Abstract
Objective
To examine rates of attempted and successful vacuum-assisted vaginal delivery by pre-pregnancy body mass index (BMI).
Methods
We conducted a retrospective cohort study of 2,084 women with singleton gestations needing operative delivery assistance and vacuum-eligible (fully dilated, ≥ +2 station, ≥34 weeks) using 2006–2014 in-patient records. Pre-pregnancy BMI was categorized as underweight (<18.5kg/m2), normal weight (18.5kg/m2≤BMI<25kg/m2), overweight (25kg/m2≤ BMI<30kg/m2), or obese (≥30kg/m2). Logistic regression models estimated odds ratios (ORs) and 95% confidence intervals (CIs) of attempted and successful vacuum-assisted vaginal delivery by pre-pregnancy BMI adjusted for age, race, marital status, parity, diabetes, labor induction–augmentation, episiotomy, gestational age, and infant birth weight.
Results
Thirty-nine percent of women requiring delivery assistance and eligible for a vacuum were overweight or obese, 79% had vacuum attempts, and 95.3% of attempted vacuum-assisted vaginal deliveries were successful. Compared to women who were normal weight pre-pregnancy (82.8%), women who were overweight or obese were less likely to have vacuum attempted (75.8%, OR=0.71, 95% CI 0.53–0.96 and 71.2%, OR=0.53, 95% CI 0.39–0.74, respectively). Among women with attempted vacuum-assisted vaginal delivery, successful delivery did not differ by pre-pregnancy BMI (92.6%, OR=0.54, 95% CI 0.21–1.37 for underweight; 94.5%, OR=1.07, 95% CI 0.57–2.00 for overweight; 96.3%, OR=1.09, 95% CI 0.51–2.33 for obese, versus 95.6% among normal weight women).
Conclusions
Among women in need of operative delivery assistance, pre-pregnancy obesity was associated with lower likelihood of attempted vacuum-assisted vaginal delivery, but if attempted, success rates were similar to rates among normal weight women. With significant morbidity of second stage cesarean delivery in obese women, research should examine whether vacuum-assisted vaginal delivery may be appropriate for additional obese patients.
Precis
Among women needing operative delivery assistance, obese women have a lower likelihood of a vacuum-assisted delivery attempt, but if attempted, have similar rates of success.
INTRODUCTION
Three-fifths of reproductive-aged women are overweight or obese,1 and the prevalence of class III obesity (BMI > 40) in the United States has increased from 4.5% to 10.1% from 1999–2014.2,3 Obesity increases the risk of maternal and neonatal complications, and managing obese laboring women is complex4 as evidenced by longer time to second stage of labor, slower cervical dilatation rates, and higher risk for emergent cesarean delivery.5–7Women with obesity have increased cesarean delivery risks, including infection, bleeding, poor wound healing, thromboembolism, and anesthetic complications.4,8–10 With studies showing that obese women are as likely as normal weight women to deliver vaginally once reaching second stage labor,5,9,11,12 research is needed to examine whether vacuum instrumentation can be utilized to facilitate vaginal deliveries among obese women, and for which obese women is vacuum-assisted vaginal delivery appropriate.
The knowledge that obesity increases risk of large for gestational age neonates and therefore risk of failed operative (vacuum and forceps) vaginal delivery may influence health care providers’ decision-making on whether to attempt a vacuum extraction.9,13 Based on a search of Pubmed, Obstetrics & Gynecology, and Google Scholar (all sources searched from inception to June 2014) using the following terms “vacuum assisted vaginal delivery, obesity, BMI, body mass index, VAVD, vacuum extraction, vacuum assisted vaginal delivery, operative delivery,” the rates of attempted and successful vacuum extraction have not been studied [0]in relation to pre-pregnancy body mass index (BMI) despite vacuum delivery becoming the more common operative vaginal approach compared to forceps delivery.14 The aim of this study was to examine rates of attempted and successful vacuum-assisted vaginal delivery among laboring women in need of operative delivery assistance in relation to pre-pregnancy BMI.
MATERIALS AND METHODS
We conducted a retrospective cohort study using data from the UMass Memorial Health Care (UMMHC) inpatient obstetric electronic medical record of women who delivered from April 2006 through March 2014. UMMHC is an academically affiliated tertiary care referral hospital providing comprehensive inpatient obstetric care for approximately 3,800 deliveries per year. Height, weight, gestational weight gain, birth weight, and delivery gestational age have been previously validated.15–17 Data retrieved from the electronic medical record was supplemented by and validated with manual chart abstraction for delivery mode, cervical dilation, and fetal station. The University of Massachusetts Medical School Institutional Review Board approved this study.
We excluded women with non-singleton gestations, deliveries not eligible for labor (e.g., planned cesarean deliveries for malpresentation, prior uterine surgery), or gestations meeting other pre-determined exclusion criteria (prior cesarean delivery, forceps-assisted deliveries, pregnancy terminations, intrauterine fetal demises, prenatal and postnatal diagnosed congenital anomalies, maternal HIV). We also excluded women without a documented pre-pregnancy weight, height, delivery mode, dilation, station, or potential confounders of interest including maternal age, marital status, current smoking status, race/ethnicity, primary language, parity, episiotomy, diabetes status, hypertension, receipt of prenatal care, oxytocin induction or augmentation, delivery gestational age, and neonatal birth weight (small for gestational age, appropriate for gestational age, or large for gestational age). For women with more than one pregnancy during the study period, we randomly selected one pregnancy using the random number generator built into Stata. We limited the analytic sample only to women eligible for attempted vacuum, i.e., women not having a spontaneous vaginal delivery, ≥34 weeks of gestation, 10 cm dilated and ≥ +2 station (out of 3) requiring operative assistance,. From this analytic sample, we also examined a subcohort of “essential nulliparous” women (those with no prior pregnancies delivered ≥20 weeks).
Body mass index (BMI; kg/m2) was calculated from pre-pregnancy weight and height obtained from the medical record or self-reported at time of delivery according to a prioritized sequence as available (1) self-reported pre-pregnancy weight as recorded in the woman’s prenatal record, (2) weight self-reported by the women upon admission for delivery or (3) measured weight at first prenatal visit as recorded in her prenatal record. Pre-pregnancy BMI was categorized as underweight (BMI<18.5kg/m2), normal weight (18.5kg/m2≤BMI<25kg/m2), overweight (25kg/m2≤ BMI<30kg/m2), or obese (30kg/m2≤BMI).18 Gestational weight gain (GWG) was calculated by subtracting pre-pregnancy weight from admission weight as self-reported at delivery admission or last documented prenatal visit weight. GWG was categorized as inadequate, appropriate, or excessive considering gestational age at delivery and using the 2009 Institute of Medicine’s (now the National Academies of Sciences, Engineering, and Medicine) pre-pregnancy BMI-specific recommended trajectories of ranges of gain achieved by the 40th week of gestation: 28–40 pounds for women who were underweight before pregnancy, 25–35 pounds for women of normal weight, 15–25 pounds for overweight women, and 11–20 pounds for obese women.18 Abstraction of medical records also included adverse maternal (third and fourth degree perineal lacerations) and neonatal (shoulder dystocia, Apgar < 7 at 1 and 5 minutes and Neonatal Intensive Care Unit admission) outcomes.
We examined the distribution of potential confounders by pre-pregnancy BMI using chi-square tests for categorical variables and ANOVA for continuous variables. We used logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for attempted and successful vacuum-assisted vaginal delivery. Variables associated with delivery mode at p<0.10 were included in adjusted regression models. We compared rates of adverse maternal and neonatal outcomes by delivery type using crude logistic regression models. Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
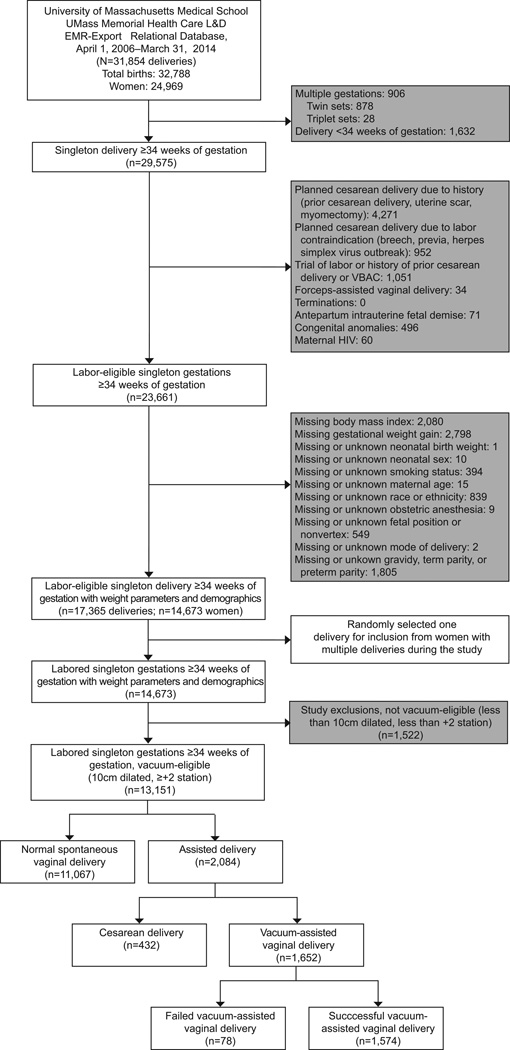
Of the 31,854 deliveries in 2006–2014, we excluded 14,489 based on our a priori study scheme (Figure 1). Of the remaining 17,365 deliveries, we randomly selected one delivery per woman, and excluded deliveries not eligible for a vacuum delivery (< 10cm dilatation, < +2 station), and spontaneous vaginal deliveries (N=11,067), resulting in an analytic sample of 2,084 women who required operative delivery assistance (Figure 1).
Figure 1.
Vacuum delivery by maternal body mass index. Values in gray boxes are not mutually exclusive. L&D, labor and delivery; EMR, electronic medical records; VBAC, vaginal birth after cesarean delivery; HIV, human immunodeficiency virus.
Characteristics of the analytic cohort in relation to pre-pregnancy BMI are shown in Table 1. Women were predominantly white (65%), English speakers (83%), married (62%), and non-smokers (89%). Three-quarters (73%) were nulliparous. The vast majority received prenatal care (99%). Half (56%) of women were normal weight pre-pregnancy, 23% were overweight, and 16% were obese. Hypertensive disease of pregnancy (10%) and combined pre-gestational and gestation DM (6%) were the most common comorbid conditions in our sample, with higher occurrence among women with higher pre-pregnancy BMI.
Table 1.
Characteristics of laboring women who require operative delivery assistance in relation to pre-pregnancy body mass index†, N (%) (N=2,084)
| Underweight (N=99) |
Normal weight (N=1,175) |
Overweight (N=476) |
Obese (N=337) |
P-value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 15–19 | 9 (9.1) | 95 (8.1) | 28 (5.9) | 19 (5.6) | 0.234 |
| 20–24 | 23 (23.2) | 188 (16.0) | 73 (15.3) | 58 (17.2) | |
| 25–29 | 22 (22.2) | 327 (27.9) | 127 (26.7) | 100 (29.7) | |
| 30–34 | 31 (31.3) | 367 (31.3) | 147 (30.9) | 95 (28.2) | |
| 35+ | 14 (14.1) | 195 (16.6) | 101 (21.2) | 65 (19.3) | |
| Race/Ethnicity | |||||
| Black | 4 (4.0) | 61 (5.2) | 40 (8.4) | 22 (6.5) | <0.001 |
| White | 54 (54.5) | 738 (63.0) | 322 (67.6) | 241 (71.5) | |
| Asian | 15 (15.2) | 104 (8.9) | 14 (2.9) | 5 (1.5) | |
| Hispanic | 15 (15.2) | 150 (12.8) | 56 (11.8) | 56 (16.6) | |
| Other | 11 (11.1) | 119 (10.2) | 44 (9.2) | 13 (3.9) | |
| Primary language | |||||
| English | 73 (75.3) | 947 (82.3) | 394 (84.5) | 291 (87.7) | 0.001 |
| Spanish | 7 (7.2) | 64 (5.6) | 30 (6.4) | 25 (7.5) | |
| Other | 17 (17.5) | 139 (12.1) | 42 (9.0) | 16 (4.8) | |
| Married | 53 (53.5) | 725 (62.4) | 311 (65.9) | 192 (57.7) | 0.032 |
| Parity | |||||
| 0 | 74 (74.7) | 870 (74.2) | 352 (73.9) | 220 (65.3) | <0.001 |
| 1 | 12 (12.1) | 228 (19.5) | 75 (15.8) | 83 (24.6) | |
| 2+ | 13 (13.1) | 74 (6.3) | 49 (10.3) | 34 (10.1) | |
| Smoking | 16 (16.2) | 114 (9.7) | 49 (10.3) | 53 (15.7) | 0.006 |
| Diabetes (Type I, II or GDM) | 4 (4.3) | 45 (4.2) | 33 (7.6) | 38 (12.3) | <0.001 |
| Hypertension: PIH, Chronic & Preeclampsia | 2 (2.0) | 83 (7.1) | 57 (12.0) | 63 (18.7) | <0.001 |
| Received prenatal care | 98 (99.0) | 1,152 (98.8) | 467 (99.2) | 334 (99.7) | 0.479 |
| Any Augmentation or Induction | 70 (70.7) | 883 (75.3) | 379 (79.6) | 287 (85.2) | <0.001 |
| Epidural Anesthesia | 84 (84.8) | 1,020 (87.1) | 413 (86.8) | 300 (89.0) | 0.663 |
| Adherence to 2009 IOM guideline | |||||
| Inadequate | 22 (22.2) | 197 (16.8) | 54 (11.3) | 68 (20.2) | <0.001 |
| Appropriate | 41 (41.4) | 374 (31.9) | 92 (19.3) | 53 (15.8) | |
| Excessive | 36 (36.4) | 601 (51.3) | 330 (69.3) | 215 (64.0) | |
| Gestational age | |||||
| EGA ≥34 –<37 weeks | 2 (2.0) | 51 (4.4) | 17 (3.6) | 16 (4.7) | 0.637 |
| EGA 37+ weeks | 97 (98.0) | 1,121 (95.6) | 459 (96.4) | 321 (95.3) | |
| Neonate weight | |||||
| SGA | 14 (14.3) | 124 (10.6) | 46 (9.7) | 21 (6.2) | 0.001 |
| LGA | 83 (84.7) | 968 (82.7) | 397 (83.4) | 277 (82.2) | |
| AGA | 1 (1.0) | 79 (6.7) | 33 (6.9) | 39 (11.6) | |
≥ 34 weeks of gestation, vertex, 10 cm dilation, ≥ +2 station
Underweight (<18.5kg/m2), Normal weight (18.5kg/m2≤BMI<25kg/m2), Overweight (25kg/m2≤ BMI<30kg/m2), Obese (≥30kg/m2)
GDM (Gestational Diabetes Mellitus), GWG (Gestational Weight Gain), IOM (Institute of Medicine), SGA (Small for Gestational Age), AGA (Average for Gestational Age), LGA (Large for Gestational Age)
Among our sample of women requiring operative delivery assistance, 79% had an attempted vacuum assisted vaginal delivery (N=1,652) and 21% (N=432) had a cesarean delivery without a trial of vacuum. The proportion of women with attempted vacuum was lower among women who were overweight or obese pre-pregnancy (75.8%, adjusted OR=0.71, 95% CI 0.53–0.96 and 71.2%, adjusted OR=0.53, 95% CI 0.39–0.74, respectively) compared to women who were normal weight (82.8%; Table 2). Women with excessive gestational weight gain (adjusted OR=0.78, 95% CI 0.59–1.04), large for gestational age neonates (adjusted OR=0.27, 95% CI 0.18–0.40), and diabetes (adjusted OR=0.55, 95% CI 0.35–0.87), were less likely to have a vacuum-assisted vaginal delivery attempted. Conversely, women who received labor augmentation or induction (adjusted OR=1.37, 95% CI 1.00–1.87), used epidural anesthesia (adjusted OR=1.61, 95% CI 1.12–2.29), gained inadequate weight gain (adjusted OR=1.80, 95% CI 1.15–2.82), were aged 15–19 years (adjusted OR= 2.37, 95% CI 1.28–4.42), delivered a small for gestational age infant (adjusted OR=3.54. 95% 1.92–6.53), and multiparous women (adjusted OR=5.22, 95% CI 2.75–9.93) were more likely to have a vacuum-assisted vaginal delivery attempted (Table 2).
Table 2.
Attempted vacuum-assisted vaginal delivery in relation to pre-pregnancy body mass index and participant characteristics among laboring women in need of operative delivery assistance* (N=2,084)
| Characteristics | Attempted vacuum-assisted vaginal delivery | ||
|---|---|---|---|
| N (%) | Crude OR [95% CI] | Adjusted† OR [95% CI] | |
| Pre-pregnancy BMI (kg/m2) | |||
| Underweight (< 18.5) | 81 (81.8) | 0.94 [0.55–1.60] | 0.68 [0.38–1.22] |
| Normal Weight (18.5 to < 25.0) | 970 (82.8) | Reference | Reference |
| Overweight (25.0 to < 30.0) | 361 (75.8) | 0.65 [0.51–0.85] | 0.71 [0.53–0.96] |
| Obese (≥30.0) | 240 (71.2) | 0.52 [0.39–0.68] | 0.53 [0.39–0.74] |
| Age (years) | |||
| 15 – 19 years | 133 (88.1) | 1.77 [1.01–3.10] | 2.37 [1.28–4.42] |
| 20 – 24 years | 276 (80.7) | Reference | Reference |
| 25 – 29 years | 451 (78.3) | 0.86 [0.62–1.20] | 0.96 [0.65–1.43] |
| 30 – 34 years | 490 (76.6) | 0.78 [0.56–1.08] | 0.77 [0.51–1.16] |
| 35+ years | 302 (80.5) | 0.99 [0.68–1.43] | 0.81 [0.51–1.29] |
| Race | |||
| Black | 106 (83.5) | 1.39 [0.86–2.26] | 0.92 [0.53–1.59] |
| White | 1,062 (78.4) | Reference | Reference |
| Asian | 118 (85.5) | 1.63 [1.00–2.66] | 1.36 [0.77–2.41] |
| Hispanic | 218 (78.7) | 1.02 [0.74–1.40] | 0.67 [0.45–0.99] |
| Other | 148 (79.1) | 1.05 [0.72–1.52] | 0.84 [0.55–1.28] |
| Marital Status | |||
| Not Married | 645 (82.3) | Reference | Reference |
| Married | 993 (77.5) | 0.74 [0.59–0.93] | 0.77 [0.57–1.05] |
| Parity | |||
| 0 | 1,149 (75.8) | Reference | Reference |
| 1 | 346 (86.9) | 2.13 [1.55–2.91] | 2.73 [1.91–3.89] |
| 2+ | 157 (92.4) | 3.85 [2.16–6.87] | 5.22 [2.75–9.93] |
| Smoking Cigarettes | |||
| No smoking | 1,458 (78.7) | 0.72 [0.51–1.04] | |
| Smoking | 194 (83.6) | Reference | Reference |
| Diabetes (Type 1, 2, GDM) | |||
| Not Diabetes | 1,415 (79.5) | Reference | Reference |
| Diabetes | 79 (65.8) | 0.50 [0.34–0.74] | 0.55 [0.35–0.87] |
| Hypertension (chronic, pregnancy-induced, preeclampsia) | |||
| Not hypertension | 1,496 (79.6) | Reference | |
| Hypertension | 156 (76.1) | 0.82 [0.52–1.15] | |
| Received Prenatal care | |||
| No | 17 (85.0) | 1.48 [0.43–5.09] | |
| Yes | 1,625 (79.2) | Reference | |
| Any Augmentation or Induction | |||
| No | 394 (84.7) | Reference | |
| Yes | 1,258 (77.7) | 0.63 [0.48–0.83] | 1.37 [1.00–1.87] |
| Epidural | |||
| No | 205 (77.1) | Reference | Reference |
| Yes | 1,447 (79.6) | 1.16 [0.86–1.58] | 1.61 [1.12–2.29] |
| Adherence to 2009 IOM guideline | |||
| Inadequate | 301 (88.3) | 1.64 [1.10–2.43] | 1.80 [1.15–2.82] |
| Appropriate | 460 (82.1) | Reference | Reference |
| Excessive | 890 (75.3) | 0.66 [0.51–0.85] | 0.78 [0.59–1.04] |
| Estimated Gestational age (weeks) | |||
| EGA ≥34 – <37 | 70 (81.4) | Reference | |
| EGA 37+ | 1,582 (79.2) | 0.87 [0.50–1.51] | |
| Neonate weight | |||
| SGA | 190 (92.7) | 3.18 [1.85–5.45] | 3.54 [1.92–6.53] |
| AGA | 1,379 (79.9) | Reference | Reference |
| LGA | 81 (53.3) | 0.29 [0.20–0.40] | 0.27 [0.18–0.40] |
≥ 34 weeks gestational, vertex, 10 cm dilation, ≥ +2 station
Adjusted for: age category, smoking, hypertension, prenatal care, epidural, adherence to GWG, race, marital status, parity, diabetes, induction, augmentation, and estimated gestational age
Significant Odds Ratios at the p< 0.05 level bolded
GDM (Gestational Diabetes Mellitus), GWG (Gestational Weight Gain), IOM (Institute of Medicine), SGA (Small for Gestational Age), AGA (Average for Gestational Age), LGA (Large for Gestational Age)
Among the women who had a vacuum-assisted vaginal delivery attempt (N=1,652), the vast majority was successful, regardless of pre-pregnancy BMI (92.6–96.3%, Table 3). Compared to women having spontaneous vaginal deliveries (N=11,067), women who had a vacuum-assisted vaginal delivery were more likely to experience a third or fourth degree perineal laceration (1.8% vs. 12.3%, OR=7.7, 95% CI: 6.3–9.4) and shoulder dystocia (1.6% vs. 2.5%, OR=1.6, 95% CI: 1.1–2.2). Among women who required operative assistance, there were no differences in infant 1-minute and 5-minute Apgar score <7 or Neonatal Intensive Care Unit (NICU) admissions between vacuum assisted vaginal delivery, cesarean delivery without vacuum attempt, or failed vacuum delivery leading to cesarean (Table 4).
Table 3.
Successful vacuum-assisted vaginal delivery in relation to participant characteristics among women on whom vacuum was attempted (N=1,652)
| Characteristics | Successful Vacuum | ||||
|---|---|---|---|---|---|
| N (%) | Crude OR [95% CI] | Adjusted OR* [95% CI] | |||
| Pre-pregnancy BMI (kg/m2) | |||||
| Underweight (< 18.5) | 75 (92.6) | 0.58 [0.24–1.41] | 0.54 [0.21–1.37] | ||
| Normal Weight (18.5 to < 25.0) | 927 (95.6) | Reference | Reference | ||
| Overweight (25.0 to < 30.0) | 341 (94.5) | 0.79 [0.46–1.36] | 1.07 [0.57–2.00] | ||
| Obese (≥30.0) | 231 (96.3) | 1.19 [0.57–2.48] | 1.09 [0.51–2.33] | ||
| Age (years) | |||||
| 15 – 19 | 128 (96.2) | 0.76 [0.25–2.38] | |||
| 20 – 24 | 268 (97.1) | Reference | |||
| 25 – 29 | 424 (94.0) | 0.47 [0.21–1.05] | |||
| 30 – 34 | 466 (95.1) | 0.58 [0.26–1.31] | |||
| 35+ | 288 (95.4) | 0.61 [0.25–1.49] | |||
| Race | |||||
| Black | 102 (96.2) | 1.34 [0.48–3.78] | 1.48 [0.44–4.98] | ||
| White | 1,009 (95.0) | Reference | Reference | ||
| Asian | 112 (94.9) | 0.98 [0.41–2.33] | 1.12 [0.42–3.02] | ||
| Hispanic | 215 (98.6) | 3.76 [1.17–12.16] | 3.39 [1.03–11.13] | ||
| Other | 136 (91.9) | 0.60 [0.31–1.14] | 0.62 [0.30–1.28] | ||
| Marital Status | |||||
| Married | 944 (95.1) | 0.91 [0.57–1.45] | |||
| Not married | 616 (95.5) | Reference | |||
| Parity | |||||
| 0 | 1.076 (93.6) | Reference | |||
| 1 | 344 (99.4) | 11.67 [2.85–47.79] | 11.85 [2.86–49.08] | ||
| 2+ | 154 (98.1) | 3.48 [1.08–11.19] | 3.38 [1.03–11.07] | ||
| Smoking Cigarettes | |||||
| No Smoking | 1,391 (95.4) | 1.25 [0.65–2.41] | |||
| Smoking | 183 (94.3) | Reference | |||
| Diabetes (Type 1, 2, GDM) | |||||
| Not Diabetes | 1,350 (95.4) | Reference | |||
| Diabetes | 72 (91.1) | 0.50 [0.22–1.12] | 0.47 [0.20–1.11] | ||
| Hypertension (chronic, pregnancy–induced, preeclampsia) | |||||
| Not hypertension | 1,424 (90.5) | Reference | |||
| Hypertension | 150 (9.5) | 1.26 [0.54–2.96] | |||
| Received Prenatal Care | |||||
| No | 17 (100.0) | ||||
| Yes | 1,547 (95.2) | Reference | |||
| Any labor induction or augmentation | |||||
| No | 382 (96.9) | Reference | Reference | ||
| Yes | 1,192 (94.7) | 0.57 [0.30–1.06] | 0.59 [0.30–1.17] | ||
| Epidural | |||||
| No | 195 (95.1) | Reference | Reference | ||
| Yes | 1,379 (95.3) | 1.04 [0.53–2.06] | 1.77 [0.84–3.71] | ||
| Adherence To 2009 IOM Guideline | |||||
| Inadequate | 285 (94.7) | 0.94 [0.49–1.81] | |||
| Appropriate | 437 (95.0) | Reference | |||
| Excessive | 851 (95.6) | 1.15 [0.68–1.95] | |||
| Estimated Gestational Age | |||||
| EGA <37 | 68 (97.1) | Reference | Reference | ||
| EGA 37+ | 1,506 (95.2) | 0.58 [0.14–2.42] | 0.26 [0.04–1.93] | ||
| Neonate weight | |||||
| SGA | 185 (97.4) | 1.89 [0.75–4.75] | 3.43 [1.05–11.25] | ||
| AGA | 1,312 (95.1) | Reference | Reference | ||
| LGA | 75 (92.6) | 0.64 [0.27–1.52] | 0.44 [0.18–1.12] | ||
Adjusted for: age category, smoking, hypertension, prenatal care, epidural, adherence to GWG, race, diabetes, induction, augmentation, and estimated gestational age
Significant Odds Ratios at the p< 0.05 level bolded
GDM (Gestational Diabetes Mellitus), GWG (Gestational Weight Gain), IOM (Institute of Medicine), SGA (Small for Gestational Age), AGA (Average for Gestational Age), LGA (Large for Gestational Age)
Table 4.
Neonatal complications of interest by mode of delivery
| NICU Admission | Apgar Score at 1 min < 7 | Apgar Score at 5 min <7 | ||||
|---|---|---|---|---|---|---|
| N (%) | OR (95% CI) | N (%) | OR (95% CI) | N (%) | OR (95% CI) | |
| VAVD | 43 (2.7) | 0.98 (0.51–1.88) | 317 (20.1) | 1.03 (0.79–1.35) | 44 (2.8) | 1.10 (0.56–2.15) |
| CD | 12 (2.8) | Ref. | 85 (19.7) | Ref. | 11 (2.6) | Ref. |
| CD after failed VAVD | 3 (3.9) | 1.42 (0.39–5.15) | 22 (28.6) | 1.63 (0.94–2.83) | 4 (5.2) | 2.10 (0.65–6.77) |
CD (Cesarean Delivery), VAVD (vacuum assisted vaginal delivery), NICU (Neonatal Intensive Care Unit)
We conducted two sensitivity analyses. First, we excluded women who had a vacuum applied but removed prior to delivery (N=55). Results were similar to main analyses (data not shown). Second, we excluded women with a prior delivery ≥20 weeks of gestation (N=1,171). Results were similar to the main analyses (data not shown).
DISCUSSION
We found that among women achieving full dilation, descent to ≥+2 station (out of 3) and gestational age ≥34 weeks, requiring operative delivery assistance, higher pre-pregnancy BMI was associated with lower likelihood of attempted vacuum-assisted vaginal delivery but similar success rates when attempted. Increased risks of large for gestational age neonates and associated shoulder dystocia among obese women4 may raise clinician concern for failure or complications deterring vacuum-assisted vaginal delivery attempts, especially since estimating fetal weight may be less accurate in obese women.19 Perineal lacerations and shoulder dystocia are known risks of vacuum-assisted vaginal delivery.20,21 Among women who underwent a vacuum assisted vaginal delivery, the rates of 3rd or 4th degree laceration (12.3%) and shoulder dystocia (2.5%) in this study were similar to rates reported in previous studies and increased relative to those who had cesarean delivery without attempted vaginally delivery.22 In our sample of women requiring operative delivery assistance, neither Apgar scores or NICU admissions differed between women with vacuum assisted vaginal delivery, cesarean delivery without vacuum attempt or cesarean delivery after failed vacuum assisted vaginal delivery, suggesting that attempting vacuum-assisted vaginal delivery may not increase these risks. This should be balanced against the significant morbidity accompanying cesarean delivery in women with obesity. The rates of neonatal SGA in the successful vacuum delivery group and macrosomia in the infants who were delivered by cesarean delivery suggest that clinicians made knowledgeable decisions about when to apply a vacuum.
Our finding of lower odds of attempted vacuum-assisted vaginal delivery in women of higher pre-pregnancy BMI contrasts with previous studies.9,23 A recent prospective cohort study of Norwegian women examined operative vaginal delivery (including vacuum-assisted vaginal delivery) in relation to pre-pregnancy BMI and found 50% higher rates of vacuum-assisted vaginal delivery among women with class III obesity compared to normal weight women.24 However, our study differs in ways that may explain the discrepancy in findings. First, rates of cesarean delivery in Norway were half of those in the United States during the study period (2001–2010).25 Thus, if cesarean deliveries are less common, it is possible that physicians in Norway relied more on operative vaginal deliveries, including for women with obesity. The Norwegian study excluded women with chronic hypertensive disease, hypertensive disease of pregnancy and gestational diabetes, all risk factors associated with higher cesarean rates. Finally, they reported a prevalence of obesity of 8% compared to 18% in our cohort.
Our study has several strengths. Characteristics of our sample are similar to the ethnic and racial composition reported in the 2010 US Census for Worcester County,26 thus speaking to generalizability for communities similar to ours. Limitations include lack of information about medical comorbidities associated with recommendations to shorten second stage labor (e.g., valvular disease, chronic obstructive pulmonary disease).20 Pre-pregnancy weight was self-reported or measured early in prenatal care, potentially resulting in misclassification of weight. However, a recent study found that self-reported and clinically measured pre-pregnancy weight are highly correlated (r=0.99) and mean underreporting of weight by ~2 pounds did not differ by race/ethnicity, gestational age, or pre-pregnancy weight.27 In a previous study derived from this patient population, categorization of pre-pregnancy BMI was identical for 87% of women when using self-reported pre-pregnancy weight or weight measured at first prenatal visit during the first trimester;28 thus, potential misclassification is unlikely to alter our findings. Given the modest number of women with class II or III obesity with whom vacuum-assisted vaginal delivery was attempted (N=240) and the small number of obese women with attempted vacuum-assisted vaginal delivery that was not successful (N=9), we were unable to examine rates of attempted or successful vacuum-assisted vaginal delivery in relation to class of obesity. Further studies with larger samples of obese women should examine rates of attempted and successful vacuum-assisted vaginal delivery in relation to classes of obesity.
When failed vacuum delivery occurs, clinicians face difficult choices. They can either attempt an alternative operative vaginal approach or do an emergent cesarean delivery. Obstetricians must contend with the higher likelihood of large-for-gestational age neonates and higher inaccuracy of estimating fetal weight in patients with obesity, with fetal macrosomia being an important shoulder dystocia risk factor,29 which may contribute to clinician hesitancy in attempting vacuum-assisted vaginal delivery versus proceeding directly to cesarean delivery. Clinicians may hesitate to attempt vacuum-assisted vaginal delivery in potentially eligible women who are obese, given the difficulty in assessing estimated weight, and higher likelihood of fetal macrosomia, a significant risk factor for shoulder dystocia. Additional research is needed to understand factors contributing to clinician decision-making about operative delivery method among laboring women who require operative assistance, and what influence, if any, maternal pre-pregnancy BMI plays in this process.
In conclusion, among women in need of operative delivery assistance, pre-pregnancy obesity was associated with a lower likelihood of a vacuum-assisted vaginal delivery attempt, but if attempted, similar rates of success. The risks accompanying vacuum extraction in this patient population should be balanced with the higher surgical risk and morbidity of cesarean delivery.23,30 Clinicians may wish to consider attempting a vacuum extraction in clinically appropriate laboring women with obesity who require operative assistance.
Acknowledgments
Partial support for Dr. Waring provided by NIH grants KL2TR000160 and U01HL105268.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Presented as a poster at the American College of Obstetricians and Gynecologists Annual Clinical Meeting, May 2–6, 2015, San Francisco, CA.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the united states, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists. Obesity in pregnancy<br />. ACOG Committee Opinion no. 549a, Obstetrics and gynecology. 2013;121(1):213. doi: 10.1097/01.aog.0000425667.10377.60. [DOI] [PubMed] [Google Scholar]
- 5.Fyfe EM, Anderson NH, North RA, et al. Risk of first-stage and second-stage cesarean delivery by maternal body mass index among nulliparous women in labor at term. Obstet Gynecol. 2011;117(6):1315–1322. doi: 10.1097/AOG.0b013e318217922a. [DOI] [PubMed] [Google Scholar]
- 6.Norman SM, Tuuli MG, Odibo AO, Caughey AB, Roehl KA, Cahill AG. The effects of obesity on the first stage of labor. Obstet Gynecol. 2012;120(1):130–135. doi: 10.1097/AOG.0b013e318259589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuthalapaty FS, Rouse DJ, Owen J. The association of maternal weight with cesarean risk, labor duration, and cervical dilation rate during labor induction. Obstet Gynecol. 2004;103(3):452–456. doi: 10.1097/01.AOG.0000102706.84063.C7. [DOI] [PubMed] [Google Scholar]
- 8.Wispelwey BP, Sheiner E. Cesarean delivery in obese women: A comprehensive review. J Matern Fetal Neonatal Med. 2013;26(6):547–551. doi: 10.3109/14767058.2012.745506. [DOI] [PubMed] [Google Scholar]
- 9.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. Am J Obstet Gynecol. 2004;190(4):1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 10.Tan T, Sia AT. Anesthesia considerations in the obese gravida. Semin Perinatol. 2011;35(6):350–355. doi: 10.1053/j.semperi.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Buhimschi CS, Buhimschi IA, Malinow AM, Weiner CP. Intrauterine pressure during the second stage of labor in obese women. Obstet Gynecol. 2004;103(2):225–230. doi: 10.1097/01.AOG.0000102706.84063.C7. [DOI] [PubMed] [Google Scholar]
- 12.Gunatilake RP, Perlow JH. Obesity and pregnancy: Clinical management of the obese gravida. Am J Obstet Gynecol. 2011;204(2):106–119. doi: 10.1016/j.ajog.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Sheiner E, Shoham-Vardi I, Silberstein T, Hallak M, Katz M, Mazor M. Failed vacuum extraction. maternal risk factors and pregnancy outcome. J Reprod Med. 2001;46(9):819–824. [PubMed] [Google Scholar]
- 14.Hehir MP, Reidy FR, Wilkinson MN, Mahony R. Increasing rates of operative vaginal delivery across two decades: Accompanying outcomes and instrument preferences. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):40–43. doi: 10.1016/j.ejogrb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Simas TA, Waring ME, Liao X, et al. Pre-pregnancy weight, gestational weight gain, and risk of growth affected neonates. J Womens Health (Larchmt) 2012;21(4):410–417. doi: 10.1089/jwh.2011.2810. [DOI] [PubMed] [Google Scholar]
- 16.Simas TA, Liao X, Garrison A, Sullivan GM, Howard AE, Hardy JR. Impact of updated institute of medicine guidelines on pre-pregnancy body mass index categorization, gestational weight gain recommendations, and needed counseling. J Womens Health (Larchmt) 2011;20(6):837–844. doi: 10.1089/jwh.2010.2429. [DOI] [PubMed] [Google Scholar]
- 17.Moore Simas TA, Doyle Curiale DK, Hardy J, Jackson S, Zhang Y, Liao X. Efforts needed to provide institute of medicine-recommended guidelines for gestational weight gain. Obstet Gynecol. 2010;115(4):777–783. doi: 10.1097/AOG.0b013e3181d56e12. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: What obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21(6):521–526. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox NS, Bhavsar V, Saltzman DH, Rebarber A, Chasen ST. Influence of maternal body mass index on the clinical estimation of fetal weight in term pregnancies. Obstet Gynecol. 2009;113(3):641–645. doi: 10.1097/AOG.0b013e3181998eef. [DOI] [PubMed] [Google Scholar]
- 20.Ali UA, Norwitz ER. Vacuum-assisted vaginal delivery. Rev Obstet Gynecol. 2009;2(1):5–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Dall'Asta A, Ghi T, Pedrazzi G, Frusca T. Does vacuum delivery carry a higher risk of shoulder dystocia? review and meta-analysis of the literature. Eur J Obstet Gynecol Reprod Biol. 2016;204:62–68. doi: 10.1016/j.ejogrb.2016.07.506. [DOI] [PubMed] [Google Scholar]
- 22.Angioli R, Gomez-Marin O, Cantuaria G, O'sullivan MJ. Severe perineal lacerations during vaginal delivery: The university of Miami experience. Am J Obstet Gynecol. 2000;182(5):1083–1085. doi: 10.1067/mob.2000.105403. [DOI] [PubMed] [Google Scholar]
- 23.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 24.Morken NH, Klungsoyr K, Magnus P, Skjaerven R. Pre-pregnant body mass index, gestational weight gain and the risk of operative delivery. Acta Obstet Gynecol Scand. 2013;92(7):809–815. doi: 10.1111/aogs.12115. [DOI] [PubMed] [Google Scholar]
- 25.SOURCE: OECD StatsExtracts. Cesarean rates OECD countries, 2001–2010. [Accessed on January 28, 2015]; http://statsoecdorg. [Google Scholar]
- 26. [Accessed January 28, 2015]; Available at quickfacts.census.gov/qgd/states/ooooo.html.
- 27.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322.e1–322.e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holland E, Moore Simas TA, Doyle Curiale DK, Liao X, Waring ME. Self-reported pre-pregnancy weight versus weight measured at first prenatal visit: Effects on categorization of pre-pregnancy body mass index. Matern Child Health J. 2013;17(10):1872–1878. doi: 10.1007/s10995-012-1210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson H, Tkatch S, Mayes DC, Bott N, Okun N. Is maternal obesity a predictor of shoulder dystocia? Obstet Gynecol. 2003;101(1):24–27. doi: 10.1016/s0029-7844(02)02448-1. [DOI] [PubMed] [Google Scholar]
- 30.Stamilio DM, Scifres CM. Extreme obesity and postcesarean maternal complications. Obstet Gynecol. 2014;124(2 Pt 1):227–232. doi: 10.1097/AOG.0000000000000384. [DOI] [PubMed] [Google Scholar]