Abstract
Background and aims
Most prior studies investigating the association of lower extremity peripheral artery disease (PAD) with physical function were small or analyzed selected populations (e.g., patients at vascular clinics or persons with reduced function), leaving particular uncertainty regarding the association in the general community.
Methods
Among 5,262 ARIC participants (age 71-90 years during 2011-2013), we assessed the cross-sectional association of ankle-brachial index (ABI) with the Short Physical Performance Battery (SPPB) score (0-12), its individual components (chair stands, standing balance, and gait speed) (0-4 points each), and grip strength after accounting for potential confounders, including a history of coronary disease, stroke, or heart failure.
Results
There were 411 participants (7.8%) with low ABI ≤0.90 and 469 (8.9%) participants with borderline low ABI 0.91-1.00. Both ABI ≤0.90 and 0.91-1.00 were independently associated with poor physical function (SPPB score ≤6) compared to ABI 1.11-1.20 (adjusted odds ratio 2.10 [95% CI 1.55-2.84] and 1.86 [1.38-2.51], respectively). The patterns were largely consistent across subgroups by clinical conditions (e.g., leg pain or other cardiovascular diseases), in every SPPB component, and for grip strength. ABI >1.3 (472 participants [9.0%]), indicative of non-compressible pedal arteries, was related to lower physical function as well but did not necessarily reach significance.
Conclusions
In community-dwelling older adults, low and borderline low ABI suggestive of PAD were independently associated with poorer systemic physical function compared to those with normal ABI. Clinical attention to PAD as a potential contributor to poor physical function is warranted in community-dwelling older adults.
Keywords: Peripheral artery disease, physical function, aging
Introduction
Lower extremity peripheral artery disease (PAD), commonly defined as ankle-brachial index (ABI) <0.9, affects 8-10 million individuals in the US1-3 and more than 200 million individuals around the globe,2 with particularly high prevalence in older adults.4 Of note, its prevalence increased by 24% globally in the last decade.2 Persons with PAD are well-known to have increased mortality risk, mainly due to cardiovascular disease.5 Indeed, patients with PAD have 3-6 fold higher risk of 10-year mortality compared to those without.5 Leg amputation is another critical consequence of PAD, and more than 150,000 legs are amputated annually due to PAD in the US.1
Reduced physical function has been investigated as a potential consequence of PAD. However, several previous studies have reported conflicting results regarding PAD and objectively assessed physical function.6-17 Many of these studies were small (n <1,000) and had limited statistical power.6-8, 12-17 More importantly, most of these studies investigated selected populations (i.e., patients from vascular labs and clinics,6-8, 12, 15-17 persons with disability13, 14 or reduced physical function plus sedentary lifestyle9, or only women10, 13, 14), leaving uncertainty regarding the impact of PAD on physical function in the community. Therefore, the aim of this study is to assess the cross-sectional association of a representative indicator of PAD, ABI, with objectively measured physical function, using data from a biracial community-based cohort, the Atherosclerosis Risk in Communities (ARIC) Study.
Materials and methods
Study participants
The ARIC Study consisted of 15,792 participants who were aged 45–64 years at visit 1 during the period 1987–1989 and were recruited from four communities in the US: Washington County, Maryland; suburban Minneapolis, Minnesota; Jackson, Mississippi; and Forsyth County, North Carolina.18 Subsequently, three follow-up visits were performed triennially (visits 2, 3, and 4 during 1990-1992, 1993-1995 and 1996-1998, respectively). Visit 5 was recently conducted during 2011–2013 and included the first assessments of physical function and ABI in both legs; visit 5 serves as baseline for the present study. Among 6,538 participants who attended visit 5, we excluded participants who reported race other than white or black (n=18), due to small numbers, or had missing values of ABI (n=868), physical function (n=123), or covariates of interest (n=267), leaving a final study population of 5,262 participants. The study was approved by the Institutional Review Boards of all participating institutions, and all participants gave informed consent.
Ankle-brachial index
Using OMRON VP-1000 plus (Kyoto, Japan), an oscillometric device, blood pressure was automatically measured twice, five minutes apart in both ankles and brachia by certified technicians.19 Using the higher value of the right or left brachial systolic blood pressure as the denominator, the ABI, the ratio of ankle systolic blood pressure to brachial systolic blood pressure,20 was calculated for right and left legs. The mean ABI of two measurements was recorded for each leg. In general, the lower value of right and left ABI was used for our analysis. Only when the higher ABI exceeded 1.3 and the lower ABI was normal (1.0-1.3), did we use the higher ABI value >1.3 for the analysis, to avoid missing potentially pathophysiological information from exceptionally high ABI indicating arterial non-compressibility.21-24
Objective measures of physical function
Short Physical Performance Battery (SPPB)
Physical function was assessed using the Short Physical Performance Battery (SPPB) as a measure of lower extremity performance25, 26 and grip strength as a measure of upper body strength.27 Briefly, the SPPB consists of three components of physical performance, chair stands, standing balance, and gait speed. A point from 0 (poorest) to 4 (best) was assigned for each of these three components, based on population-based norms. The sum of the scores provided a composite score ranging from 0 to 12 for each participant, and SPPB score ≤6 was considered as poor physical function as previously reported.26 We analyzed both SPPB score as a composite measure of physical function and each component of chair stands, standing balance, and gait speed, separately.
Chair stands
Participants were timed standing from and sitting on a chair five times within 60 seconds, with their arms crossed on their chest. The ability of and time for accomplishing the task determined the score (0 points if unable to accomplish, 1 point if it took 16.7 to 60 seconds, 2 points if 13.7 to <16.7 seconds, 3 points if 11.2 to <13.7 seconds, and 4 points if <11.2 seconds). We also investigated time to accomplish five chair stands as a continuous variable. For those who could not complete five stands within 60 seconds, we conservatively allocated 60 seconds.
Standing balance
The ability to maintain standing posture for 10 seconds with three different foot positions, side-by-side (easiest), semi-tandem (intermediate), and tandem (hardest), beginning with the semi-tandem position.28 Participants who could not complete semi-tandem were tested for side-by-side. Those who completed semi-tandem were assumed to be able to complete side-by-side and subsequently evaluated in the tandem stand. Two trials were allowed for tandem standing balance if needed. Upon accomplishment, one point was provided for each of side-by-side stand and semi-tandem stand; one point was given if the tandem stand was held 3 to <10 seconds and two points if the tandem stand was held for 10 seconds (minimum of 0 to maximum of 4 points in total for this test of standing balance). We also analyzed time of holding stand with maximum of 10 seconds for each feet position as continuous variables.
Gait speed
Participants were timed walking 4 meters at their usual speed, and the faster of the two trials was recorded for analysis. Participants were encouraged not to use walking aids but were allowed per participants’ discretion. The point was determined by ability and time as follows: 0 point if unable to accomplish, 1 point if it took ≥8.70 seconds, 2 points if 6.21 to <8.70 seconds, 3 points if 4.82 to <6.21 seconds, and 4 points if <4.82 seconds. Dividing 4 meters by the time required to walk this distance, we calculated walking velocity (m/sec) and used it as a continuous variable in the analysis as well.
Grip strength
Grip strength in kilograms of force was assessed using Jamar Hydraulic Hand Dynamometer in participant's preferred hand (usually the dominant). The better of two trials was used for the analysis.
Other variables
ARIC study participants provided information on demographic and behavioral variables and medical history to a trained interviewer. Physical assessment and blood sample collection were performed according to standardized procedures.29 Age, gender, race, education level, smoking, alcohol intake, leg pain, and physical activity were based on self-report. Completed years of education were categorized into <12, 12 to 16, or >16 years. Smoking status and alcohol intake were dichotomized as current vs. former/never. The presence of leg pain due to artery blockage was based on the questionnaire during annual telephone follow-ups prior to visit 5. Physical activity was categorized into five categories regarding whether they do any exercise during leisure time (never, seldom, sometimes, often, and very often). For systolic blood pressure as a covariate, we used the average of the last two of three readings, which were measured by certified technicians with participants in the sitting position after 5-minute rest using a validated automatic sphygmomanometer (the OMRON HEM-907 XL). Participants were asked to bring all medications including antihypertensive, antidyslipidemic, and antidiabetic drugs, which were coded by trained personnel. Diabetes mellitus was defined as a fasting glucose ≥7.0 mmol/L, non-fasting glucose ≥11.1 mmol/L, self-reported physician diagnosis of diabetes, or use of glucose lowering medications. Total cholesterol and high-density lipoprotein cholesterol were determined using enzymatic methods meeting the National Cholesterol Education Program's accuracy performance criteria. Body mass index was defined as weight (kg) divided by the square of height (m2). Prevalent coronary heart disease and stroke were defined as self-reported history at visit 1 or adjudicated clinical events between visit 1 and visit 5. Hospitalization for heart failure, physician diagnosis of heart failure, and self-reported treatment for heart failure between visits 1 and 5 were considered prevalent heart failure.
Statistical analysis
According to previous literature9, 30 and the distribution in the ARIC Study, ABI was divided into the following six categories: ≤0.90 (low ABI31), 0.91-1.00 (borderline low20), 1.01-1.10, 1.11-1.20, 1.21-1.30, and >1.30 (high ABI31). The category of 1.11-1.20 was used as reference since this category was most prevalent in our study and was used as a reference in the ABI Collaboration.32 First, baseline characteristics were compared across these ABI categories based on ANOVA and chi-square tests, as appropriate.
Subsequently, multivariable linear regression models were examined with the SPPB score or its components as dependent variables. To specifically capture the contribution of ABI to poor physical function, we also constructed multivariable logistic regression models with SPPB score ≤6 defined as poor performance26 and ≤2 points of each of three components (threshold of ≤6 divided by three components) of chair stands, standing balance, and gait speed as dependent variables. We also modeled time required for chair stands, time held while standing, and walking velocity as well as grip strength as continuous dependent variables using linear regression models.
We evaluated two sets of covariates. Our primary model included a list of potential confounders: age, gender, race, education, smoking, alcohol intake, body mass index, diabetes, systolic blood pressure, antihypertensive drugs, total and high-density lipoprotein cholesterols, lipid-lowering drugs, and history of coronary heart disease, stroke, and heart failure. Our secondary model additionally included potential mediators, leg pain and physical activity.
Finally, we repeated our analyses in several prespecified subgroups by age, gender, race, diabetes, smoking status, history of cardiovascular disease (a composite of coronary disease, stroke, and heart failure), and presence/absence of leg pain related to PAD. Interaction by these factors was assessed with likelihood ratio test by comparing two models with and without product terms of ABI categories and each of those factors. All analyses were performed with Stata version 14.0, and a p-value <0.05 was considered statistically significant.
Results
The mean age of 5,262 participants was 75.3 (SD 5.0) years. There were 411 participants (7.8%) with a low ABI ≤0.90 and 469 (8.9%) with a borderline low ABI of 0.91-1.00. High ABI >1.3 was observed in 472 subjects (9.0%). As compared to participants with ABI of 1.11-1.20 in the reference range, those with low and borderline low ABI were likely to be older and blacks and to have worse cardiovascular risk factor profiles, such as higher prevalence of current smoking, diabetes, antihypertensive use, and history of cardiovascular disease (Table 1). Participants with low ABI were more likely to be men, whereas those with borderline low ABI were more likely to be women. Those with high ABI also demonstrated a worse profile for diabetes, antihypertensive use, and prevalent coronary disease and stroke, compared to those with ABI 1.11-1.20. There was generally a dose-response relationship of lower ABI to higher prevalence of leg pain and physical inactivity (“never” and “seldom” for leisure time exercise).
Table 1.
Baseline characteristics according to ABI categories.
| Characteristics | Overall | ABI categories | |||||
|---|---|---|---|---|---|---|---|
| ≤0.90 | 0.91-1.00 | 1.01-1.10 | 1.11-1.20 | 1.21-1.30 | >1.30 | ||
| Total N | 5262 | 411 | 469 | 1329 | 1866 | 715 | 472 |
| Age, years | 75 (5) | 77 (5) | 76 (6) | 75 (5) | 75 (5) | 75 (5) | 75 (5) |
| Female, % | 57.4 | 54.0 | 68.9 | 71.0 | 59.0 | 41.5 | 28.4 |
| Black, % | 21.5 | 40.2 | 29.0 | 27.6 | 18.5 | 9.7 | 10.0 |
| Systolic blood pressure, mmHg | 130 (18) | 133 (21) | 129 (18) | 130 (18) | 130 (17) | 130 (17) | 127 (17) |
| Antihypertensive medication, % | 74.4 | 89.8 | 78.5 | 77.7 | 70.4 | 70.2 | 70.3 |
| Diabetes, % | 36.2 | 49.4 | 39.5 | 36.1 | 33.9 | 31.9 | 37.3 |
| Body mass index, kg/m2 | 29 (5) | 29 (6) | 29 (6) | 29 (6) | 28 (5) | 28 (5) | 29 (5) |
| High school, no degree, % | 13.1 | 24.1 | 16.2 | 15.7 | 11.6 | 7.1 | 8.5 |
| High school graduate or some college, % | 41.9 | 40.9 | 43.3 | 44.8 | 41.9 | 41.5 | 34.5 |
| College and above, % | 44.9 | 35.0 | 40.5 | 39.6 | 46.6 | 51.3 | 57.0 |
| Total cholesterol, mmol/L | 4.7 (1.1) | 4.6 (1.2) | 4.7 (1.2) | 4.8 (1.1) | 4.7 (1.1) | 4.6 (1.0) | 4.4 (1.0) |
| HDL cholesterol, mmol/L | 1.3 (0.4) | 1.3 (0.3) | 1.3 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.3 (0.3) | 1.3 (0.3) |
| Lipid lowering medication, % | 56.3 | 63.5 | 57.6 | 56.1 | 54.1 | 55.8 | 58.5 |
| Current smoker, % | 5.9 | 14.8 | 13.0 | 6.0 | 3.9 | 2.4 | 3.8 |
| Current drinker, % | 50.9 | 37.0 | 42.4 | 46.7 | 54.5 | 59.3 | 56.6 |
| History of coronary heart disease, % | 14.4 | 28.0 | 17.5 | 12.3 | 12.1 | 13.0 | 16.7 |
| History of stroke, % | 3.4 | 6.8 | 6.0 | 2.8 | 2.7 | 2.7 | 3.2 |
| History of heart failure, % | 15.4 | 32.9 | 19.8 | 15.7 | 12.3 | 10.6 | 14.6 |
| Leg pain, % | 7.9 | 20.2 | 7.9 | 7.7 | 6.3 | 7.1 | 5.7 |
| Exercise in leisure time | |||||||
| Never % | 27.7 | 36.6 | 36.3 | 30.9 | 26.2 | 18.7 | 21.4 |
| Seldom, % | 21.0 | 20.5 | 22.0 | 21.5 | 19.4 | 24.1 | 20.6 |
| Sometimes, % | 27.2 | 27.3 | 24.2 | 26.8 | 28.8 | 26.1 | 26.1 |
| Often, % | 17.2 | 11.5 | 13.9 | 14.5 | 18.6 | 20.0 | 23.4 |
| Very often, % | 6.9 | 4.2 | 3.6 | 6.2 | 6.9 | 11.1 | 8.5 |
ABI, ankle-brachial index; HDL, high-density lipoprotein.
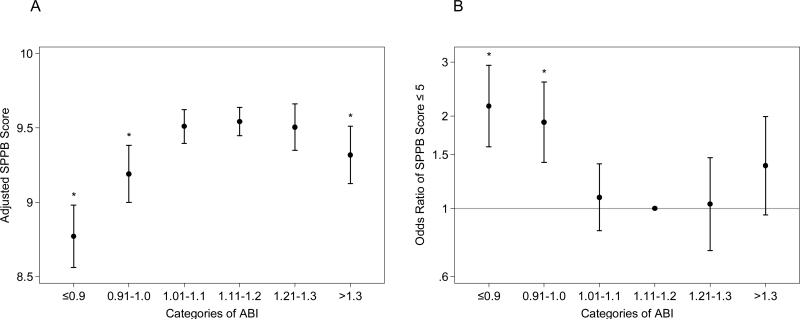
Even after accounting for the differences in these characteristics, significantly lower SPPB score was observed in both low ABI ≤0.9 and borderline low ABI 0.91-1.00 compared to the referent ABI (8.77 [95% CI 8.56-8.98], p<0.001, and 9.19 [9.00-9.38], p=0.002, respectively, vs. 9.53 [9.44-9.63]) (Fig. 1A). High ABI >1.3 showed significantly lower SPPB score compared to the reference ABI as well. Similarly, low ABI and borderline low ABI categories were associated with poor overall physical function (SPPB score ≤6) (odds ratio 2.10 [95% CI 1.55-2.84], 1.86 [1.38-2.51], and 1.35 [0.93-1.94], respectively, with ABI 1.11-1.20 as the reference) (Fig. 1B). The associations with high ABI in this logistic regression model did not reach significance (p-value 0.111). Further adjustment for leg pain and physical activity did not materially change the results (Supplemental Fig. 1). Although the number of participants was limited, the dose-response relationship was observed after subdividing ABI ≤0.9 into three categories of ≤0.7 (n=133), 0.71-0.8 (n=97), and 0.81-0.9 (n=181) (Supplemental Fig. 2).
Fig. 1. Association of ABI with total score of the Short Physical Performance Summary (SPPB).
Adjusted SPPB score (A) and adjusted odds ratio of low SPPB score (≤ 6) (B) according to ABI categories. The results were adjusted for age, race, sex, education, smoking, alcohol, body mass index, antihypertensive drugs, systolic blood pressure, lipid lowering drugs, total and HDL cholesterols, diabetes, and history of coronary disease, heart failure, and stroke. * Indicates statistical significance with ABI 1.11-1.20 as the reference.
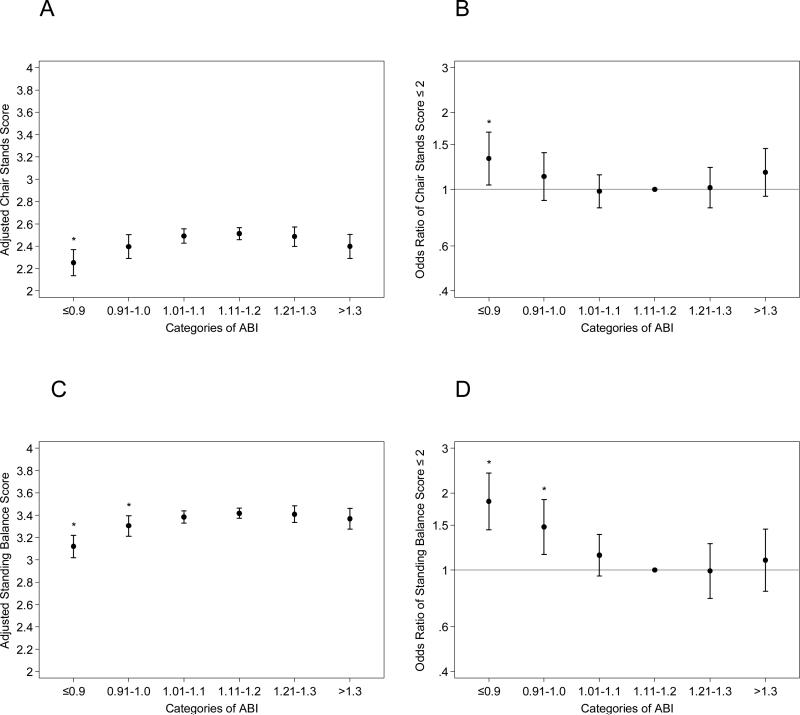
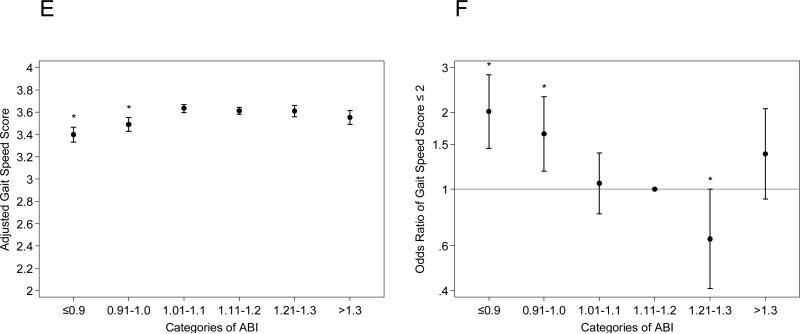
Low ABI ≤0.90 and borderline low ABI 0.91-1.00 were largely consistently associated with poorer scores of each SPPB component compared to ABI 1.11-1.20, regardless of the additional adjustment for leg pain and physical activity (Fig. 2 and Supplemental Fig. 3). The poorer performance was more evident when time required for chair stand, time able to hold standing, and walking velocity were analyzed as continuous variables (Table 2). For example, compared to those with ABI 1.11-1.20, those with low ABI ≤0.9 took 2.82 (95% CI 1.50-4.13) seconds more for chair stands on average (19.26 [18.07-20.44] seconds vs. 16.44 [15.90-16.98] seconds), held the semi-tandem stand 0.40 (0.16-0.64) seconds less comparing the medians (9.01 [interquartile interval, 8.80-9.23] seconds vs. 9.42 [9.32-9.51] seconds), and walked 0.07 (0.05-0.09) m/s slower on average (0.89 [0.87-0.91] meter/second vs. 0.96 [0.95-0.97] meter/second) (all p-values <0.05). Both low ABI and borderline low ABI groups demonstrated lower grip strength compared to ABI 1.11-1.20. Although the high ABI group demonstrated worse performance in each of the SPPB components and grip strength compared to the reference ABI group, none of them were statistically different. Largely consistent results were seen when we stratified by gender (Supplemental Tables 1 and 2) or further adjusted for leg pain and physical activity (Supplemental Table 3).
Fig. 2. Association of ABI with each component of the Short Physical Performance Summary (SPPB).
Adjusted score of each component in the SPPB ((A) [chair stand score], (C) [standing balance score], and (E) [gait speed score]) and adjusted odds ratio of having low score (≤2) of each component ((B) [chair stand score], (D) [standing balance score], and (F) [gait speed score]) according to ABI categories. *Indicates statistical significance with ABI 1.11-1.20 as the reference.
Table 2.
Adjusteda measures of physical function according to ABI categories.
| Measured elements of physical function | ABI categories | |||||
|---|---|---|---|---|---|---|
| ≤0.90 | 0.91-1.00 | 1.01-1.10 | 1.11-1.20 | 1.21-1.30 | >1.30 | |
| N | 411 | 469 | 1329 | 1866 | 715 | 472 |
| Time required for five chair stands (sec) | 19.26 (18.07, 20.44)b | 17.51 (16.43, 18.60) | 16.78 (16.13, 17.90) | 16.44 (15.90, 16.98) | 17.01 (16.13, 17.90) | 17.43 (16.33, 18.52) |
| Time able to hold standing with side-by-side feet (sec) | 9.79 (9.69, 9.90) | 9.96 (9.86, 10.05) | 9.86 (9.81, 9.92) | 9.88 (9.83, 9.93) | 9.90 (9.82, 9.98) | 9.84 (9.75, 9.94) |
| Time able to hold standing with semi-tandem feet (sec) | 9.01 (8.80, 9.23)b | 9.32 (9.12, 9.52) | 9.34 (9.22, 9.45) | 9.42 (9.32, 9.51) | 9.23 (9.07, 9.39) b | 9.31 (9.11, 9.50) |
| Time able to hold standing with tandem feet (sec) | 7.03 (6.70, 7.37)b | 7.61 (7.31, 7.91)b | 7.92 (7.74, 8.10) b | 8.17 (8.02, 8.32) | 7.97 (7.73, 8.22) | 7.86 (7.55, 8.16) |
| Walking velocity (m/s) | 0.89 (0.87, 0.91)b | 0.91 (0.89, 0.92)b | 0.95 (0.94, 0.96) | 0.96 (0.95, 0.97) | 0.96 (0.94, 0.97) | 0.94 (0.92, 0.96) |
| Grip strength (kg) | 28.09 (27.38, 28.81)b | 28.81 (28.16, 29.46)b | 29.05 (28.67, 29.44)b | 29.80 (29.48, 30.13) | 29.78 (29.25, 30.31) | 29.58 (28.93, 30.23) |
Adjusted for age, race, sex, education, smoking, alcohol, body mass index, antihypertensive drugs, systolic blood pressure, lipid lowering drugs, total and HDL cholesterols, diabetes, and history of coronary disease, heart failure, and stroke.
Indicates statistical significance with ABI 1.11-1.20 as the reference.
ABI, ankle-brachial index.
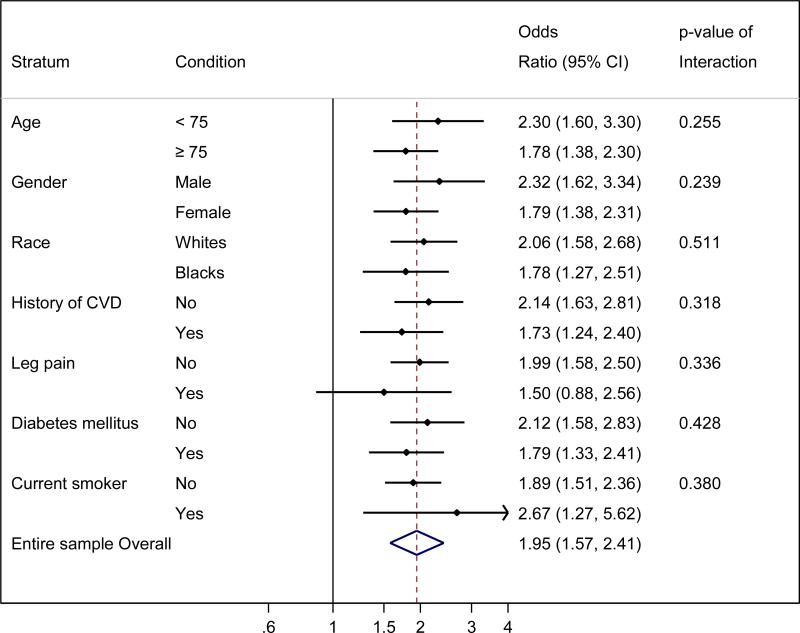
Given the results above and to obtain reliable estimates in each of subgroups, we dichotomized ABI as ≤1.00 vs. 1.01-1.30 for subgroup analysis (Fig. 3). Participants with ABI ≤1.00 compared to ABI 1.01-1.30 showed higher odds of poor physical function in all demographic and clinical subgroups tested. The association did not reach significance only in the subgroup of participants with leg pain, but the interaction was not statistically significant (p for interaction 0.342).
Fig. 3. Adjusted odds ratio of low SPPB score (≤6) according to lower ABI ≤1.00 vs. 1.01-1.30 by demographic and clinical subgroups.
The results were adjusted for age, race, sex, education, smoking, alcohol, body mass index, antihypertensive drugs, systolic blood pressure, lipid lowering drugs, total and HDL cholesterols, diabetes, and history of coronary disease, heart failure, and stroke, as appropriate.
Discussion
To our knowledge, this is the largest study exploring the associations between ABI and objectively measured physical function. We observed that lower ABI was robustly associated with poor physical function in community-dwelling older adults. Of importance, the association was independent of a history of other cardiovascular diseases, coronary heart disease, stroke, and heart failure. Lower ABI was consistently associated with three different components of SPPB. Of note, those with high ABI, indicating non-compressibility of the artery with or without PAD, had lower physical function as well, although the results did not necessarily reach statistical significance.
Our results are in line with and build upon previous studies showing positive associations of lower ABI or clinical diagnosis of PAD with objective measurements of impaired physical function.6-9, 11-15, 17 Importantly, we, for the first time, confirmed this association in a community-based sample of older men and women unselected for PAD status or reduced physical function. The significant results for each of the individual SPPB components in the present study would be of value, since there was considerable heterogeneity among those previous positive studies regarding which individual SPPB components related to PAD and each component of the SPPB reflects different physical functions. Specifically, chair stands reflect leg strength, balance, and endurance,8 whereas walking involves multiple organ systems including muscles, nerves, vision, joints, the heart and lungs. Moreover, this is one of few studies reporting lower physical function among those with ABI of 0.91-1.00,9, 33 a group currently considered borderline but not as PAD.20 As most of the previous studies excluded persons with high ABI,6-8, 10-17 our observations between high ABI and lower physical function are of interest.
There are a few plausible mechanisms linking PAD to the reduction of physical function. In terms of leg-specific mechanisms, it has been suggested that low blood perfusion of leg muscle during physical activity contributes to functional impairment related to PAD.34 In severe PAD cases, skeletal muscle atrophy in the legs through ongoing ischemia has been reported,35 which can further impact leg-specific physical function. In this connection, we confirmed the association of lower ankle blood pressure with reduced physical function (Supplemental Fig. 4). However, the independent associations of lower ABI with lower grip strength suggest the involvement of systemic pathophysiology. Low ABI is an indicator of systemic atherosclerosis and thus, those with low ABI often have other manifestations of cardiovascular disease such as stroke and heart failure,36 which may lead to poor systemic physical function. However, of note, the association of ABI with physical function was independent of other cardiovascular subtypes. Thus, further investigations are needed to understand potential pathophysiological mechanisms linking PAD to reduced systemic physical function and muscle strength.
High ABI is usually considered indicative of non-compressible pedal arteries, but some investigators consider as an indicator of PAD since the proportion of PAD is high in this group.37 Thus, the association of high ABI, similar to low ABI, with lower physical function performance in our study may not be unexpected. From another perspective, high ABI is also considered as a marker of arterial stiffness.38 Importantly, several potential mechanisms have been suggested regarding the link between arterial stiffness and impaired physical function. Increased arterial stiffness is known to associate with microvascular and endothelial abnormalities, which may potentially influence capillary perfusion in the exercising extremity and thus reduce the functional capacity of the lower extremities.39 Also, as reflected on the terminology of ventricular-vascular coupling, structural and functional alterations of the arterial system are known to adversely impact left ventricular function and structure, which could limit functional capacity.39, 40
These results suggest several public health and clinical implications. The association of low ABI with poor physical function independently of history of other cardiovascular diseases suggests a unique value of knowing PAD status. Screening of PAD using ABI is controversial since data are not conclusive regarding whether ABI provides information beyond the risk of other cardiovascular diseases.31, 41 Thus, it would be of interest to include the impact on physical function in this discussion. Indeed, identification of PAD as a cause of impaired lower-extremity physical function may have therapeutic implications since several studies have demonstrated that lifestyle (e.g., smoking cessation42 or exercise43) or medical (e.g., cilostazol44 or revascularization45, 46) interventions may improve physical function among patients with PAD and leg symptoms. This is particularly relevant to older adults since lower extremity performance is an important predictor to sustain their independent living.47 Furthermore, unhindered functional mobility also allows an older adult to take part in a range of productive and social activities. In this regard, the difference in walking speed between low ABI ≤0.90 vs. 1.11-1.20 in our study exceeded the thresholds of meaningfully different walking speed of 0.04-0.06 meter/second.9 Whether those interventions are effective among those without PAD-related leg symptoms are yet to be investigated.
Several limitations of this study should be acknowledged. The cross-sectional design does not allow us to infer temporality between ABI and physical function. ABI was measured using an oscillometric device due to feasibility in a large community cohort study, although clinical guidelines of PAD recommend using a Doppler probe for measuring ABI.20 However, the main weakness of oscillometric device is inaccuracy of ABI in severe PAD cases.20 Oscillometry-derived ABI has been validated against Doppler-determined ABI among healthy individuals and mild PAD cases,20 which seems relevant to a community-based cohort study like ours. Finally, although we controlled for potential confounders, as true for any observational studies, we cannot eliminate the possibility of residual confounding.
In conclusion, lower ABI was robustly and consistently associated with lower physical function in community-dwelling older adults. Since physical function is a key element for independent living in older adults and several therapeutic options for PAD exist, clinical attention could be warranted to PAD as a potential contributor to poor lower-extremity physical function among older adults.
Supplementary Material
Highlights.
In community-dwelling older adults, lower ankle-brachial index (ABI) was robustly associated with objective measures of poor physical function.
Lower ABI was consistently associated with three different components of lower-extremity function, squatting, walking speed, and standing balance.
The association was independent of a history of other cardiovascular diseases, namely, coronary heart disease, stroke, and heart failure.
Those with high ABI, indicating non-compressibility of the artery with or without PAD, tended to have lower physical function as well.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Financial support
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The funding source had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
All authors critically reviewed and provided substantial input on all manuscript outlines and drafts. All authors approved the final version of this manuscript for submission.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- 1.Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet. 2013;382:1312–1314. doi: 10.1016/S0140-6736(13)61576-7. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UKA, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. The Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. Journal of Vascular Surgery. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. The New England journal of medicine. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA : the journal of the American Medical Association. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA : the journal of the American Medical Association. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Applegate WB, Bonds DE, Buford TW, Church T, Espeland MA, Gill TM, Guralnik JM, Haskell W, Lovato LC, Pahor M, Pepine CJ, Reid KF, Newman A. Ankle brachial index values, leg symptoms, and functional performance among community-dwelling older men and women in the lifestyle interventions and independence for elders study. J Am Heart Assoc. 2013;2:e000257. doi: 10.1161/JAHA.113.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Functional status and mobility among elderly women with lower extremity arterial disease: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 1994;42:923–929. doi: 10.1111/j.1532-5415.1994.tb06581.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuo HK, Yu YH. The relation of peripheral arterial disease to leg force, gait speed, and functional dependence among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:384–390. doi: 10.1093/gerona/63.4.384. [DOI] [PubMed] [Google Scholar]
- 12.Parmenter BJ, Raymond J, Dinnen PJ, Lusby RJ, Fiatarone Singh MA. Preliminary evidence that low ankle-brachial index is associated with reduced bilateral hip extensor strength and functional mobility in peripheral arterial disease. J Vasc Surg. 2013;57:963–973. e961. doi: 10.1016/j.jvs.2012.08.103. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Ferrucci L, Simonsick EM, Balfour J, Fried L, Ling S, Gibson D, Guralnik JM. The ankle brachial index and change in lower extremity functioning over time: the Women's Health and Aging Study. J Am Geriatr Soc. 2002;50:238–246. doi: 10.1046/j.1532-5415.2002.50054.x. [DOI] [PubMed] [Google Scholar]
- 14.McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women's health and aging study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Ferrucci L, Guralnik JM, Dyer AR, Liu K, Pearce WH, Clark E, Liao Y, Criqui MH. The ankle-brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc Med. 2010;15:251–257. doi: 10.1177/1358863X10365181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szuba A, Oka RK, Harada R, Cooke JP. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vasc Med. 2006;11:155–163. doi: 10.1177/1358863x06074828. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Liu K, Guralnik JM, Mehta S, Criqui MH, Martin GJ, Greenland P. The ankle brachial index independently predicts walking velocity and walking endurance in peripheral arterial disease. J Am Geriatr Soc. 1998;46:1355–1362. doi: 10.1111/j.1532-5415.1998.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 18.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Study TARiCA Manual 2 Home and Field Center Procedures ARIC Visit 5 and NCS Study Protocol. 2013 [Google Scholar]
- 20.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 21.Fleg JL, Forman DE, Berra K, Bittner V, Blumenthal JA, Chen MA, Cheng S, Kitzman DW, Maurer MS, Rich MW, Shen WK, Williams MA, Zieman SJ. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. 2013;128:2422–2446. doi: 10.1161/01.cir.0000436752.99896.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison MA, Laughlin GA, Barrett-Connor E, Langer R. Association between the ankle-brachial index and future coronary calcium (the Rancho Bernardo study). Am J Cardiol. 2006;97:181–186. doi: 10.1016/j.amjcard.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Ix JH, Katz R, Peralta CA, de Boer IH, Allison MA, Bluemke DA, Siscovick DS, Lima JAC, Criqui MH. A High Ankle Brachial Index is Associated with Greater Left Ventricular Mass: The Multi-Ethnic Study of Atherosclerosis. Journal of the American College of Cardiology. 2010;55:342–349. doi: 10.1016/j.jacc.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wattanakit K, Folsom AR, Duprez DA, Weatherley BD, Hirsch AT. Clinical significance of a high ankle-brachial index: insights from the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2007;190:459–464. doi: 10.1016/j.atherosclerosis.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. The New England journal of medicine. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon RW. Dynamometer measurements of grip and knee extension strength: are they indicative of overall limb and trunk muscle strength? Percept Mot Skills. 2009;108:339–342. doi: 10.2466/PMS.108.2.339-342. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 29.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study—I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 30.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (Updating the 2005 Guideline)A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2011;58:2020–2045. doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr., White CJ, White J, White RA, Antman EM, Smith SC, Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 32.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of borderline and low normal ankle-brachial index values with functional decline at 5-year follow-up: the WALCS (Walking and Leg Circulation Study). Journal of the American College of Cardiology. 2009;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner G, Bismuth J, Nambi V, Ballantyne CM, Taylor AA, Lumsden AB, Morrisett JD, Shah DJ. Calf muscle perfusion as measured with magnetic resonance imaging to assess peripheral arterial disease. Med Biol Eng Comput. 2016;54:1667–1681. doi: 10.1007/s11517-016-1457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parmenter BJ, Raymond J, Dinnen PJ, Lusby RJ, Fiatarone Singh MA. Preliminary evidence that low ankle-brachial index is associated with reduced bilateral hip extensor strength and functional mobility in peripheral arterial disease. Journal of Vascular Surgery. 2013;57:963–973. e961. doi: 10.1016/j.jvs.2012.08.103. [DOI] [PubMed] [Google Scholar]
- 36.Gupta DK, Skali H, Claggett B, Kasabov R, Cheng S, Shah AM, Loehr LR, Heiss G, Nambi V, Aguilar D, Wruck LM, Matsushita K, Folsom AR, Rosamond WD, Solomon SD. Heart failure risk across the spectrum of ankle-brachial index: the ARIC study (Atherosclerosis Risk In Communities). JACC Heart Fail. 2014;2:447–454. doi: 10.1016/j.jchf.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. Journal of vascular surgery. 2008;48:1197–1203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Ix JH, Katz R, De Boer IH, Kestenbaum BR, Allison MA, Siscovick DS, Newman AB, Sarnak MJ, Shlipak MG, Criqui MH. Association of chronic kidney disease with the spectrum of ankle brachial index the CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2009;54:1176–1184. doi: 10.1016/j.jacc.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amoh-Tonto CA, Malik AR, Kondragunta V, Ali Z, Kullo IJ. Brachial-ankle pulse wave velocity is associated with walking distance in patients referred for peripheral arterial disease evaluation. Atherosclerosis. 2009;206:173–178. doi: 10.1016/j.atherosclerosis.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saba PS, Cameli M, Casalnuovo G, Ciccone MM, Ganau A, Maiello M, Modesti PA, Muiesan ML, Novo S, Palmiero P, Sanna GD, Scicchitano P, Pedrinelli R. Ventricularvascular coupling in hypertension: methodological considerations and clinical implications. J Cardiovasc Med (Hagerstown) 2014;15:773–787. doi: 10.2459/JCM.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 41.Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:342–348. doi: 10.7326/0003-4819-159-5-201309030-00008. [DOI] [PubMed] [Google Scholar]
- 42.Gardner AW. The effect of cigarette smoking on exercise capacity in patients with intermittent claudication. Vasc Med. 1996;1:181–186. doi: 10.1177/1358863X9600100302. [DOI] [PubMed] [Google Scholar]
- 43.Bendermacher BL, Willigendael EM, Nicolai SP, Kruidenier LM, Welten RJ, Hendriks E, Prins MH, Teijink JA, de Bie RA. Supervised exercise therapy for intermittent claudication in a community-based setting is as effective as clinic-based. J Vasc Surg. 2007;45:1192–1196. doi: 10.1016/j.jvs.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 44.Regensteiner JG, Ware JE, Jr., McCarthy WJ, Zhang P, Forbes WP, Heckman J, Hiatt WR. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. Journal of the American Geriatrics Society. 2002;50:1939–1946. doi: 10.1046/j.1532-5415.2002.50604.x. [DOI] [PubMed] [Google Scholar]
- 45.Regensteiner JG, Hargarten ME, Rutherford RB, Hiatt WR. Functional benefits of peripheral vascular bypass surgery for patients with intermittent claudication. Angiology. 1993;44:1–10. doi: 10.1177/000331979304400101. [DOI] [PubMed] [Google Scholar]
- 46.Fakhry F, Spronk S, van der Laan L, Wever JJ, Teijink JA, Hoffmann WH, Smits TM, van Brussel JP, Stultiens GN, Derom A, den Hoed PT, Ho GH, van Dijk LC, Verhofstad N, Orsini M, van Petersen A, Woltman K, Hulst I, van Sambeek MR, Rizopoulos D, Rouwet EV, Hunink MG. Endovascular Revascularization and Supervised Exercise for Peripheral Artery Disease and Intermittent Claudication: A Randomized Clinical Trial. JAMA. 2015;314:1936–1944. doi: 10.1001/jama.2015.14851. [DOI] [PubMed] [Google Scholar]
- 47.Ganesh SP, Fried LP, Taylor DH, Jr., Pieper CF, Hoenig HM. Lower extremity physical performance, self-reported mobility difficulty, and use of compensatory strategies for mobility by elderly women. Archives of physical medicine and rehabilitation. 2011;92:228–235. doi: 10.1016/j.apmr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.