Abstract
Sirolimus used in transplantation is often associated with hypercholesterolemia. We measured serum lipid and PCSK9 levels in 51 heart transplant recipients who had their immunosuppressive therapy switched from calcineurin inhibitors to sirolimus. The switch resulted in a 23% increase in LDL cholesterol, and 46% increase in triglycerides and PCSK9 levels increased from 316 ± 105 ng/mL to 343 ± 107 ng/mL (p=0.04), however the change in PCSK9 levels did not correlate with an increase in lipid levels (p=0.2). To investigate the mechanism for the variability in the change in PCSK9 levels, lymphoblastoid cell lines were incubated with both sirolimus and everolimus, resulting in a 2–3 fold increase in PCSK9 expression and protein levels in mTOR inhibitor sensitive but not in mTOR inhibitor resistant cell lines. This first in human study demonstrates that sirolimus therapy is associated with elevation in PCSK9 levels which is not associated with sirolimus-induced hypercholesterolemia.
Keywords: Sirolimus, mTOR inhibitors, PCSK9, hypercholesterolemia, cardiac transplant
Introduction
Sirolimus (Rapamycin) is a novel immunosuppressive agent which binds to the kinase enzyme, mammalian target of rapamycin (mTOR), leading to cell cycle arrest, and subsequent inhibition of T cell activation and proliferation in response to cytokine stimulation [1]. Because of its potent anti-rejection and anti-proliferative effects that translate into less cardiac allograft vasculopathy, and the absence of nephrotoxicity, it is increasingly used as a primary immunosuppressant in cardiac transplant patients.
Sirolimus is also the first pharmacological agent that has been shown to extend maximal lifespan in a mammalian species and is being increasingly used in anti-aging research [2]. However, use of sirolimus is often associated with development of significant hypercholesterolemia and hypertriglyceridemia, with an average 15–20% increase over baseline levels [3–6]. Despite its efficacy in reducing cardiac allograft vasculopathy [7], there is concern that sirolimus-induced dyslipidemia can contribute to atherosclerosis. Indeed, studies in LDL receptor knock-out mice do suggest that the potential of mTOR inhibitors to ameliorate atherosclerosis might be attenuated by concomitant hypercholesterolemia [8]. A better understanding of the mechanisms of sirolimus-induced hypercholesterolemia is therefore likely to have a significant bearing on cardiac allograft vasculopathy and long-term survival of cardiac transplant recipients.
The exact mechanism by which mTOR inhibitors cause dyslipidemia is not known. Proprotein convertase subtilisin/kexin Type 9 (PCSK9) is a serine protease enzyme which plays a critical role in regulation of LDL cholesterol levels by binding to LDL receptors, leading to their ultimate lysosomal degradation [9]. Sirolimus, which blocks the mTOR pathway, could potentially increase LDL cholesterol by increasing PCSK9 levels, as mTOR signaling is known to regulate various aspects of lipid metabolism [10]. Ai et al [11] showed that administration of Sirolimus in wild type mice leads to increased PCSK9 expression, reduced LDL receptors and increase in serum cholesterol. This effect was not seen in PCSK9 knock-out mice, thus suggesting the critical role of PCSK9 in mediating the hypercholesterolemic effect of sirolimus. Whether a similar mechanism occurs in humans is not known.
The primary purpose of this study was to perform the first in human study to investigate the effects of sirolimus therapy on serum PCSK9 levels in heart transplant recipients. We then sought to evaluate the effect of the change in PCSK9 levels on the change in lipids, to evaluate whether a change in PCSK9 levels explains the hyperlipidemia observed in heart transplant recipients receiving sirolimus. We also performed in-vitro studies using human lymphoblastoid cell lines to investigate the variable effect of mTOR inhibition on PCSK9 gene expression and protein levels.
Methods
Clinical study
This is an analysis of patients who underwent cardiac transplantation at the Mayo Clinic, Rochester within the past 7 years and had been switched to sirolimus-based immunosuppressive therapy. Transition from calcineurin inhibitor (CNI) based immunosuppressive therapy to sirolimus-based therapy is part of a routine protocol in our program, and a fasting serum lipid profile is obtained before and after the transition. The most recent blood sample before switching to sirolimus, and the first sample obtained 8 weeks after the switch were chosen for the pre and post-sirolimus measurements, respectively. Total cholesterol, triglycerides and HDL cholesterol were measured by an automated colorimetric enzymatic assay, and LDL cholesterol was calculated using the Friedewald equation. Serum PCSK9 levels were measured using the commercially available CircuLex Human PCSK9 ELISA kit. The intra and inter-assay coefficient of variation for this assay varies from 1.5–2.6% and 2.9–7.1%, respectively (CycLex Co., Ltd., Nagano, Japan). The study protocol was approved by the Mayo Clinic Institutional Review Board, and all patients provided informed written consent.
Statistical analysis
All continuous values are reported as mean ± standard deviation. Weight, lipid parameters and PCSK9 levels before and after sirolimus therapy were compared using the Students’ paired t test, and p≤0.05 was considered significant. Pearson correlation was used to analyze the relationship between PCSK9 and LDL levels.
In-vitro cell culture studies
The human variation panel Lymphoblastoid Cell Lines (LCL) are derived from 96 African–American (AA), 96 Caucasian–American (CA) and 96 Han Chinese–American (HCA) healthy unrelated individuals (sample sets HD100AA, HD100CAU, HD100CHI). The LCLs were obtained from the Coriell Cell Repository (Camden, NJ) and were collected, anonymized and deposited by the National Institute of General Medical Sciences. LCLs were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 15% heat-inactivated Fetal Bovine Serum (FBS) (Atlanta Biologicals, Flowery Branch, GA). In accordance with Coriell Institute instructions, LCLs were maintained at a density of 2–8 × 105 cells/mL, and were split with fresh medium every 3 days, depending on the growth status of each cell line.
Drug treatment and expression detection
Cytotoxicity data for mTOR inhibitor sirolimus (Sigma, St. Louis, MO) and everolimus (Sigma, St. Louis, MO) were performed in all LCLs as described before [12]. Based on the half maximal effective concentration that results in cytotoxicity (EC50) for all the cell lines, five LCL cell lines were selected from sirolimus and everolimus resistant or sensitive groups, respectively. Drug treatments were performed in triplicate for each selected LCLs. Specifically, 1×106 cells were plated into 6-well plates and treated with sirolimus or everolimus for 3 days. The concentration is based on the mean EC50 for all the LCLs, which is 0.2 μM for sirolimus or 0.33 μM for everolimus. DMSO was added as the control at a final concentration of <0.1% in the medium.
Total RNA was isolated from treated cells with the Qiagen RNeasy kit (QIAGEN, Hilden, Germany), and 100 ng of total RNA was used to perform qRT-PCR using the PCSK9 primer (QIAGEN). All experiments were performed in triplicate with Beta-ACTIN (QIAGEN) as an internal control.
Proteins from treated cells were extracted using NETN buffer (100 mM NaCl, 20 mM Tris-Cl (pH 8.0), 0.5 mM EDTA, 0.5 % Nonidet P-40) with protease and phosphatase inhibitor cocktail (Roche, Basel, Switzerland), and were separated by electrophoresis on 4%–20% SDS-PAGE gels followed by transferring onto the polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA). Membranes were probed with anti-PCSK9 antibody (Abcam, Cambridge, United Kingdom), anti-mTOR antibody (Cell Signalling, Danvers, MA), anti- phosphorylated Ser2448 mTOR antibody (Cell Signalling) and anti-Actin antibody (Sigma, St. Louis, MO) in 1% BSA blocking buffer. Protein bands were visualized by SuperSignal West Pico chemiluminescence (Thermo Scientific, Waltham, MA).
Results
Clinical study
The baseline characteristics of the 51 patients who underwent cardiac transplantation, and were switched to sirolimus-based immunosuppressive therapy are summarized in Table 1. The mean age of the patients was 55 years, ranging from 30 to 74 years. About 75% of the subjects were male, and the most common cause for heart failure was idiopathic dilated cardiomyopathy. In addition to cardiac transplantation, 9 patients received a simultaneous liver transplant, 5 received a simultaneous kidney transplant, and one patient underwent a combined heart, liver and kidney transplant.
TABLE 1.
Baseline characteristics
| Patient characteristics (n=51) | |
|---|---|
| Age (y) | 55 ± 12.8 |
| Sex | 38 M and 13 F |
| Diabetes | 8 |
| Underlying cause for heart failure | |
| Dilated cardiomyopathy | 14 |
| Amyloidosis | 11 |
| Ischemic cardiomyopathy | 9 |
| Restrictive cardiomyopathy | 6 |
| Hypertrophic cardiomyopathy | 3 |
| Giant cell myocarditis | 3 |
| Congenital heart disease | 2 |
| Arrhythmogenic right ventricle | 1 |
| Lymphocytic myocarditis | 1 |
| Chemotherapy induced | 1 |
| Allografts | |
| Heart only | 36 |
| Heart and liver | 9 |
| Heart and kidney | 5 |
| Heart, liver and kidney | 1 |
They were transitioned from a calcineurin-based immunosuppressive regimen consisting of either tacrolimus or cyclosporine to sirolimus-based immunosuppressive regimen at an average of 51 ± 30.2 weeks (median, 40 weeks) after transplantation. The mean sirolimus dose was 3.3 ± 1.6 mg/day. Baseline pre-sirolimus samples were obtained 34 ± 30.5 weeks after the transplant, while post-sirolimus measurements were obtained 72 ± 31.9 weeks after the transplant.
At baseline (pre-sirolimus), 8 subjects had diabetes, of which 5 were on insulin therapy. One additional patient developed diabetes after sirolimus conversion. A 4.1 ± 8.8% mean increase in weight was noted after patients were switched to sirolimus (81.2 ± 16.3 kg to 84.1 ± 15.8 kg, p=0.002), but no change in fasting plasma glucose levels was observed (99.5 ± 18.7 mg/dL to 101.5 ± 19.8 mg/dL, p=0.529). There was also a significant reduction in prednisone dose from 10.5 ± 5.9 mg/day to 6 ± 4 mg/day as part of a routine steroid weaning protocol.
At baseline, 39 of the 51 subjects were on statin therapy, pravastatin 20–40 mg/day being the most common (87%) drug used. Statin therapy was intensified in 10 subjects and initiated in an additional 7 subjects after sirolimus conversion. Changes in lipid profile are shown in Table 2. There was a 23–39% increase in serum total, LDL and non HDL cholesterol, and a 56% increase in serum triglycerides, with no changes in HDL cholesterol (Table 2).
TABLE 2.
Changes in lipid profile during sirolimus therapy
| Lipid parameter | Pre-sirolimus | Post-sirolimus | P value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 189.9 ± 47.6 | 225.3 ± 64 | <0.001 |
| Triglycerides (mg/dL) | 142.5 ± 75.7 | 208.8 ± 126.4 | <0.001 |
| HDL cholesterol (mg/dL) | 59 ± 19 | 57.7 ± 16.1 | 0.5 |
| LDL cholesterol (mg/dL) | 102.1 ± 37.8 | 125.7 ± 50.6 | <0.002 |
| Non HDL cholesterol (mg/dL) | 130.9 ± 46.4 | 167.3 ± 58.8 | <0.001 |
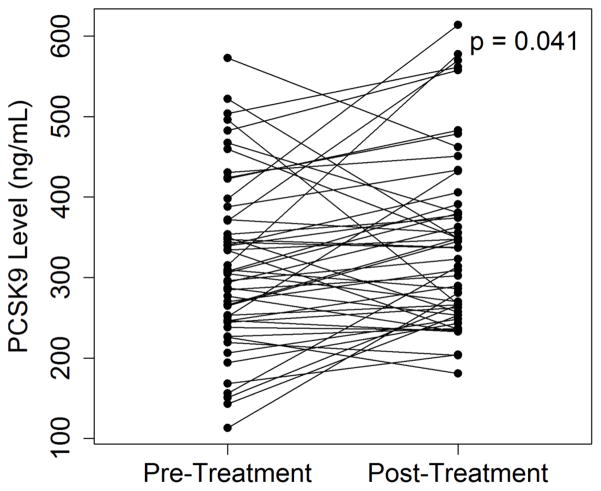
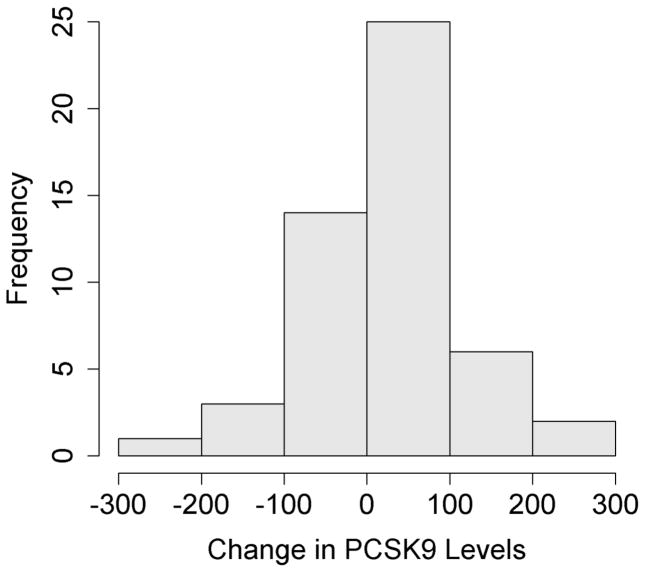
Serum PCSK9 levels also increased from 316 ± 105 ng/mL to 343 ± 107 ng/mL (p=0.041) during this period (Figure 1). There was however significant inter-individual variability observed in the change in PCSK9 levels with sirolimus use. There were 18 patients who had no change or a decrease in PCSK9 levels, however the vast majority of patients (n=33) had an increase in PCSK9 levels. Plasma sirolimus levels were similar between the two groups. The increase in LDL cholesterol was also similar between both groups (22.1 ± 29.7 mg/dL vs 26.2 ± 68.3 mg/dL, p=0.8).
Figure 1.
(A) Changes in plasma PCSK9 levels in 51 cardiac transplant patients after being switched to sirolimus based immunosuppressive therapy. (B) Distribution of patient cohort based on changes in PCSK9 levels.
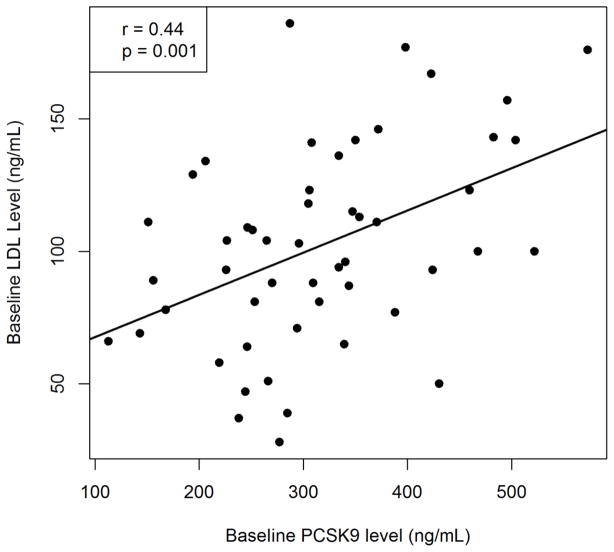
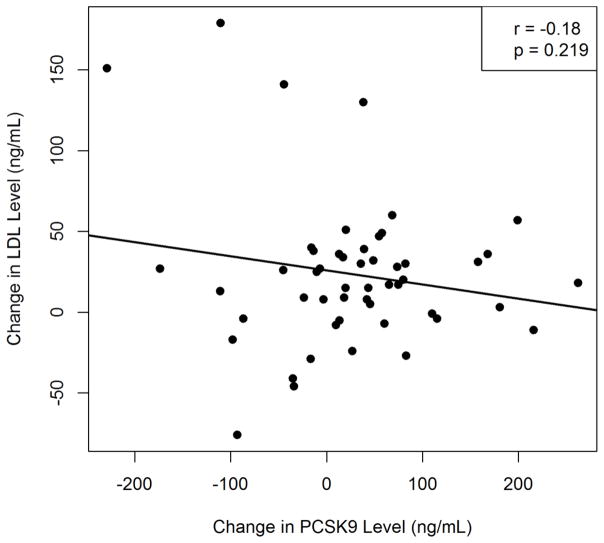
Baseline serum PCSK9 levels showed a significant positive correlation with baseline LDL cholesterol levels (r = 0.44, p = 0.001, Figure 2A), but there was no correlation between changes in PCSK9 and LDL cholesterol levels (r = −0.17, p = 0.2, Figure 2B). By controlling for sex, we found no significant difference in the change in LDL-C given a change in PCSK9 (slope = −0.091791, p=0.2087). Similarly, we found that sex was not a significant predictor of the change in LDL-C (p=0.7433).
Figure 2.
Correlation between (A) Baseline serum PCSK9 and LDL cholesterol levels. (B) Change in PCSK9 level with change in LDL cholesterol levels after sirolimus therapy
In-vitro study
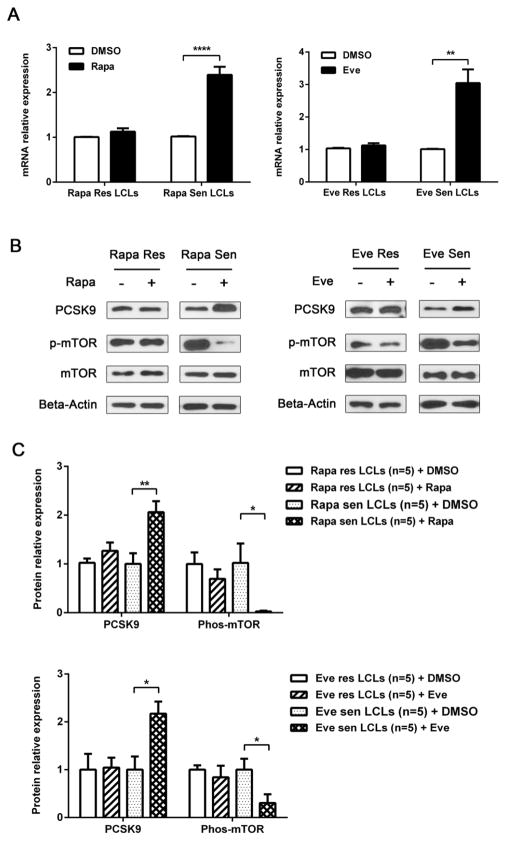
To further examine the inter-individual variability in change in PCSK9 levels with mTOR inhibition, we selected 5 LCLs from two ends of the distribution of EC50 values for all of the LCLs to test PCSK9 expression and protein levels before and after treatment with mTOR inhibitors. In sirolimus or everolimus-sensitive LCLs, the mTOR activity, determined by phosphorylated Ser2448 mTOR level, decreased dramatically as expected after treatment with mTOR inhibitors, while both mRNA and protein level of PCSK9 was inversely upregulated (Figure 3). In the resistant LCLs, mTOR activity did not change significantly; neither did the expression of PCSK9 after treatment with sirolimus or everolimus (Figure 3). These results suggested that mTOR activity directly affects PCSK9 expression, and variability in PCSK9 response is dependent on variability in mTORi sensitivity.
Figure 3.
(A) Effect of mTOR inhibitors on PCSK9 mRNA expression. Five selected resistant and sensitive Lymphoblastoid Cell Lines (LCLs) for mTOR inhibitors were treated with sirolimus (0.2 μM), everolimus (0.33 μM) or DMSO for 3 d. QRT-PCR was performed to measure the expression of PCSK9 pre and post drug treatment. (B) Representative Western blot for protein expression. Western blot analysis with antibodies against mTOR, phosphorylated Ser2448mTOR, PCSK9 was performed using protein lysate from cells treated under the same conditions as those in (A). Actin was used as a loading control. (C) Quantification of relative protein levels for all 5 LCLs. Results were calculated with Grey-scan value. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. Rapa = rapamycin, Eve = everolimus, Res = resistant, Sen = sensitive.
Discussion
This is the first study in humans, to the best of our knowledge, which demonstrates that treatment with mTORi therapy can result in an increase in PCSK9 levels. Recently, circulating PCSK9 levels have been shown to predict the occurrence of cardiovascular events in the general population independent of lipid levels and other cardiovascular risk factors [13]. Therefore the observation that circulating PCSK9 levels can change with pharmacological therapy such as sirolimus may be significant. Second, we demonstrate that the mTORi associated hyperlipidemia is likely not due to elevation in PCSK9 levels, which has broader implications for treatment of not only cardiac transplant recipients but also the general population if used for its anti-aging potential. Third, we observe that there is significant variability in the change in PCSK9 levels with sirolimus use that may result from the variability in sensitivity to mTORi as demonstrated in our in-vitro LCL model system. Further studies are needed to identify the determinants of mTORi sensitivity.
Sirolimus is the first FDA approved drug that has been shown to prolong life in both sexes in the mammalian species and has beneficial effects by decreasing cancers and cognitive impairment [14]. Sirolimus-based immunosuppressive therapy also offers many advantages in cardiac transplant recipients including decreased cardiac allograft vasculopathy and nephrotoxicity [7], but its use can also lead to hypercholesterolemia [4] which can increase the risk for atherosclerotic vascular disease. While the genesis of cardiac allograft vasculopathy is not similar to atherosclerotic cardiovascular disease, hypercholesterolemia can still exacerbate this condition. A clear understanding of the mechanisms by which mTORi causes dyslipidemia will help address this potential complication that may, in turn, improve outcomes not only in cardiac transplant patients but in the general population if used to retard the aging process. The current study clearly shows that in most individuals, mTORi increase the expression and circulating levels of PCSK9. It has been well established that PCSK9 increases the lysosomal degradation of LDL receptors, thus interfering with receptor mediated uptake of LDL particles leading to elevated LDL cholesterol in the circulation at baseline [15,16]. However, based on our study findings, this phenomenon may not have a linear relationship (higher increase in PCSK9 levels lead to higher LDL levels) in sirolimus-induced hyperlipidemia.
Following transition to sirolimus based therapy, we noticed a 23% increase in serum LDL cholesterol levels. This may have been partly attenuated by concomitant statin therapy in many patients, but is similar to the magnitude of dyslipidemia reported in previous studies [4,5,17,18]. While majority of our patients were already on statin therapy after transplant and before being switched to sirolimus, the increase in lipid levels necessitated further changes in lipid-lowering therapy in some patients. These included 7 patients in whom statin therapy was initiated, 6 in whom the dose was increased, and 4 who were switched to a more potent statin. These changes could potentially have masked an even greater effect of sirolimus on LDL cholesterol levels. A modest weight gain of about 3 kg was noted, but this is also unlikely to have a significant effect on PCSK9 levels. Arsenault et al [19] reported no correlation between PCSK9 levels and BMI, and no significant change in PCSK9 levels after an average 6.7 kg weight loss. It therefore appears that the most likely reason for the increase in PCSK9 levels in our patients was introduction of sirolimus therapy.
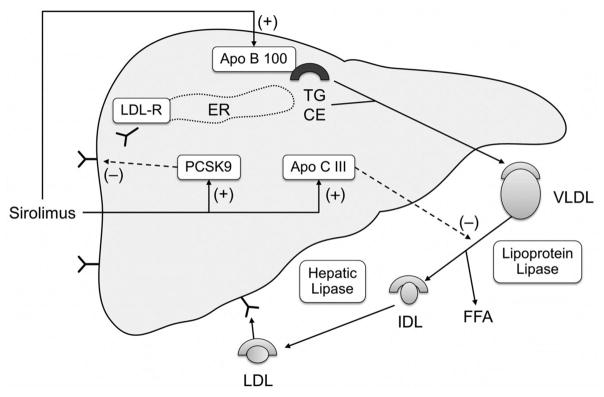
There is a wealth of data supporting the role of PCSK9 in the regulation of LDL cholesterol levels [15,16] as observed in our study which shows a significant correlation between baseline PCSK9 levels and baseline LDL levels (p=0.001). However our study does not support the concept that an increase in PCSK9 levels with sirolimus use results in an increase in lipid levels, hence making it unlikely that sirolimus-induced hyperlipidemia is due to an elevation in PCSK9 levels. This finding is supported by a prior observation that the magnitude of PSCK9 levels in itself is unlikely to account for the observed increase in LDL cholesterol levels [20]. Additionally, a recent report demonstrating elevated PCSK9 levels in patients with nephrotic syndrome failed to show a correlation between changes in PCSK9 and lipid levels despite a significant decline in both PCSK9 and lipid levels after resolution of nephrotic syndrome [21]. Other contributing factors to sirolimus-induced hyperlipidemia could be an increase in apolipoprotein B synthesis leading to increased production of VLDL particles [22] ultimately leading to increased LDL particles with triglyceride hydrolysis. Increased apolipoprotein B synthesis could also contribute to hypertriglyceridemia observed with sirolimus therapy, as could also an increase in the synthesis of apolipoprotein CIII which inhibits triglyceride hydrolysis and remnant particle uptake [23]. Sirolimus, therefore, has multiple effects on lipoprotein metabolism (Figure 4) that could contribute to the dyslipidemia observed with therapy.
Figure 4.
(Central illustration). Mechanism of sirolimus-induced hyperlipidemia. Sirolimus increases the expression of Apo B100 leading to increased VLDL secretion. It also increases the expression of Apo CIII which inhibits lipoprotein lipase, and thus reduces hydrolysis and clearance of triglyceride rich lipoproteins. Both these effects contribute to hypertriglyceridemia. It also increases PCSK9 levels leading to decreased LDL receptor expression, and a resultant increase in LDL cholesterol levels. LDL-R, low density lipoprotein receptor; VLDL, very low density lipoprotein, FFA, free fatty acid; IDL, intermediate density lipoprotein; LDL, low density lipoprotein; ER endoplasmic reticulum; TG, triglyceride; CE, cholesterol ester.
In our first in human study, we confirmed not only the laboratory based observation of an increase in PCSK9 levels with sirolimus therapy but we also demonstrated the inter-individual variability that exists in these measurements, and by performing in-vitro studies in human lymphoblastoid cell lines, attempted to explain this inter-individual variability. Incubation of cells with sirolimus and everolimus lead to a more than 2–3 fold elevation in PCSK9 mRNA and protein levels only in mTOR sensitive cells, thus demonstrating that increase in PCSK9 expression is dependent on mTOR inhibition. It is known that mTOR signaling influences the expression of sterol regulatory element-binding protein (SREBP), and other transcription factors which regulate lipid homeostasis [10]. Recently, Liu et al [24] showed that in mice with non-alcoholic fatty liver disease, blocking mTORC1 signaling leads to decreased SREBP translocation and increased PCSK9 expression and LDL receptor degradation. This interesting observation may explain the possible molecular mechanism of the interaction between mTOR signaling and lipid homeostasis.
Our in-vitro data also demonstrated the presence of variability in PCSK9 expression and levels in distinct mTOR sensitive and resistant LCL. We observed a similar dichotomous response in changes in PCSK9 levels in our patient cohort. Plasma sirolimus levels between the two groups were not different, and hence the bioavailability of the drug is unlikely to account for the difference in PCSK9 levels.
In summary, our human and in-vitro cell culture studies together suggest that mTOR inhibition with sirolimus use increases PCSK9 expression and levels, however the increase in serum PCSK9 levels does not correlate with sirolimus-induced hypercholesterolemia. These observations have important implications for the use of mTORi in the management of transplant patients, as an anti-aging agent and in our overall understanding of mTOR signaling in maintaining lipid homeostasis. The cause for variability in sirolimus-induced elevation in circulating PCSK9 levels, its effect on graft vasculopathy, and the potential use of novel PCSK9 blocking therapy in attenuating this long-term complication needs to be further explored.
Acknowledgments
Funding: This study was funded by the Mayo Clinic Transplant Center
Abbreviations
- AA
African–American
- CNI
calcineurin inhibitor
- CA
Caucasian–American
- HCA
Han Chinese–American
- LCL
Lymphoblastoid Cell Lines
- PCSK9
Proprotein convertase subtilisin/kexin Type 9
- SREBP
sterol regulatory element-binding protein
Footnotes
Conflict of Interest: All of the authors report no conflict of interest pertaining to this manuscript.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Neuhaus P, Klupp J, Langrehr JM. mTOR inhibitors: an overview. Liver Transplantation. 2001;7(6):473–484. doi: 10.1053/jlts.2001.24645. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bak T, Wachs M, Trotter J, Everson G, Trouillot T, Kugelmas M, et al. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transplantation. 2001;7(8):680–686. doi: 10.1053/jlts.2001.26509. [DOI] [PubMed] [Google Scholar]
- 4.Brattstrom C, Wilczek H, Tyden G, Bottiger Y, Sawe J, Groth CG. Hyperlipidemia in renal transplant recipients treated with sirolimus (rapamycin) Transplantation. 1998;65(9):1272–1274. doi: 10.1097/00007890-199805150-00023. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71(2):271–280. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 6.Neff GW, Montalbano M, Slapak-Green G, Berney T, Bejarano PA, Joshi A, et al. A retrospective review of sirolimus (Rapamune) therapy in orthotopic liver transplant recipients diagnosed with chronic rejection. Liver Transplantation. 2003;9(5):477–483. doi: 10.1053/jlts.2003.50119. [DOI] [PubMed] [Google Scholar]
- 7.Raichlin E, Bae JH, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116(23):2726–2733. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 8.Beutner F, Brendel D, Teupser D, Sass K, Baber R, Mueller M, et al. Effect of everolimus on pre-existing atherosclerosis in LDL-receptor deficient mice. Atherosclerosis. 2012;222(2):337–343. doi: 10.1016/j.atherosclerosis.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. The Journal of Biological Chemistry. 2007;282(25):18602–18612. doi: 10.1074/jbc.M702027200. [DOI] [PubMed] [Google Scholar]
- 10.Lamming DW, Sabatini DM. A central role for mTOR in lipid homeostasis. Cell Metabolism. 2013;18(4):465–469. doi: 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ai D, Chen C, Han S, Ganda A, Murphy AJ, Haeusler R, et al. Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. Journal of Clinical Investigation. 2012;122(4):1262–1270. doi: 10.1172/jci61919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang J, Fridley BL, Feng Q, Abo RP, Brisbin A, Batzler A, et al. Genome-wide association study for biomarker identification of Rapamycin and Everolimus using a lymphoblastoid cell line system. Frontiers in Genetics. 2013;4:166. doi: 10.3389/fgene.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leander K, Malarstig A, Van’t Hooft FM, Hyde C, Hellenius ML, Troutt JS, et al. Circulating proprotein convertase subtilisin/kexin type 9 (PCSK9) predicts future risk of cardiovascular events independently of established risk factors. Circulation. 2016;133(13):1230–1239. doi: 10.1161/circulationaha.115.018531. [DOI] [PubMed] [Google Scholar]
- 14.Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cellular and Molecular Life Sciences. 2014;71(22):4325–4346. doi: 10.1007/s00018-014-1677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz R, Schluter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9) Basic Research in Cardiology. 2015;110(2):4. doi: 10.1007/s00395-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavori H, Rashid S, Fazio S. On the function and homeostasis of PCSK9: reciprocal interaction with LDLR and additional lipid effects. Atherosclerosis. 2015;238(2):264–270. doi: 10.1016/j.atherosclerosis.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neff GW, Montalbano M, Slapak-Green G, Meyer D, Berney T, Safdar K, et al. Sirolimus therapy in orthotopic liver transplant recipients with calcineurin inhibitor related chronic renal insufficiency. Transplantation Proceedings. 2003;35(8):3029–3031. doi: 10.1016/j.transproceed.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Trotter JF, Wachs ME, Trouillot TE, Bak T, Kugelmas M, Kam I, et al. Dyslipidemia during sirolimus therapy in liver transplant recipients occurs with concomitant cyclosporine but not tacrolimus. Liver Transplantation. 2001;7(5):401–408. doi: 10.1053/jlts.2001.23916. [DOI] [PubMed] [Google Scholar]
- 19.Arsenault BJ, Pelletier-Beaumont E, Almeras N, Tremblay A, Poirier P, Bergeron J, et al. PCSK9 levels in abdominally obese men: association with cardiometabolic risk profile and effects of a one-year lifestyle modification program. Atherosclerosis. 2014;236(2):321–326. doi: 10.1016/j.atherosclerosis.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. Journal of Clininical Endocrinology & Metabolism. 2009;94(7):2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas ME, Levenson AE, Sun X, Liao WH, Rutkowski JM, de Ferranti SD, et al. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation. 2016;134(1):61–72. doi: 10.1161/circulationaha.115.020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, et al. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. Journal of Lipid Research. 2002;43(8):1170–1180. [PubMed] [Google Scholar]
- 23.Tur MD, Garrigue V, Vela C, Dupuy AM, Descomps B, Cristol JP, et al. Apolipoprotein CIII is upregulated by anticalcineurins and rapamycin: implications in transplantation-induced dyslipidemia. Transplantation Proceedings. 2000;32(8):2783–2784. doi: 10.1016/s0041-1345(00)01884-4. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Ma KL, Zhang Y, Wu Y, Hu ZB, Lv LL, et al. Activation of mTORC1 disrupted LDL receptor pathway: a potential new mechanism for the progression of non-alcoholic fatty liver disease. The International Journal of Biochemistry & Cell Biology. 2015;61:8–19. doi: 10.1016/j.biocel.2015.01.011. [DOI] [PubMed] [Google Scholar]