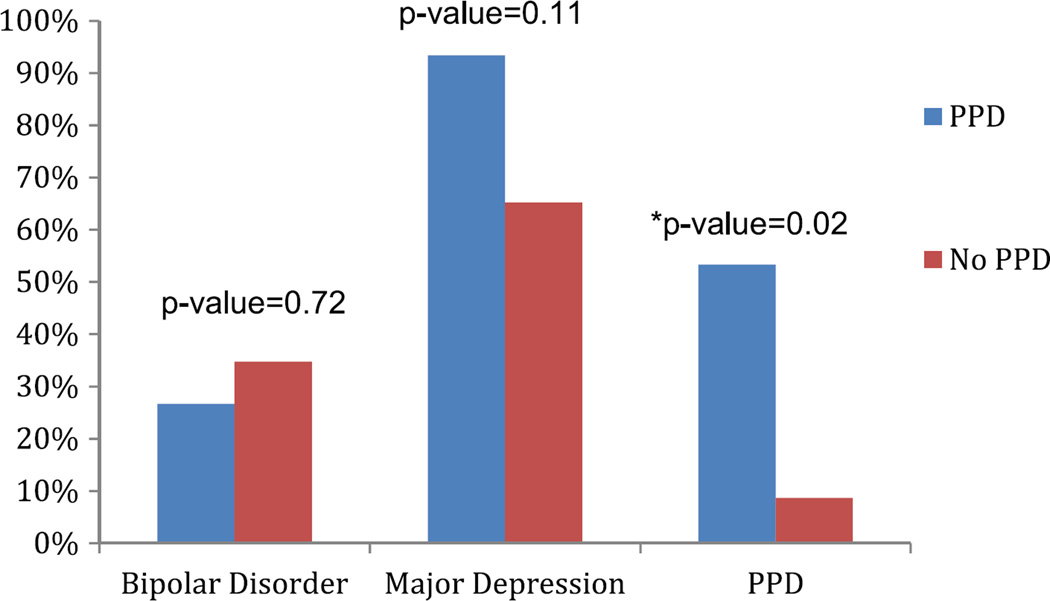
In Table 3, we examine the clinical characteristics in women who developed PPD and those that did not in the sample of women who were clinically well during the third trimester. There were no differences in course of illness between those that developed PPD and those that did not. There was also no significant difference between the two groups in the average number of hospitalizations. When looking at a personal history of PPD, 42.9% of those that developed PPD had a personal history and 42.1 % of those that did not develop PPD had a history. In contrast, 53.3 % of those that developed PPD had an immediate family member with a history of PPD, while 11.8 % of those who did not develop PPD (n=17 with available data) had a family history of PPD (Fisher’s exact test, p= 0.02) (Fig. 1). A family history of MDD or BP did not differ between the two groups (Fig. 1). Other clinical characteristics including a history of abuse, premenstrual symptoms, and a history of an anxiety disorder did not differ between the two groups. A history of substance abuse or dependence was more common in those that developed PPD (Fisher’s exact test, p= 0.001).
Table 3.
Clinical characteristics of women with and without PPD
| No postpartum depression (total N=23) |
Postpartum depression (total N=15) |
p value (Fisher’s exact test) |
|
|---|---|---|---|
| Remitting course | 65.2 % (23) | 66.7 % (15) | 1.00 |
| Chronic course | 13.0 % (23) | 33.3 % (15) | 0.22 |
| Frequent/brief course | 13.0 % (23) | 0.0 % (15) | 0.54 |
| Personal Hx PPD | 42.1 % (19) | 42.9 % (15) | 1.00 |
| Family Hx PPD | 11.8 % (17) | 53.3 % (15) | 0.02* |
| Family Hx MDD | 68.2 % (22) | 93.3 % (15) | 0.11 |
| Family Hx BP | 36.4 % (22) | 26.7 % (15) | 0.72 |
| Hospitalization | 39.1 % (23) | 33.3 % (15) | 1.00 |
| On meds T3 | 73.9 % (23) | 80.0 % (15) | 1.00 |
| Childhood abuse | 40.9 % (22) | 26.7 % (15) | 0.49 |
| Adult abuse | 9.1 % (22) | 0.0 % (15) | 0.50 |
| PMS | 60.9 % (23) | 67.7 % (15) | 1.00 |
| Significant PMS | 4.8 % (21) | 26.7 % (15) | 0.14 |
| Lifetime substance abuse | 8.7 % (23) | 60.0 % (15) | 0.001* |
| Lifetime any anxiety disorder | 75.0 % (20) | 53.3 % (15) | 0.28 |
Statistically Significant
Fig. 1.
Family history in women with and without PPD
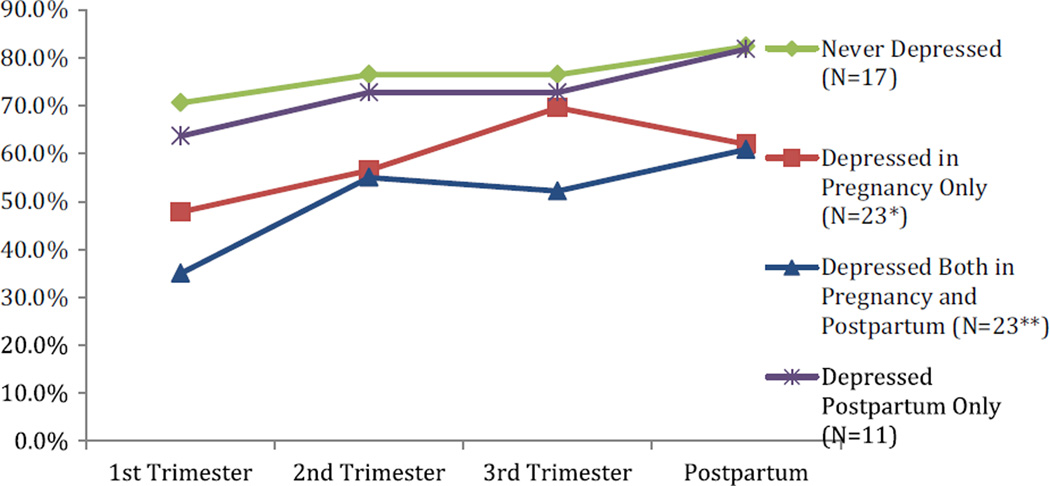
We were interested in whether medication use differed among the different clinical groups. Figure 2 shows the percentage of medication usage in (a) those that were never depressed (green circles), (b) those that were depressed during pregnancy only (red squares), (c) those that were depressed during pregnancy and postpartum (blue triangles), and (d) those depressed postpartum only (purple x’s). Those that were never depressed (green circles) had the highest rate of medication use while those that were chronically depressed both during and after pregnancy (blue triangles) had the least. Further, medication use was common in those that were well during pregnancy but depressed postpartum (purple x’s).
Fig. 2.
Percentage of women using medication during pregnancy and postpartum * We had data for 20 women at the 1st and 2nd trimester time point *We had data for 21 women at the 3rd trimester and postpartum time point
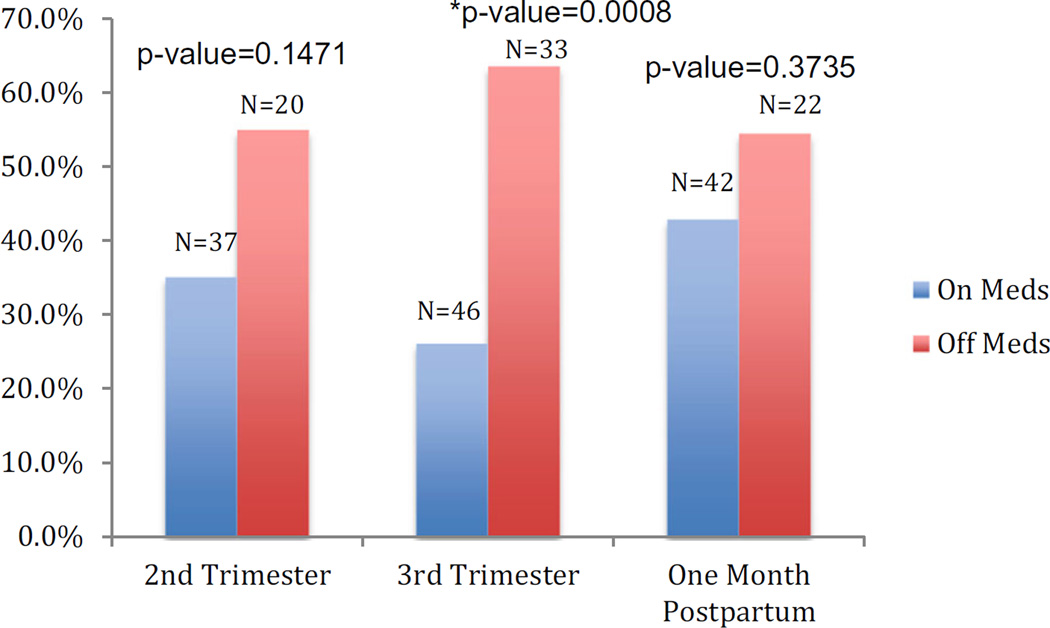
We also examined whether medication use or lack of medication use was associated with a MDE at the next evaluation period. For example, those not using medication in the second trimester experienced higher rates of depression in the third trimester. Figure 3 shows the percentage of women depressed at each time period (second and third trimester and 1 month postpartum) and whether or not they were taking medication during the previous time period (first, second, or third trimester, respectively). At each time period, more women were depressed who had been off of medications during the previous time period, though this was only significant at the third trimester visit (p=0.0008).
Fig. 3.
Percentage of women depressed based on whether medication use was reported during the previous visit
To assess the overall effect of medication usage and to account for within subject correlation due to repeated measures, we included all the aforementioned periods into a single longitudinal model: a generalized linear mixed model with depression status (yes/no) as the outcome and medication usage in the previous time period as the predictor. We found that the lack of medication use in the previous time period was associated with an increased risk of being depressed in the following time period with an odds ratio of 2.79 (95 % CI of 1.38 to 5.66, p value=0.0048). That is, the odds of being depressed for those who were off medication at the preceding time period were 2.8 times that of those who were on medication (mood stabilizer/antipsychotic or antidepressant).
Discussion
There are a number of observations that can be drawn from our data. First, medication use was common in this high risk population. Despite this, the development of a DSM-IV-defined MDE was also common in this sample with almost 75 % of the sample meeting the criteria for a MDE at some point during or after pregnancy. Thus, despite the high rate of medication use, most of our sample became psychiatrically ill during the perinatal time period. When we examined medication use based on presence of and timing of (during pregnancy and/or postpartum) MDE’s, we found that women who were never depressed during or after pregnancy and women who were only depressed postpartum had a higher rate of medication use during pregnancy compared to women who were depressed either during pregnancy or both during and after pregnancy. In addition, our analyses revealed that the odds of depression were higher in women who did not take medication during the preceding time point indicating that medication use decreased the odds of depression perinatally. Thus, medication use during pregnancy appears to be protective for some women during pregnancy. In contrast, women who developed PPD developed it despite a high rate of medication use during the third trimester: 80 % of those that developed PPD were taking psychiatric medications. Perhaps, our most striking finding was that in women who were well during the third trimester prior to delivery who then developed PPD, there was a very high rate of a family history of PPD (53.3 %) compared to women who were well in pregnancy but did not develop PPD (11.8 %). Thus, the use of psychiatric medications during pregnancy may not protect against PPD in women at high risk, particularly those with a family history of PPD. This finding supports the hypothesis that women who develop PPD, as defined as the development of a MDE within 4 weeks of delivery, may represent a more genetically homogenous group with an underlying biological vulnerability to times of hormonal change.
A genetic basis for postpartum mood syndromes had been suggested by several studies. Family studies of postpartum psychosis have supported a genetic susceptibility to a postpartum trigger in BPAD, as well as an overlap in genetic factors predisposing to postpartum psychosis and BPAD (Jones et al. 2000 and 2001). Dean et al. found a higher risk of postpartum mood illness in relatives of probands with postpartum psychosis (1989). A genome-wide linkage study of postpartum psychosis (Jones et al. 2007) identified a gene on chromosome 16 (16p13) that had previously been demonstrated to be associated with bipolar disorder. There have been a number of studies specifically examining the genetic basis of PPD. Forty et al. showed that the trait of postpartum depression exhibited familiality in pedigrees with recurrent MDD (2006). We reported familial aggregation of postpartum depressive symptoms developing within 4 weeks of delivery in families with recurrent early onset MDD as well as BPAD (Murphy-Eberenz et al. 2006; Payne et al. 2008). We went on to perform a genome-wide linkage in families with either MDD or BPAD and found that genetic variations on chromosomes 1q21.3-q32.1 and 9p24.3-p22.3 may increase susceptibility to postpartum mood symptoms (Mahon et al. 2009). More recently, we have identified two epigenetically modified biomarker loci at the HP1BP3 (chromosome 1) and TTC9B (chromosome 7) genes that predicted PPD in antenatally euthymic women from the same sample described here (Guintivano et al. 2013). These studies support the supposition of a genetic susceptibility to the hormonal changes associated with pregnancy, delivery, and the postpartum time period in women with mood disorders who develop PPD.
The finding that medication use during pregnancy did not prevent the development of PPD in women who were affectively well during pregnancy is a new one. There have been surprisingly few randomized trials examining the use of medication in the prevention of PPD. All have begun medication use after delivery. Wisner and Wheeler (1994) conducted an open-label study of postpartum monitoring versus postpartum monitoring plus a previously effective antidepressant or nortriptyline in 23 women with a personal history of PPD. In the monitored group, 62.5 % developed PPD versus 6.7 % in the medication treated group over the course of 3 months postpartum. There have been two placebo-controlled trials for the prevention of recurrent PPD using conventional antidepressants: one using nortriptyline postpartum in 26 women (Wisner et al. 2001) and the other using sertraline postpartum in 14 women all with personal histories of PPD (Wisner et al. 2004). Interestingly, nortriptyline failed to decrease the rate of PPD unlike Sertraline, although the nortriptyline study may have been underpowered (Wisner et al. 2001). Further, though nortriptyline levels were therapeutic, three of the six women who developed PPD in the nortriptyline group did so in the first 2 weeks postpartum while dose titration was taking place indicating that a less than therapeutic blood level may have contributed to the ineffectiveness of nortriptyline in preventing PPD (Wisner et al. 2001). The only other trial examining the pharmacological prevention of PPD was a non-blinded study using high-dose estrogen postpartum (begun at 10 mg daily tapered over 28 days to 0.625 mg daily) following delivery in four women with a history of PPD (Sichel et al. 1995). The authors report that the intervention prevented the expected relapse rate of 35–60 %. There have been no studies specifically examining whether the use of medication during pregnancy decreases the risk of PPD nor have the studies available to date taken into account family history of PPD. It remains unclear if additional pharmacological interventions (other than continuing medication taken during pregnancy) postpartum could reduce the risk of PPD in a high-risk population.
The finding that almost 75 % of the sample met DSM-IV criteria for a MDE at some point either during or after pregnancy deserves mention. Previous studies in community samples have found a much lower rate (Yonkers et al. 2011). Cohen et al. found a similar rate of depression in a high-risk clinic in women who discontinued their medications for pregnancy (2004 and 2006). The women in our study all had a prior history of a mood disorder and many continued their medications during pregnancy indicating that severity of illness may have played a role in the observed rate of MDEs. Many women and their psychiatrists are reluctant to increase medication during pregnancy even though drug levels are likely to drop across pregnancy leading to the undertreatment of depression during pregnancy (Sit et al. 2008). Future work should examine how often medication is increased and whether increasing is effective in the setting of depression during pregnancy. Despite the high rate of MDEs occurring in our sample, our data also suggests that the use of antidepressants decreased the risk of MDEs. As mentioned, evidence available to date has been conflicting: Cohen et al. found that discontinuation of antidepressants led to relapse at a high rate (2004 and 2006) and Yonkers et al. (2011) found that the use of antidepressants did not alter the risk of developing depression either during or after pregnancy. The fact that we found that the use of medication decreased the risk of MDEs may reflect the underlying characteristics of our sample population.
There are a number of limitations to our study. The study was conducted through a high risk clinic at a prominent university hospital. Therefore, women with more severe illness and complicated histories are likely to have participated (as supported by the high rate of psychiatric comorbidities in the population) and thus these observations may not apply to a less ill population. The sample size is small and we examined data from subjects with both MDD and BP. Although it did not appear that there were differences in terms of the rates of PPD between subjects with MDD and BP, the sample sizes were small making it difficult to make a precise estimate of whether they were different. We did not correct for multiple comparisons. We also did not confirm compliance with medications. Strengths include the prospective nature of the design, the rigorous definition of PPD, and the use of a DSM-IV-based psychiatric interview conducted by a trained psychiatrist to diagnose the presence or absence of a MDE. We were also very careful to define the timing of onset off MDEs so that we could easily determine if a woman met criteria for PPD.
In summary, our primary findings included the following: (1) major depressive episodes were common even in women who took psychiatric medication during pregnancy; (2) overall, medication use was associated with a decreased risk of MDEs during pregnancy; (3) in women who were clinically well prior to delivery, we observed a 39.4 % rate of the development of a postpartum depressive episode within the first 4 weeks after delivery; (4) 80 % of the women who developed PPD were taking psychiatric medications indicating that medication use during pregnancy may not prevent the onset of PPD in a high-risk population; (5) a family history of PPD was associated with the development of PPD in women who were clinically well prior to delivery. Future work should further explore the role of family history in the development of PPD and interventions that can be done to decrease the rate of PPD in high risk populations.
Acknowledgments
Conflict of interest Dr. Payne has received research support from Corcept Pharmaceuticals in the past year and legal consulting fees from Pfizer, Johnson and Johnson, and Astra Zeneca. Dr. Meltzer-Brody has received research support from Astra Zeneca. This study was funded by NIH Grant K23 MH074799 at the Johns Hopkins site and by the Foundation of Hope for the University of North Carolina site.
Footnotes
Ethical statement The study was approved by the Johns Hopkins IRB. All participants gave written informed consent prior to participation in the study.
References
- Akman I, Kuscu K, Ozdemir N, Yurdakul Z, Solakoglu M, Orhan L, Karabekiroglu A, Ozek E. Mothers’ postpartum psychological adjustment and infantile colic. Arch Dis Child. 2006;91(5):417–419. doi: 10.1136/adc.2005.083790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bratfos O, Hang JO. Puerperal mental disorders in manic depressive females. Acta Psychiatr Scand. 1966;42:285–294. doi: 10.1111/j.1600-0447.1966.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Cohn JF. Prevalence and correlates of postpartum depression in first-time mothers. J Abnorm Psychol. 1992;100(4):594–599. doi: 10.1037//0021-843x.100.4.594. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Nonacs RM, Bailey JW, Viguera AC, Reminick AM, Altshuler LL, Stowe ZN, Faraone SV. Relapse of depression during pregnancy following antidepressant discontinuation: a preliminary prospective study. Arch Women Mental Health. 2004;7(4):217–221. doi: 10.1007/s00737-004-0059-3. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- Davidson J, Robertson E. A follow-up study of postpartum illness. Acta Psychiatr Scand. 1985;71:451–457. doi: 10.1111/j.1600-0447.1985.tb05057.x. [DOI] [PubMed] [Google Scholar]
- Dean C, Williams RJ, Brockington IF. Is puerperal psychosis the same as bipolar manic-depressive disorder? A family study. Psychol Med. 1989;19:637–647. doi: 10.1017/s0033291700024235. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatr. 2007;164(10):1515–1520. doi: 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- Field T. Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14. doi: 10.1016/j.infbeh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Flynn HA, Davis M, Marcus SM, Cunningham R, Blow FC. Rates of maternal depression in pediatric emergency department and relationship to child service utilization. Gen Hosp Psychiatry. 2004;26(4):316–322. doi: 10.1016/j.genhosppsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Forty L, Jones L, Macgregor S, Caesar S, Cooper C, Hough A, Dean L, Dave S, Farmer A, McGuffin P, Brewster S, Craddock N, Jones I. Familiality of postpartum depression in unipolar disorder: results of a family study. Am J Psychiatry. 2006;163(9):1549–1553. doi: 10.1176/ajp.2006.163.9.1549. [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Jacob M, Blumenthal SJ, Jarrett DB. Pregnancy-related affective episodes among women with recurrent depression. Am J Psychiatry. 1987;144(3):288–293. doi: 10.1176/ajp.144.3.288. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Women Mental Health. 2003;6(4):263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Guintivano J, Arad M, Gould TD, Payne JL, Kaminsky ZA. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Molecular Psych. 2013:1–8. doi: 10.1038/mp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I, Craddock N. Familiality of the puerperal trigger in bipolar disorder: results of a family study. Am J Psychiatry. 2001;158(6):913–917. doi: 10.1176/appi.ajp.158.6.913. [DOI] [PubMed] [Google Scholar]
- Jones I, Middle F, McCandless F, Coyle N, Robertson E, Brockington I, Lendon C, Craddock N. Molecular genetic studies of bipolar disorder and puerperal psychosis at two polymorphisms in the estrogen receptor alpha gene (ESR 1) Am J Med Genet. 2000;96(6):850–853. doi: 10.1002/1096-8628(20001204)96:6<850::aid-ajmg31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Jones I, Hamshere M, Nangle JM, Bennett P, Green E, Heron J, Segurado R, Lambert D, Holmans P, Corvin A, Owen M, Jones L, Gill M, Craddock N. Bipolar affective puerperal psychosis: genome-wide significant evidence for linkage to chromosome 16. Am J Psychiatry. 2007;164(7):1099–1104. doi: 10.1176/ajp.2007.164.7.1099. [DOI] [PubMed] [Google Scholar]
- Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–673. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Payne JL, MacKinnon DF, et al. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. 2009;166(11):1229–1237. doi: 10.1176/appi.ajp.2009.09030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM. Depression during pregnancy: rates, risks and consequences—Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e15–e22. [PubMed] [Google Scholar]
- Misri S, Eng A, Abizadeh J, Blackwell E, Spidel A, Oberlander T. Factors impacting decisions to decline or adhere to antidepressant medication in perinatal women with mood and anxiety disorders. Depress Anxiety. 2013;30(11):1129–1136. doi: 10.1002/da.22137. [DOI] [PubMed] [Google Scholar]
- Murphy-Eberenz K, Zandi PP, March D, et al. Is perinatal depression familial? J Affect Disord. 2006;90(1):49–55. doi: 10.1016/j.jad.2005.10.006. [DOI] [PubMed] [Google Scholar]
- O'hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65(12):1258–1269. doi: 10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- Payne JL, Roy PS, Murphy-Eberenz K, Weissman MM, Swartz KL, McInnis MG, Nwulia E, Mondimore FM, MacKinnon DF, Miller EB, Nurnberger JI, Levinson DF, DePaulo JR, Potash JB. Reproductive cycle-associated mood symptoms in women with major depression and bipolar disorder. J Affect Disord. 2007;99:221–229. doi: 10.1016/j.jad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Payne JL, MacKinnon DF, Mondimore FM, et al. Familial aggregation of postpartum mood symptoms in families with bipolar disorder. J Bipolar Disord. 2008;10(1):38–44. doi: 10.1111/j.1399-5618.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T, Winokur Postpartum psychosis in patients with manic depressive disease. J Nerv Merit Dis. 1970;151:60–68. doi: 10.1097/00005053-197007000-00008. [DOI] [PubMed] [Google Scholar]
- Shadigian E, Bauer ST. Pregnancy-associated death: a qualitative systematic review of homicide and suicide. Obstet Gynecol Surv. 2005;60(3):183–190. doi: 10.1097/01.ogx.0000155967.72418.6b. [DOI] [PubMed] [Google Scholar]
- Shivakumar G, Johnson NL, McIntire DD, Leveno K. Progression of major depression during pregnancy and postpartum: a preliminary study. J Matern Fetal Neonatal Med. 2013 doi: 10.3109/14767058.2013.825599. [DOI] [PubMed] [Google Scholar]
- Sichel DA, Cohen LS, Robertson LM, Ruttenberg A, Rosenbaum JF. Prophylactic estrogen in recurrent postpartum affective disorder. Biol Psychiatry. 1995;38(12):814–818. doi: 10.1016/0006-3223(95)00063-1. [DOI] [PubMed] [Google Scholar]
- Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69(4):652–658. doi: 10.4088/jcp.v69n0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli MG. Maternal infanticide associated with mental illness: prevention and the promise of saved lives. Am J Psychiatry. 2004;161(9):1548–1557. doi: 10.1176/appi.ajp.161.9.1548. [DOI] [PubMed] [Google Scholar]
- Viguera AC, Tondo L, Koukopoulos AE, Reginaldi D, Lepri B, Baldessarini RJ. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. Am J Psychiatry. 2011;168(11):1179–1185. doi: 10.1176/appi.ajp.2011.11010148. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Wheeler SB. Prevention of recurrent postpartum major depression. Hosp Community Psychiatry. 1994;45(12):1191–1196. doi: 10.1176/ps.45.12.1191. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Findling RL, Rapport DJ. Prevention of recurrent postpartum depression: a randomized clinical trial. J Clin Psychiatry. 2001;62:82–86. doi: 10.4088/jcp.v62n0202. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of recurrent postpartum depression: a pilot randomized clinical trial. Am J Psychiatry. 2004;161:1290–1292. doi: 10.1176/appi.ajp.161.7.1290. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Ramin SM, Rush AJ, Navarrete CA, Carmody T, March D, Heartwell SF, Leveno KJ. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. Am J Psychiatry. 2001;158(11):1856–1863. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Gotman N, Smith MV, Forray A, Belanger K, Brunetto WL, Lin H, Burkman RT, Zelop CM, Lockwood CJ. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848–854. doi: 10.1097/EDE.0b013e3182306847. [DOI] [PMC free article] [PubMed] [Google Scholar]