Abstract
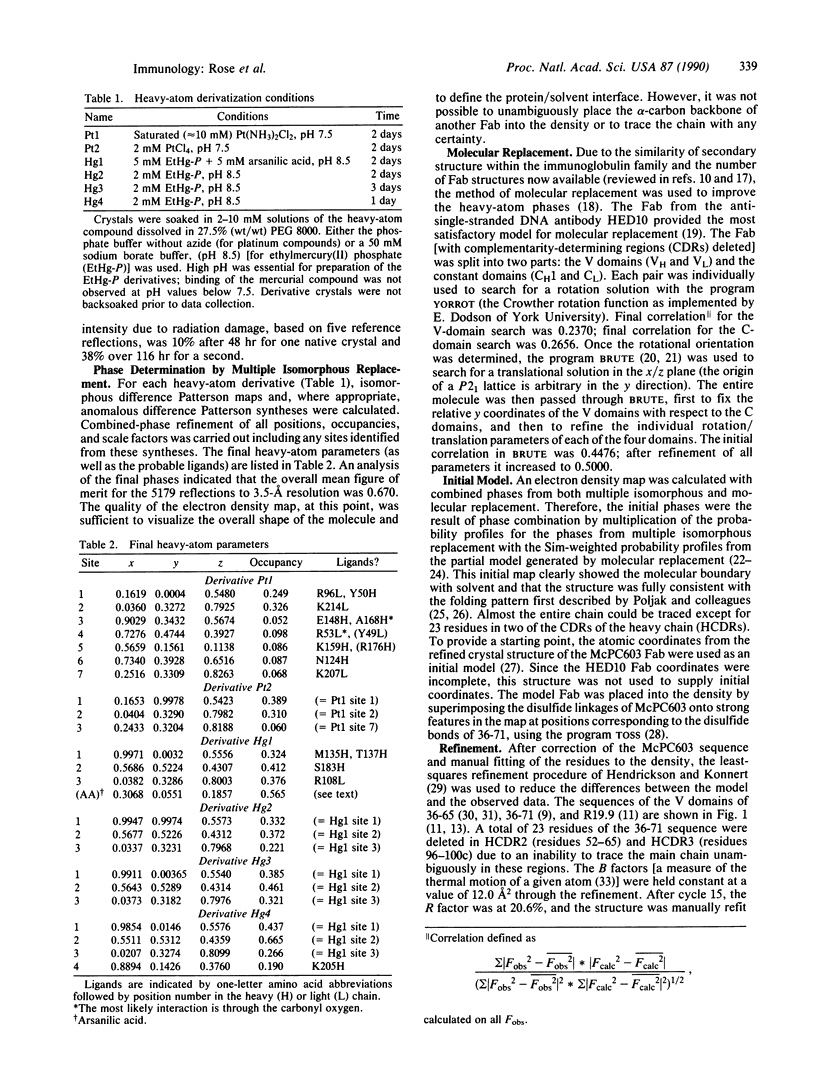
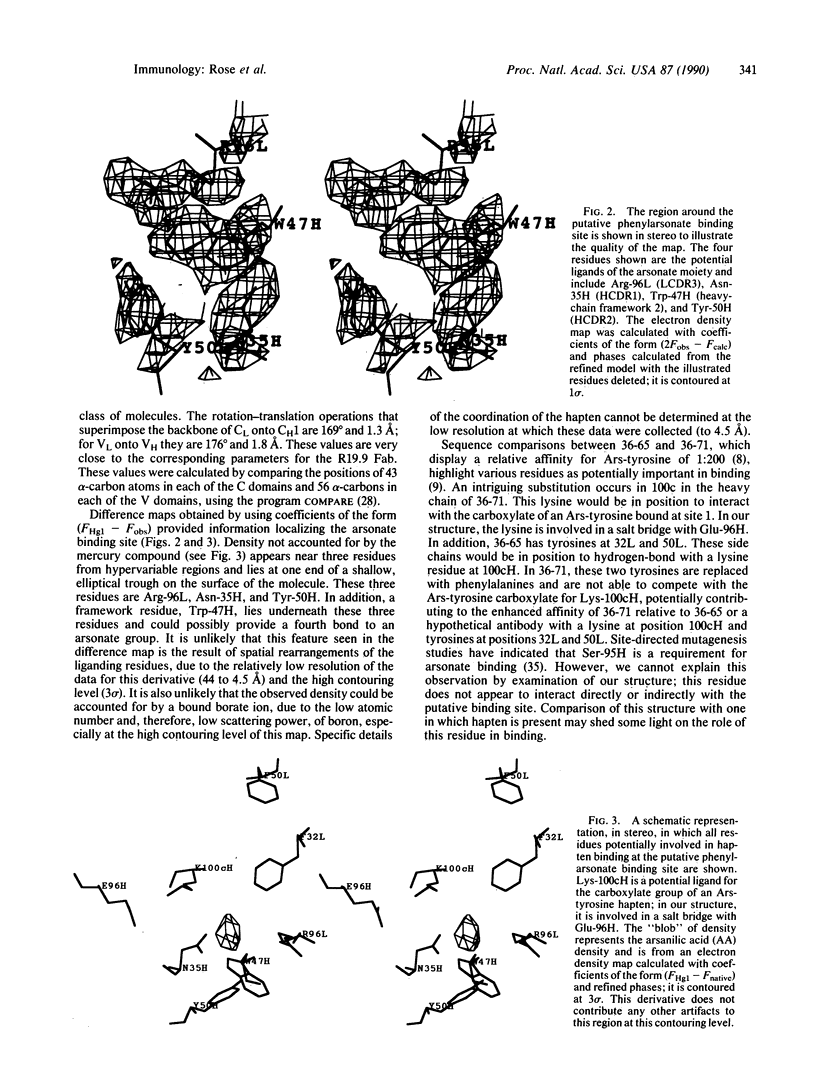
The structure of the antigen-binding fragment (Fab) of an anti-phenylarsonate monoclonal antibody (36-71) bearing a major crossreacting idiotype of A/J mice has been solved and refined to an R factor of 19.3% at a resolution of 2.9 A. An initial electron density map was obtained with phase information from a total of six isomorphous heavy-atom derivatives (from two different compounds) and a molecular replacement solution using the HED10 Fab crystal structure as a model. The structure of the McPC603 Fab was used to provide an initial set of atomic coordinates. The electron density maps are clear and easily interpretable for the entire sequence except for sections from two of the heavy-chain complementarity-determining regions totaling 21 residues. These residues have been left out of the refinement and are not represented in our current model. The antigen-combining site was located by means of a difference Fourier synthesis with one of the heavy-atom derivatives, which contained arsanilic acid. It lies in a small pocket formed by residues from the hypervariable regions of both the heavy and the light chains. Interactions with the hapten from framework residues are also possible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzari P. M., Lascombe M. B., Poljak R. J. Three-dimensional structure of antibodies. Annu Rev Immunol. 1988;6:555–580. doi: 10.1146/annurev.iy.06.040188.003011. [DOI] [PubMed] [Google Scholar]
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Cygler M., Boodhoo A., Lee J. S., Anderson W. F. Crystallization and structure determination of an autoimmune anti-poly(dT) immunoglobulin Fab fragment at 3.0 A resolution. J Biol Chem. 1987 Jan 15;262(2):643–648. [PubMed] [Google Scholar]
- Davies D. R., Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87–117. doi: 10.1146/annurev.iy.01.040183.000511. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Hodgkin D. C., Reynolds C. D. Structural relationships in the two-zinc insulin hexamer. Can J Biochem. 1979 Jun;57(6):469–479. doi: 10.1139/o79-060. [DOI] [PubMed] [Google Scholar]
- Gorini G., Medgyesi G. A., Doria G. Heterogeneity of mouse myeloma gamma G globulins as revealed by enzymatic proteolysis. J Immunol. 1969 Nov;103(5):1132–1142. [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascombe M. B., Alzari P. M., Boulot G., Saludjian P., Tougard P., Berek C., Haba S., Rosen E. M., Nisonoff A., Poljak R. J. Three-dimensional structure of Fab R19.9, a monoclonal murine antibody specific for the p-azobenzenearsonate group. Proc Natl Acad Sci U S A. 1989 Jan;86(2):607–611. doi: 10.1073/pnas.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Wysocki L. J., Margolies M. N., Gefter M. L. Evolution of antibody variable region structure during the immune response. Immunol Rev. 1987 Apr;96:141–162. doi: 10.1111/j.1600-065x.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Margolies M. N., Marshak-Rothstein A., Gefter M. L. Structural diversity among anti-p-azophenylarsonate monoclonal antibodies from A/J mice; comparison of Id- and Id+ sequences. Mol Immunol. 1981 Dec;18(12):1065–1077. doi: 10.1016/0161-5890(81)90022-5. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Margolies M. N., Benedetto J. D., Gefter M. L. Two structurally distinct and independently regulated idiotypic families associated with the A/J response to azophenylarsonate. Eur J Immunol. 1981 Jul;11(7):565–572. doi: 10.1002/eji.1830110709. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Siekevitz M., Margolies M. N., Mudgett-Hunter M., Gefter M. L. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun G., Sanz I., Meek K., Tucker P., Capra J. D. The molecular genetics of the arsonate idiotypic system of A/J mice. Adv Immunol. 1988;42:95–164. doi: 10.1016/s0065-2776(08)60843-3. [DOI] [PubMed] [Google Scholar]
- Rothstein T. L., Gefter M. L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983 Feb;20(2):161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Gefter M. L., Manser T., Ptashne M. Site-directed mutagenesis of an invariant amino acid residue at the variable-diversity segments junction of an antibody. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2628–2631. doi: 10.1073/pnas.83.8.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J., Gefter M. L., Wysocki L. J., Margolies M. N. Recurrent somatic mutations in mouse antibodies to p-azophenylarsonate increase affinity for hapten. J Immunol. 1989 Jan 15;142(2):596–601. [PubMed] [Google Scholar]
- Siekevitz M., Gefter M. L., Brodeur P., Riblet R., Marshak-Rothstein A. The genetic basis of antibody production: the dominant anti-arsonate idiotype response of the strain A mouse. Eur J Immunol. 1982 Dec;12(12):1023–1032. doi: 10.1002/eji.1830121208. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Gridley T., Huang S., Grandea A. G., 3rd, Gefter M. L. Single germline VH and V kappa genes encode predominating antibody variable regions elicited in strain A mice by immunization with p-azophenylarsonate. J Exp Med. 1987 Jul 1;166(1):1–11. doi: 10.1084/jem.166.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L., Manser T., Gefter M. L. Somatic evolution of variable region structures during an immune response. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1847–1851. doi: 10.1073/pnas.83.6.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]



