Abstract
Background/objectives
Cardiovascular (CV) risk is increased in patients with rheumatoid arthritis (RA), but not fully explained by traditional risk factors such as LDL and HDL cholesterol concentrations. The cholesterol efflux capacity of HDL may be a better CV risk predictor than HDL concentrations. We hypothesized that HDL’s cholesterol efflux capacity is impaired and inversely associated with coronary atherosclerosis in patients with RA.
Methods
We measured the net cholesterol efflux capacity of apolipoprotein B depleted serum and coronary artery calcium score in 134 patients with RA and 76 control subjects, frequency-matched for age, race and sex. The relationship between net cholesterol efflux capacity and coronary artery calcium score and other clinical variables of interest was assessed in patients with RA.
Results
Net cholesterol efflux capacity was similar among RA (median [IQR]: 34% removal [28, 41%]) and control subjects (35% removal [27%, 39%]) (P=0.73). In RA, increasing net cholesterol efflux capacity was not significantly associated with decreased coronary calcium score (OR=0.78 (95% CI 0.51–1.19), P=0.24, adjusted for age, race and sex, Framingham risk score and presence of diabetes). Net cholesterol efflux capacity was not significantly associated with RA disease activity score, C-reactive protein, urinary F2-isoprostanes, or degree of insulin resistance in RA.
Conclusions
Net cholesterol efflux capacity is not significantly altered in patients with relatively well-controlled RA nor is it significantly associated with coronary artery calcium score.
Keywords: rheumatoid arthritis, HDL, lipoprotein, athero, cholesterol efflux
1. Introduction
Rheumatoid arthritis (RA) is associated with increased cardiovascular (CV) risk and mortality, independent of traditional CV risk factors [1–3]. In most populations, increased low density lipoprotein cholesterol (LDL-C) and decreased high density lipoprotein cholesterol (HDL-C) concentrations are among the strongest modifiable CV risk factors identified [4]. However, HDL-C concentration may not be a good predictor of atherosclerosis in all populations. For example, cholesterol concentrations, including HDL-C, are not substantially altered and do not account for increased CV risk in patients with RA [3,5].
Recent work suggests that HDL function may be a better predictor of atherosclerotic risk than HDL-C concentrations. The anti-atherogenic functions of HDL include its ability to mediate removal of cholesterol from macrophages (termed cholesterol efflux, the first step of reverse cholesterol transport) as well as anti-inflammatory and anti-oxidant effects [6]. Of these functions, HDL-mediated cholesterol efflux is considered critical to its anti-atherosclerotic effect [6,7]. Indeed, cholesterol efflux capacity was inversely associated with subclinical atherosclerosis measured by carotid intima-media thickness in healthy subjects and with obstructive coronary artery disease in patients undergoing cardiac catheterization, independent of HDL-C concentrations [8]; however, not all studies have found this relationship. For example, in another study higher cholesterol efflux capacity was paradoxically associated with increased risk of non-fatal MI or stroke and major adverse cardiovascular events [9].
The observations that the usual inverse relationship between HDL-C concentration and atherosclerosis is altered in RA [10,11] suggest that HDL function may be altered. We have shown previously that high HDL-C concentrations in the setting of high oxidative stress were associated paradoxically with greater risk of atherosclerosis in RA [10]. Thus, oxidative stress and inflammation may impair HDL’s function. However, the relationship between cholesterol efflux capacity and atherosclerosis in RA is not known. We hypothesized that net cholesterol efflux capacity is impaired in RA and inversely associated with coronary atherosclerosis.
2. Methods
2.1 Study population
From a previous cross-sectional study of 169 patients with RA and 92 control subjects focusing on CV risk factors [3], we used samples from 134 patients with RA and 76 control subjects. Recruitment and study procedures for the original study have been described previously [3]. All subjects were older than 18 years of age and patients with RA fulfilled American College of Rheumatology 1987 classification criteria for RA [12]. RA and control groups were frequency-matched for age, race and sex; control subjects did not have RA or other inflammatory disease. The study was approved by the Vanderbilt Institutional Review Board, and all subjects gave written informed consent.
2.2 Clinical and laboratory information
Clinical information, laboratory measurements, and coronary artery calcium scores were obtained as described previously [3]. RA disease activity was determined by the 28 joint count disease activity score (DAS28) [13]. Body mass index (BMI) was calculated and expressed as kg/m2. Patients were categorized as having the metabolic syndrome based on the modified World Health Organization criteria [14]. The degree of insulin resistance was measured by the homeostatic model of insulin resistance (HOMA) calculated as: [fasting glucose (mmol/l) × fasting insulin (µU/ml)]/22.5] [15,16]. Framingham risk score was calculated based on age, total and HDL-C, blood pressure and smoking [17,18].
Fasting lipid and high-sensitivity C-reactive protein (CRP) concentrations were measured by the Vanderbilt University Medical Center Clinical Laboratory or enzyme-linked immunosorbent assay (ELISA) (Millipore). Urinary F2-isoprostane excretion, a robust indicator of oxidative stress, was measured as previously described [10,19,20]. Fasting insulin and serum amyloid A (SAA) was measured by multiplex ELISA (Lincoplex Multiplex Immunoassay Kit, Millipore Corp., Billerica MA, USA).
Coronary artery calcium score was measured by electron beam computed tomography (EBCT) with an Imatron C-150 scanner (GE/Imatron, South San Francisco, CA, USA) as described previously [3] and quantified in Agatston units [21].
2.3 Measurement of net cholesterol efflux capacity of HDL enriched serum
Net cholesterol efflux capacity was measured as we have previously described [22] with minor modifications. Human monocyte THP-1 cells were plated in 12 multi-well plates (1×106 cells/ 1 ml RPMI1640 with 10% fetal bovine serum and 0.1% phorbol myristate acetate). After 72 hours, the cells were incubated with 100µg/ml acetylated LDL (Intracel, #RP-045) for 72 hours, resulting in foam cell formation. Medium was changed to RPMI containing 4mg/ml fatty acid free bovine serum albumin (Sigma, #A6003) for one hour. Patient serum (250 µl) was added to 100 µl of polyethylene glycol (PEG) solution (20% PEG 8000 in 200mM glycine) and incubated at room temperature for 15 minutes and then centrifuged at 1900g. The supernatant was removed and used as apolipoprotein B (apoB) depleted serum or HDL enriched serum [23]. Cells were then washed and incubated with medium containing apoB depleted serum (18µg/ml cholesterol concentration). Acetylated LDL exposed cells exposed to medium only were used as a comparator. After 24 hours of incubation, cells were washed twice and air dried. Cellular lipids were extracted with high performance liquid chromatography grade isopropanol. Total cellular cholesterol was determined by gas liquid chromatography [24,25]. Cholesterol content was corrected for total cellular protein for each well. Cholesterol efflux capacity was defined as the % change in total cellular cholesterol content (in µg/mg protein) between wells exposed to medium and apoB depleted serum [26,27]. Samples were run in duplicate in batches. The mean intra-assay and inter-assay coefficient of variation was 14.9% and 16.5%, respectively.
2.4 Statistical analysis
Given our sample size of 134 patients with RA and 76 control subjects and a standard deviation of 12% within each group, we had over 80% power to detect a difference in cholesterol efflux between RA and control subjects of 5% cholesterol removal.
Descriptive statistics were calculated as median with interquartile range (median [IQR: 25th, 75th]) for continuous variables and frequency and proportions for categorical variables. To compare variables between RA and control subjects, Wilcoxon’s rank sum tests were used to compare continuous variables and Pearson’s chi-square test to compare categorical variables.
To assess the adjusted association of disease status on net cholesterol efflux capacity, multiple linear regression was used with net cholesterol efflux capacity as dependent variable and disease status as an independent variable with adjustment for age, race and sex.
For patients with RA, the relationship between coronary artery calcium score and net cholesterol efflux capacity was assessed by proportional odds logistic regression with coronary artery calcium score as dependent variable and net cholesterol efflux capacity as the independent variable was performed with adjustment for age, race and sex. Also, these models were adjusted additionally for Framingham risk score and diabetes.
An exploratory analysis was performed to determine factors that influence net cholesterol efflux capacity in RA. Multiple linear regressions were used with net cholesterol efflux capacity as dependent and variables of interest as independent with adjustment for age, race and sex.
CRP, SAA, HOMA, and urinary F2-isoprostanes were natural logarithm-transformed to improve normality of residuals. Statistical analyses were performed using R version 2.15.1 (http://www.r-project.org) and IBM SPSS Statistics version 22. Two-sided P values less than or equal to 0.05 were considered statistically significant.
3. Results
3.1 Clinical characteristics
Patients with RA and controls were of similar age, race and sex (Table 1). In patients with RA the median [IQR] DAS28 score was 3.9 units [2.6, 4.9 units], 73% were rheumatoid factor positive, and the majority (73%) were receiving methotrexate. As noted previously (3), LDL-C concentrations were lower in patients with RA than controls (P=0.02), but HDL-C concentrations were similar (P=0.32).
Table 1.
Clinical characteristics of RA patients and control subjects
| RA (N=134) | Control (N=76) | P value | |
|---|---|---|---|
| Demographics/ anthropomorphic measures | |||
| Age, years | 54 [45, 64] | 54 [46, 59] | 0.67 |
| Race, % Caucasian | 90% (120) | 83 (63) | 0.34 |
| Sex, % female | 72% (96) | 63% (48) | 0.20 |
| Body mass index, kg/m2 | 28.5 [23.9, 33.3] | 27.2 [24.8, 32.3] | 0.51 |
| RA disease related | |||
| DAS28, units | 3.9 [2.6, 4.9] | - | - |
| Rheumatoid factor positivity, % | 73% (93)* | - | - |
| Disease duration, years | 3 [2, 17.8] | - | - |
| CV risk factors | |||
| Hypertension, % | 54% (72) | 38% (29) | 0.03 |
| Diabetes, % | 12% (16) | 4% (3) | 0.05 |
| Metabolic syndrome, % | 37% (50) | 12% (9) | <0.001 |
| Total-C, mg/dl | 184 [155, 210] | 195 [168, 216] | 0.07 |
| HDL-C, mg/dl | 43 [37, 54] | 46 [39, 54] | 0.32 |
| LDL-C, mg/dl | 110 [87, 134] | 122 [104, 145] | 0.02 |
| Triglycerides, mg/dl | 113 [80, 158] | 108 [75, 130] | 0.21 |
| Smoker, % | 22% (29) | 9% (7) | 0.02 |
| CRP, mg/dl | 4.0 [1.2, 10.0] | 0.6 [0.2, 1.9] | <0.001 |
| Known CAD, % | 10% (14) | 11% (8) | 0.99 |
| Coronary calcium score, Agatston units |
0 [0, 137.5] | 0 [0, 11.8] | 0.02 |
| Medication use | |||
| Methotrexate, % | 73% (98) | - | - |
| Leflunomide, % | 19% (26) | - | - |
| Hydroxychloroquine, % | 28% (38) | - | - |
| Anti-TNF, % | 21% (28) | - | - |
| Corticosteroid, % | 54% (72) | - | - |
| Statin, % | 13% (18) | 15% (11) | 0.85 |
| NSAIDs, % | 33% (44) | 33% (25) | 0.98 |
Available for 127 RA patients.
3.2 Net cholesterol efflux capacity by HDL enriched serum in RA vs Controls and relationship to coronary artery calcium score in RA
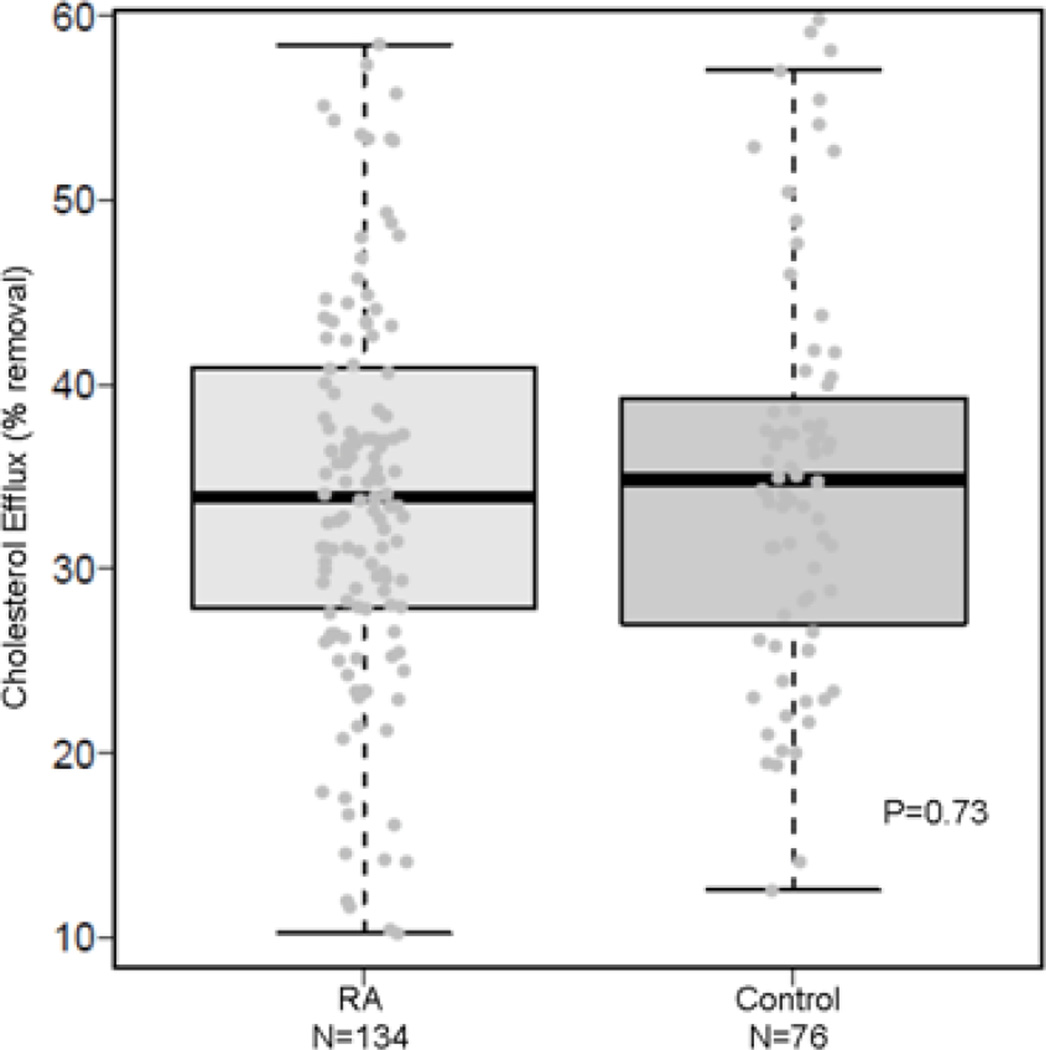
Net cholesterol efflux capacity of HDL enriched serum did not differ significantly among patients with RA (34% removal [28, 41%] and controls (35% removal [27, 39%]) (P=0.73) (Figure). Among patients with RA, net cholesterol efflux capacity was not significantly inversely associated with coronary artery calcium score in patients with RA (OR per IQR increase in net cholesterol efflux capacity=0.74 (95% CI: 0.52–1.05; P=0.10). Similarly, there was no significant protective association between net cholesterol efflux capacity and coronary artery calcium score in models that adjusted for age, race and sex, (OR=0.78 (95% CI: 0.51–1.17; P=0.23)and additionally for Framingham risk score and presence of diabetes (OR=0.78 (95% CI 0.51, 1.18; P=0.24).
Fig. 1. Net cholesterol efflux capacity of HDL enriched serum in patients with RA and control subjects.
Net cholesterol efflux of HDL enriched serum was measured in 134 patients with RA and 76 control subjects. In RA, the median [IQR] net cholesterol efflux capacity was 34% [28%, 41%], which was similar to controls (35% [27%, 39%], P=0.73).
3.3 Relationship between clinical factors and net cholesterol efflux in RA
In exploratory analyses to determine the factors that influence net cholesterol efflux capacity in RA (Table 2), RA disease activity (DAS28), CRP, SAA, and oxidative stress (urinary F2-isoprostane excretion) were not significantly associated with net cholesterol efflux capacity (all P>0.05). Moreover, net cholesterol efflux capacity did not differ significantly between those with high disease activity (DAS28>5.1) (median [IQR] net cholesterol efflux capacity 32% [26%, 41%] and those with the very low/clinical remission (DAS28<2.6) (35% [28%, 43%], P=0.52). Insulin resistance (measured as HOMA), presence of metabolic syndrome or diabetes, and smoking status were not significantly associated with net cholesterol efflux capacity (Table 3) (all P>0.05). Additionally, there was no significant relationship between use of any particular disease modifying anti-rheumatic drug, corticosteroids, or statins and net cholesterol efflux capacity (Table 3) (all P>0.05).
Table 2.
Association between increasing net cholesterol efflux capacity and variables of interest in RA
| β (95% CI) | P value | |
|---|---|---|
| DAS28 | −1.18 (−4,21, 1.86) | 0.45 |
| CRP | 0.91 (−1.95, 3.78) | 0.53 |
| SAA | 2.64 (−0.61, 5.89) | 0.11 |
| F2-isoprostanes | 0.59 (−2.47, 3.65) | 0.71 |
| HDL-C | 0.49 (−2.52, 3.49) | 0.75 |
| LDL-C | −0.18 (−3.03, 2.66) | 0.90 |
| HOMA | 1.53 (−1.04, 4.10) | 0.25 |
| Metabolic syndrome | 0.90 (−3.56, 5.36) | 0.69 |
| Diabetes | 0.15 (−6.37, 6.68) | 0.96 |
| Smoking | −4.40 (−9.63, 0.83) | 0.10 |
Assays were performed using standardized concentrations of HDL-C (apoB depleted serum with a cholesterol concentration of 18µg/ml). Beta-coefficients presented for continuous variables are per increase in interquartile range and adjusted for age, race and sex.
Table 3.
Relationship between net cholesterol efflux capacity and medication use among patients with RA
| Current Use | No Current Use | Adjusted* | |||
|---|---|---|---|---|---|
| N | Net cholesterol efflux, % [IQR] |
N | Net cholesterol efflux, % [IQR] |
P value | |
| Methotrexate | 98 | 34% [27, 41%] | 36 | 34% [29, 41%] | 0.41 |
| Leflunomide | 26 | 34% [29, 42%] | 108 | 34% [27, 41%] | 0.27 |
| Hydroxychloroquine | 38 | 32% [26, 37%] | 96 | 35% [28, 43%] | 0.15 |
| Anti-TNF | 28 | 34% [26, 41%] | 106 | 34% [28, 40%] | 0.79 |
| Corticosteroid | 72 | 35% [29, 41%] | 62 | 34% [28, 39%] | 0.34 |
| NSAID | 44 | 36% [25, 44%] | 90 | 34% [28, 39%] | 0.98 |
| Statin | 18 | 32% [27, 37%] | 116 | 34% [28, 43%] | 0.27 |
IQR= Interquartile range
Adjusted for age, race and sex
4. Discussion
The main results of this study are that the net cholesterol efflux capacity of HDL enriched serum did not differ significantly among patients with RA and controls and was not significantly associated with coronary artery calcium score in RA. We also found that net cholesterol efflux capacity was not associated significantly with systemic inflammation, oxidative stress (urinary F2-isoprostanes), or disease activity in RA.
Among patients with RA, traditional lipoprotein measures, such as LDL-C and HDL-C concentrations, are not as helpful as they are in the general population for assessing CV risk. Moreover, several lines of evidence suggest that the anti-atherosclerotic effect of HDL is impaired in RA. For example, RA patients have what is termed a “lipid paradox”, where in the setting of high inflammation, a low total-C to HDL-C ratio is no longer protective against CVD [11]. We also have previously made a similar observation; in the setting of increased oxidative stress (measured as high urinary F2-isoprostane excretion), high HDL-C concentrations were paradoxically associated with increased coronary atherosclerosis [10]. Thus, we hypothesized that cholesterol efflux capacity, one of HDL’s functions, would be impaired in RA.
We found that net cholesterol efflux capacity did not differ significantly in patients with RA compared to a well-matched control group. This finding is concordant with the results of a smaller study of comparable design that included 40 patients with RA and 40 matched control subjects [28]. Our findings were remarkably similar (HDL cholesterol efflux was 35% ± 12% (mean ± standard deviation) in both RA and controls in our study and 40.2% ± 11.1% for RA and 39.5% ± 8.9% for controls in the previous report [28]). Another study compared cholesterol efflux capacity in 38 patients with RA, 18 of whom had known cardiovascular disease, to that of 20 extremely healthy controls none of whom smoked, or had coronary artery disease, hypertension or diabetes and reported that cholesterol efflux capacity was decreased in RA, though there was no difference between RA patients with and without cardiovascular disease [29].
We also found that high RA disease activity and measures of systemic inflammation were not associated with impaired net cholesterol efflux capacity. This lack of correlation with inflammation is consistent with recent findings from a non-RA population including 2924 subjects without cardiovascular disease in the Dallas Heart Study, where CRP was not associated with cholesterol efflux capacity [30]. Moreover, it is consistent with a small study which showed that neither disease activity nor systemic inflammation measured as CRP or ESR was associated with cholesterol efflux capacity in RA [29]. Two studies reported an inverse relationship between cholesterol efflux capacity and RA disease activity and systemic inflammation [28,31]. In one, cholesterol efflux capacity was not different between RA and controls, yet efflux capacity was inversely associated with RA disease activity and inflammation [28]. In the other, the association between decreased cholesterol efflux and inflammation in RA was found in only one of three cholesterol transporters, ABCG1, in a Chinese hamster ovary (CHO-K1) cell line [31]; studies suggest that ABCA1-mediated efflux is particularly important in prevention of atherosclerosis [32–34], and this was not associated with inflammation and did not differ among patients with RA and control subjects [31].
Several methods have been used to examine HDL-mediated cholesterol efflux in the clinical setting and there is no gold-standard method. Most studies use apoB depleted (HDL enriched) serum as we did, but measured cholesterol efflux in different cell types by different methods [8,28,30,31,35]. Our assay [22] to assess cholesterol efflux capacity has some advantages. First, we used a human cell line to better reflect cholesterol uptake and removal seen in humans. Second, because HDL has the ability to not only remove cholesterol, but also to donate cholesterol to cells [36, 37], we measured net cellular cholesterol changes by gas liquid chromatography. Although this method is more demanding, it is considered to better represent physiology [37] than techniques using radiolabeled cholesterol that only measure cholesterol removal from the cell.
We found that net cholesterol efflux capacity, although trending in the expected direction, was not a significant predictor of coronary calcium score in patients with RA. This is similar to what was shown in the Dallas Heart Study, where there was no significant relationship between cholesterol efflux capacity and coronary calcium score in 2924 subjects [30]. Lower cholesterol efflux capacity was associated with carotid intima thickness in 203 healthy subjects and with the presence of coronary artery disease in 793 subjects undergoing cardiac catheterization [8], suggesting that these measures may differ from coronary calcium score regarding their relationship with cholesterol efflux.
Similarly, studies of the relationships between cholesterol efflux capacity and cardiovascular events have been mixed. Higher cholesterol efflux capacity, despite being associated with lower prevalent coronary artery disease, was paradoxically associated with increased risk of non-fatal MI or stroke and major adverse cardiovascular events [9]. In the Dallas Heart Study, despite no significant association between cholesterol efflux capacity and coronary artery calcification, there was a 67% reduction in CV events in the lowest quartile of cholesterol efflux capacity compared to the highest quartile [30]. Thus, despite our finding of negligible relationship between net cholesterol efflux capacity and coronary artery calcification in RA, we cannot exclude the possibility that cholesterol efflux capacity may be related to cardiovascular events.
HDL is composed of many different lipids and proteins with a variety of functions. Changes to these protein constituents may contribute to HDL dysfunction. For example, impaired anti-oxidant capacity of HDL is linked to decreased paraoxonase-1 activity [38], and an enrichment of acute phase proteins such as SAA on HDL [39, 40]. Moreover, oxidation products such as 3-chlorotyrosine on HDL may impair cholesterol efflux capacity [29]. We thus examined whether systemic inflammation and oxidative stress affected cholesterol efflux. We found no relationship between inflammation and oxidative stress and cholesterol efflux in RA. A potential explanation is that apoA1 on HDL may not to be decreased in RA, and in fact, may be increased among those with HDL which cannot prevent LDL oxidation (termed “proinflammatory” HDL) [38, 39]. Such an increase in HDL-apoA1 may compensate for other processes detrimental to HDL efflux function that result from inflammation.
The anti-atherosclerotic functions of HDL are thought to include not only cholesterol efflux and reverse cholesterol transport but also anti-inflammatory and anti-oxidant functions. Studies that have focused on the other putative anti-atherosclerotic functions of HDL have found that approximately 20% of patients with RA, particularly those with high disease activity have impaired anti-oxidant capacity (or ”proinflammatory HDL”) [28, 40, 41]. The relationship between impaired HDL anti-oxidant function and atherosclerosis in RA is currently not known, but this mechanism appears to play a role in accelerated atherosclerosis in systemic lupus erythematosus [41–43]. Moreover, the anti-oxidant effects of HDL appear to be closely linked to systemic inflammation and disease activity in RA [40], unlike our findings for cholesterol efflux capacity. Lastly, the impact of HDL’s anti-inflammatory capacity (tested as the ability of HDL to decrease cytokine production) on atherosclerosis in RA is also not known. Considering our findings, altered anti-oxidant and anti-inflammatory effects of HDL may be more important to risk of CV disease in patients with RA than altered cholesterol efflux. However, specific studies will be required to address those questions.
Our study had some limitations. We performed a cross-sectional study, thus we do not have long term prospective outcome data on participants to determine relationship between CV events or mortality with cholesterol efflux capacity. Moreover, patients had relatively well-controlled disease; however, this reflects the status of most treated RA patients in modern practice. We used coronary artery calcium score as a measure of atherosclerotic burden, which has the limitation of not showing non-calcified plaque which may be more susceptible to rupture. Lastly, we did not evaluate the amount of cholesterol efflux mediated by individual receptors, however, our assay importantly measured overall net cholesterol efflux as determined by changes in cellular cholesterol mass, which is more clinically relevant.
5. Conclusion
We conclude that net cholesterol efflux capacity by HDL enriched serum is not significantly altered in patients with relatively well-controlled RA nor is it associated with coronary artery calcium score, disease activity or systemic inflammation.
Acknowledgments
Funding: Arthritis Foundation Clinical to Research Transition Award, Rheumatology Research Foundation Disease Targeted Research Investigator Award, NIH grants: KL2 TR00046, K23 AR068443, P60 AR056116, P01 HL116263, and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Authors’ Contributions: MJO: study design, assays, analysis and interpretation of data; PGY: study design, assay development, and interpretation of data; SY: study design, and assay development; AMO: acquisition of patient-related data; TG: study design and statistical analysis; AS: study design and statistical analysis; MFL: study design and interpretation of data; SF: study design and interpretation of data; SSD: study design and interpretation of data; LJR: study design and interpretation of data; KCV: study design and interpretation of data; PR: study design and acquisition of coronary artery calcium data; VK: study design, assay development and interpretation of data; CMS: study design, data analysis and interpretation, and oversight of all aspects of the study. All authors read and approved the final manuscript.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994 Apr;37(4):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum. 2001 Jun;44(6):1234–1236. doi: 10.1002/1529-0131(200106)44:6<1234::AID-ART213>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005 Oct;52(10):3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 4.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 5.Chung CP, Oeser A, Raggi P, Sokka T, Pincus T, Solus JF, et al. Lipoprotein subclasses determined by nuclear magnetic resonance spectroscopy and coronary atherosclerosis in patients with rheumatoid arthritis. J Rheum. 2010 Aug;37(8):1633–1638. doi: 10.3899/jrheum.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragbir S, Farmer JA. Dysfunctional high-density lipoprotein and atherosclerosis. Curr Atheroscler Rep. 2010 Sep;12(5):343–348. doi: 10.1007/s11883-010-0091-x. [DOI] [PubMed] [Google Scholar]
- 7.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010 Jun;21(3):229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011 Jan;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li XM, Tang WH, Mosior MK, et al. Paradoxical Association of Enhanced Cholesterol Efflux With Increased Incident Cardiovascular Risks. Arterioscler Thromb Vasc Biol. 2013 Jul;33(7):1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rho YH, Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, et al. Interaction between oxidative stress and high-density lipoprotein cholesterol is associated with severity of coronary artery calcification in rheumatoid arthritis. Arthritis Care Res. 2010 Oct;62(10):1473–1480. doi: 10.1002/acr.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011 Mar;70(3):482–487. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995 Jan;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 14.Reilly MP, Wolfe ML, Rhodes T, Girman C, Mehta N, Rader DJ. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004 Aug;110(7):803–809. doi: 10.1161/01.CIR.0000138740.84883.9C. (2004) [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008 Jul;58(7):2105–2112. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 18.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec;106(25):3143–3421. [PubMed] [Google Scholar]
- 19.Morrow JD, Zackert WE, Yang JP, Kurhts EH, Callewaert D, Dworski R, et al. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999 May;269(2):326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990 Mar;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Ormseth MJ, Yancey PG, Solus JF, Bridges SL, Curtis JR, Linton MF, Fazio S, Davies SS, Roberts LJ, Vickers KC, Kon V, Stein CM TETRAD Investigators. Arthritis Rheumatol. 2016 Mar 18; doi: 10.1002/art.39675. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010 Apr;30(4):796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerome WG, Cox BE, Griffin EE, Ullery JC. Lysosomal cholesterol accumulation inhibits subsequent hydrolysis of lipoprotein cholesteryl ester. Microsc Microanal. 2008 Apr;14(2):138–149. doi: 10.1017/S1431927608080069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klansek JJ, Yancey P, St Clair RW, Fischer RT, Johnson WJ, Glick JM. Cholesterol quantitation by GLC: artifactual formation of short-chain steryl esters. Journal of lipid research. 1995 Oct;36(10):2261–2266. [PubMed] [Google Scholar]
- 26.Zhou H, Shiu SW, Wong Y, Tan KC. Impaired serum capacity to induce cholesterol efflux is associated with endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2009 Oct;6(4):238–243. doi: 10.1177/1479164109344934. [DOI] [PubMed] [Google Scholar]
- 27.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003 May;23(5):712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 28.Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012 Jul;71(7):1157–1162. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivekanandan-Giri A, Slocum JL, Byun J, Tang C, Sands RL, Gillespie BW, et al. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann Rheum Dis. 2013 Oct;72(10):1725–1731. doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol capacity and incident cardiovascular events. N Engl J Med. 2014 Dec;371(25):2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014 Mar;73(3):609–615. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 32.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009 Apr;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldan A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MW, et al. Impaired development of atherosclerosis in hyperlipidemic Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006 Oct;26(10):2301–2307. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 34.Ranalletta M, Wang N, Hans S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006 Oct;26(10):2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012 Dec;60(23):2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson WJ, Mahlberg FH, Rothblat GH, Phillips MC. Cholesterol transport between cells and high-density lipoproteins. Biochim Biophys Acta. 1991 Oct;1085(3):273–298. doi: 10.1016/0005-2760(91)90132-2. [DOI] [PubMed] [Google Scholar]
- 37.Weibel GL, Drazul-Schrader D, Shivers DK, Wade AN, Rothblat GH, Reilly MP, et al. Importance of evaluating cell cholesterol influx with efflux in determining the impact of human serum on cholesterol metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014 Jan;34(1):17–25. doi: 10.1161/ATVBAHA.113.302437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isik A, Koca SS, Ustundag B, Celik H, Yildirim A. Paraoxonase and arylesterase levels in rheumatoid arthritis. Clin Rheum. 2007 Mar;26(3):342–348. doi: 10.1007/s10067-006-0300-8. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012 Jun;64(6):1828–1837. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009 Oct;60(10):2870–2879. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006 Aug;54(8):2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 42.McMahon M, Skaggs BJ, Grossman J, Sahakian L, Fitzgerald J, Wong WK, et al. A panel of biomarkers is associated with increased risk for the presence and progression of atherosclerosis in women with Systemic Lupus Erythematosus. Arthritis Rheum. 2014 Jan;66(1):130–139. doi: 10.1002/art.38204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon M, Grossman J, Skaggs B, Fitzgerald J, Sahakian L, Ragavendra N, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009 Aug;60(8):2428–2437. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]