Abstract
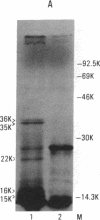
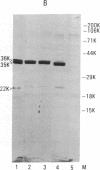
We recently identified a set of mammalian cell receptors for Neisseria gonorrhoeae that are glycolipids. These receptors, lactosylceramide [Gal(beta 1-4)Glc(beta 1-1)Cer], gangliotriosylceramide [GalNAc( beta 1-4)Gal(beta 1-4)Glc(beta 1-1)Cer], and gangliotetraosylceramide [Gal(beta 1-3)GalNAc(beta 1-4)Gal(beta 1-4)Glc(beta 1-1)Cer], were shown to be specifically bound by a gonococcal outer membrane protein distinct from pilin and protein II. Here we report the isolation of the gene encoding the gangliotetraosylceramide-binding adhesin from a N. gonorrhoeae MS11 gene bank in Escherichia coli. Transposon mutagenesis studies in E. coli indicate that the adhesion is a protein with a molecular mass of 36,000 Da. The gene encoding the 36-kDa protein is duplicated in MS11 since two transposon insertions were required to abolish expression of the gene in this bacterium. This protein is present on the surface of the gonococcus and is not associated with the pilus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Sun D., Dale J. B., Beachey E. H. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988 Dec 15;336(6200):682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- Babu J. P., Abraham S. N., Dabbous M. K., Beachey E. H. Interaction of a 60-kilodalton D-mannose-containing salivary glycoprotein with type 1 fimbriae of Escherichia coli. Infect Immun. 1986 Oct;54(1):104–108. doi: 10.1128/iai.54.1.104-108.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Simnad V. I., Seifert H. S., So M., Sparling P. F. Genetics of protein I of Neisseria gonorrhoeae: construction of hybrid porins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6841–6845. doi: 10.1073/pnas.85.18.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Normark S. Gene products specifying adhesion of uropathogenic Escherichia coli are minor components of pili. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1891–1895. doi: 10.1073/pnas.83.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal C., Perrin D., Hedgpeth J., Hofnung M. Synthesis and maturation of lambda receptor in Escherichia coli K-12: in vivo and in vitro expression of gene lamB under lac promoter control. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1491–1495. doi: 10.1073/pnas.77.3.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri D. K., Harshey R. M. Flagellar variation in Serratia marcescens is associated with color variation. J Bacteriol. 1987 Jan;169(1):61–65. doi: 10.1128/jb.169.1.61-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Wrightsman R. A., Manning J. E. Cloning of a major surface-antigen gene of Trypanosoma cruzi and identification of a nonapeptide repeat. Nature. 1986 Aug 7;322(6079):566–568. doi: 10.1038/322566a0. [DOI] [PubMed] [Google Scholar]
- Segal E., Billyard E., So M., Storzbach S., Meyer T. F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985 Feb;40(2):293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Ajioka R. S., Marchal C., Sparling P. F., So M. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature. 1988 Nov 24;336(6197):392–395. doi: 10.1038/336392a0. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988 Jun;56(6):1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg N., Deal C., Nyberg G., Normark S., So M., Karlsson K. A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Robbins K., Barrera O., Corwin D., Boslego J., Ciak J., Blake M., Koomey J. M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987 May 1;165(5):1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]