Abstract
The role of microglia in the pathophysiology of injury to the developing brain has been extensively studied. In children under the age of 4 who have sustained a traumatic brain injury (TBI), markers of microglial/macrophage activation were increased in the cerebrospinal fluid and were associated with worse neurologic outcome. Minocycline is an antibiotic that decreases microglial/macrophage activation following hypoxic-ischemia in neonatal rodents and TBI in adult rodents thereby reducing neurodegeneration and behavioral deficits. In study 1, 11-day-old rats received an impact to the intact skull and were treated for 3 days with minocycline. Immediately following termination of minocycline administration, microglial reactivity was reduced in the cortex and hippocampus (p<0.001) and was accompanied by an increase in the number of fluoro-Jade B profiles (p<0.001) suggestive of a reduced clearance of degenerating cells; however, this effect was not sustained at 7 days post-injury. Although microglial reactivity was reduced in the white matter tracts (p<0.001), minocycline treatment did not reduce axonal injury or degeneration. In the thalamus, minocycline treatment did not affect microglial reactivity, axonal injury and degeneration, and neurodegeneration. Injury-induced spatial learning and memory deficits were also not affected by minocycline. In study 2, to test whether extended dosing of minocycline may be necessary to reduce the ongoing pathologic alterations, a separate group of animals received minocycline for 9 days. Immediately following termination of treatment, microglial reactivity and neurodegeneration in all regions examined were exacerbated in minocycline-treated brain-injured animals compared to brain-injured animals that received vehicle (p<0.001), an effect that was only sustained in the cortex and hippocampus up to 15 days post-injury (p<0.001). Whereas injury-induced spatial learning deficits remained unaffected by minocycline treatment, memory deficits appeared to be significantly worse (p<0.05). Sex had minimal effects on either injury-induced alterations or the efficacy of minocycline treatment. Collectively, these data demonstrate the differential effects of minocycline in the immature brain following impact trauma and suggest that minocycline may not be an effective therapeutic strategy for TBI in the immature brain.
Keywords: Pediatric TBI, microglia, cognition, axonal injury, cortex, thalamus, subiculum, corpus callosum
Introduction
With close to half a million children affected annually, traumatic brain injury (TBI) remains one of the most common causes of disability and death in infants and children (Coronado et al., 2011; Faul, 2010; Langlois et al., 2005). The youngest age group (≤ 4 years old) exhibit worse outcome following moderate to severe TBI compared to older children (Anderson et al., 2005; Coronado et al., 2011). Pediatric TBI patients commonly exhibit traumatic axonal injury (TAI) and brain atrophy which are associated with prolonged cognitive deficits such as impairments of learning and memory, attention, and executive function (Anderson et al., 2005; Anderson et al., 2009; Catroppa et al., 2008; Catroppa et al., 2007; Duhaime and Raghupathi, 1999; Ewing-Cobbs et al., 2006; Ewing-Cobbs et al., 2004; Tong et al., 2004). Injury to the immature brain may also have adverse effects on the development of cognitive abilities (Babikian et al., 2015). Unfortunately, no specific therapies exist, with supportive care in the acute post-traumatic period being the only current treatment option.
While the mechanisms underlying neuropathologic alterations following TBI in the immature brain are not completely understood, inflammation may play an important role in the sequelae of secondary injury. Activation of microglia, the resident immuno-competent cells in the brain, is thought to play an important role in the acute and chronic neurodegeneration observed following brain injury (Beynon and Walker, 2012; Graeber and Streit, 2010; Hanisch and Kettenmann, 2007; Kreutzberg, 1996; Nimmerjahn et al., 2005; Ransohoff and Perry, 2009). Markers of microglial/macrophage activation such as sCD163, ferritin, and interleukins-6,-8 and −10 were increased in cerebrospinal fluid (CSF) from children after TBI with more prominent increases observed in the youngest age group (≤ 4 years of age), suggesting that these patients may be at higher risk for worse neurologic outcome (Bell et al., 1997; Newell et al., 2015; Whalen et al., 2000). In neonatal rodents, hypoxic-ischemia (HI) or ischemia resulted in robust microglial/macrophage activation (Denker et al., 2007; Ferrazzano et al., 2013; Ivacko et al., 1996; McRae et al., 1995; Vexler and Yenari, 2009). Increased microglial reactivity in the injured hemisphere following TBI in the immature mouse brain corresponded to areas containing degenerating neurons and was associated with an expansion of the cortical lesion and spatial learning deficits (Pullela et al., 2006; Tong et al., 2002). In addition, microglial reactivity has also been observed in the white matter tracts that was associated with an increase in tissue loss of the injured hemisphere and working and recognition memory deficits in a rabbit model of pediatric TBI (Zhang et al., 2015). These data suggest that microglial activity may be involved in ongoing pathogenesis following TBI in the immature brain and may potentially serve as a therapeutic target.
Minocycline is a second generation tetracycline derivative antibiotic with anti-inflammatory properties, effectively crosses the blood-brain barrier after systemic administration and has demonstrated neuroprotection in many models of brain injury and neurodegenerative diseases (Elewa et al., 2006; Garrido-Mesa et al., 2013; Kim and Suh, 2009; Plane et al., 2010). Following neonatal rodent models of HI, minocycline decreased microglial activation which was associated with a reduction in injury-induced neuronal damage, oligodendroglial cell death, hypomyelination, white matter atrophy and locomotor deficits (Cai et al., 2006; Carty et al., 2008; Cikla et al., 2016; Fan et al., 2006). In a model of pediatric cardiac arrest, acute treatment with minocycline reduced microglial activation along with neurodegeneration and apoptosis (Tang et al., 2010). Treatment with minocycline following TBI in the adult mouse resulted in a reduction of microglial activation and proliferation which was associated with a decrease in pro-inflammatory cytokine response, cerebral edema, lesion volume and attenuation of locomotor and spatial memory deficits (Homsi et al., 2009; Homsi et al., 2010; Siopi et al., 2011; Siopi et al., 2012). Similarly, minocycline administration following moderate-severe contusive trauma to the adult rat brain reduced microglial activation and improved behavioral function (Abdel Baki et al., 2010; Lam et al., 2013). In contrast, minocycline did not attenuate cell death, axonal injury or tissue loss despite reducing active microglia following either diffuse brain trauma in the adult mouse (Bye et al., 2007) or repetitive brain trauma in the neonate rat (Hanlon et al., 2016). Depleting the brain of its resident microglia exacerbates cell death following neonatal stroke (Faustino et al., 2011) but appears to not affect the extent of white matter injury following TBI (Bennett and Brody, 2014). It must be noted that not all effects of minocycline can be attributed to its effects on reducing microglial activation following brain injury. Fox et al., (2005) observed that minocycline administration following focal ischemia in the neonate rat did not reduce the extent of microglial activation but did reduce the volume of injury. Although microglial activation was not evaluated, minocycline treatment reduced both apoptotic and excitotoxic cell death following HI in the neonatal rat (Arvin et al., 2002), reduced caspase activation following contusive brain trauma in the adult mouse (Sanchez Mejia et al., 2001) and attenuated inflammatory protein expression in a rat model of mild blast TBI (Kovesdi et al., 2012). By contrast, minocycline worsened injury-induced infarction and tissue atrophy in a mouse model of HI (Tsuji et al., 2004). Collectively, these data underscore the complicated relationship between injury-induced microglial activation and ensuing brain damage.
With the goal of understanding the role of microglial activation in neonatal brain trauma, the present study sought to test the hypothesis that minocycline treatment following TBI will attenuate microglia reactivity along with neuronal and axonal degeneration leading to decreases in brain atrophy and a reversal of spatial learning and memory deficits. The effects of both short-term (3 days) and extended (9 days) administration of minocycline (45mg/Kg/dose) were tested in a well characterized model of single TBI in the 11-day-old rat. This injury results in neuronal and axonal degeneration, TAI, microglial reactivity, tissue atrophy and cognitive deficits that last up to 4 weeks post-injury (Raghupathi and Huh, 2007).
Methods
Brain Injury
All surgical and behavioral procedures were done in accordance with the rules and regulations of the Institutional Animal Care and Use Committee of Drexel University College of Medicine and were in compliance with the Guide for the Care and Use of Animals. Animals were placed on a heating pad set to 37°C to maintain body temperature throughout all procedures and recovery. Brain injuries were induced in isoflurane-anesthetized 11-day-old male and female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) using the electronically driven controlled cortical impact (eCCI) device (Custom Design International, Richmond, VA), as previously described (Raghupathi and Huh, 2007); the convex indenter (5mm diameter) was driven 3mm from the point of contact with the skull with a velocity of 5m/s and a dwell time of 100ms. Animals were injured at 45 seconds following the removal of anesthesia, and the total time from anesthesia to impact was 5 min; sham-injured animals were exposed to anesthesia for 4 min during surgical preparation and the zeroing of the impactor tip to the skull (which was not fired). On the day of injury, animals were randomly assigned to injury and treatment conditions. Animals had similar weights on the day of injury irrespective of sex and injury/treatment status (F2,129=0.76, p=0.38; Table 1). Apnea latency times were recorded for brain-injured animals from the time of impact to when they took their first breath. The loss of a righting reflex was measured by the length of time taken by the animal to return onto all four limbs after being placed on their side immediately following injury. Once the animal regained normal breathing, the skull was evaluated for the presence of fracture and herniation (if brain tissue was extruding via the fracture) and hematoma which was defined as mild (if the area of the hematoma was qualitatively smaller than the diameter of the impactor), moderate (if the area was as large as the diameter of the impactor or severe (if the area was larger than the diameter of the impactor) (Raghupathi and Huh, 2007). Pups were weaned from the dam on postnatal day 21.
Table 1.
Acute neurologic status of groups used in studies 1 and 2.
| Group | N | Sex | Body weight at injury (g) |
Apnea (s) |
Latency to Righting Reflex (s) |
Hematoma | Herniation | |
|---|---|---|---|---|---|---|---|---|
| Mild (N) | Moderate (N) | (N) | ||||||
| Sham-injured | 19 | Male | 23 ± 1 | n/a | 137 ± 21 | n/a | n/a | n/a |
| Sham-injured | 19 | Female | 22 ± 0 | n/a | 119 ± 14 | n/a | n/a | n/a |
| Brain-injured + vehicle | 21 | Male | 23 ± 1 | 13 ± 2 | 168 ± 23 | 11 | 8 | 2 |
| Brain-injured + vehicle | 20 | Female | 22 ± 0 | 13 ± 2 | 183 ± 18 | 9 | 7 | 4 |
| Brain-injured + minocycline |
29 | Male | 23 ± 1 | 14 ± 2 | 185 ± 17 | 11 | 16 | 2 |
| Brain-injured + minocycline |
25 | Female | 22 ± 1 | 15 ± 2 | 187 ± 22 | 9 | 13 | 3 |
For purposes of comparison, animals used in study 1 and 2 were combined. Apnea times and latency to regain righting reflex were calculated as described in the Methods. Values represent group Means and standard errors of the Mean.
Treatment paradigms
Study 1
Immediately following the injury, animals were randomly assigned to receive a 45mg/Kg dose of minocycline hydrochloride (Sigma, St. Louis MO) dissolved in phosphate-buffered saline (PBS), or vehicle (PBS, 0.2mL/Kg) via an intraperitoneal (i.p.) injection. Sham-injured animals were also randomly assigned to receive either vehicle or minocycline. Minocycline or vehicle was injected every 12 hours for 3 days for a total of 6 injections (45 mg/Kg/injection or 0.2 mL/Kg/injection). This dose and dosing paradigm was successful in reducing acute microglial activation in neonate repetitive TBI (Hanlon et al., 2016) and other models of neonate HI (Buller et al., 2009). Details of animal numbers as a function of outcome measure and time point are presented in Table 2.
Table 2.
Animal numbers used in studies 1 and 2 as a function of outcome measure and survival time point(s).
| Study | Outcome | Time point (N) | Excluded Animals |
|---|---|---|---|
| 1 | Histology | 3d (4S, 7 IV, 9 IM) | -------- |
| 7d (3S, 4 IV, 5 IM) | -------- | ||
| Spatial Learning |
10–14d (11S, 12 IV, 14 IM) |
1 IM died at 3d post-injury 1 IM and 1 SM displayed thigmotactic behavior and were excluded |
|
| 2 | Histology | 10d (5S, 5 IV, 6 IM) | --------- |
| 15d (7S, 6 IV, 6 IM) | --------- | ||
| Spatial Learning |
10–14d (14S, 14 IV, 20 IM) |
1 IV and 6 IM displayed thigmotactic behavior and were excluded |
Sham- and brain-injured animals were randomly assigned to receive either vehicle or minocycline as described in the Methods. Included are the animals that were excluded from the study due to either mortality or behavioral abnormality. S, sham; IV, injured + vehicle; IM, injured + minocycline.
Study 2
Immediately after injury, animals were randomly assigned to receive intraperitoneal injections of either minocycline (45mg/Kg) or PBS vehicle (0.2mL/Kg); following a second injection of minocycline or vehicle 12 hours later, animals received once daily intraperitoneal injections of minocycline or vehicle (45mg/Kg/injection or 0.2mL/Kg/injection) for 9 days for a total of 11 injections. Sham-injured animals were also randomly assigned to receive either vehicle or minocycline. Similar extended dosing paradigms have been successful in reducing microglial activation in the chronic post-injury period following HI in neonate rats (Carty et al., 2008; Wixey et al., 2011), diffuse TBI in the adult mouse (Ng et al., 2012) and contusive TBI in the adult rat (Lam et al., 2013). Details of animal numbers as a function of outcome measure and time point are presented in Table 2.
Histologic and immunohistochemical staining and quantification
Animals were transcardially perfused with 10% formalin (Thermofisher), and brains were processed for histology and immunohistochemistry as previously described (Hanlon et al., 2016; Huh et al., 2007). Adjacent sets of sections (500µm apart), representing the rostral-caudal extent of the injury from Bregma to 5.6mm posterior to bregma, were mounted on gelatin-coated slides and stained for Fluoro-Jade B (FJB) (Huh et al., 2008) and Nissl-myelin (2% Cresyl violet and 0.2% Cyanine R) respectively. The areas of the cortex (from midline to rhinal fissure) of the injured hemisphere and white matter (corpus callosum, cingulum and lateral aspects up to the rhinal fissure) were measured in Nissl-myelin-labeled sections via manual tracing using Image J software (NIH) (Hanlon et al., 2016). Additional sets of sections were evaluated for microglia/macrophages using anti-ionized calcium-binding adaptor molecule 1 (Iba1, Wako, Richmond, VA, 1:20,000) and traumatic axonal injury (TAI) using a polyclonal antibody to the C-terminal end of amyloid precursor protein (APP, Zymed, San Francisco, CA, 1:2,000) as previously described (Hanlon et al., 2016). Quantification in the cortex (layers 2 through 5 of the retrosplenial, motor and somatosensory cortices), hippocampus (dorsal subiculum) and thalamus (dorsolateral and lateral geniculate nuclei) was conducted by counting labeled profiles (Iba1, FJB, APP) in 3–5 high power field (HPF) images (20x magnification) per section across 5 non-adjacent sections. Counts of Iba1(+) profiles were based on the presence of a discernable cell body with elongated processes (resting) or an amoeboid appearance (activated); Iba1(+) profiles that exhibited an activated morphology were counted and presented as a percent of total Iba1(+) profiles in that region. Fluoro-Jade B(+) and APP(+) profiles (regardless of size) were counted in 3 HPF images (20x magnification) per section that covered the area between the corpus callosum, cingulum and the lateral aspects of the white matter up to the rhinal fissure across 5 sections. The high density of Iba1 immunoreactive profiles in these white matter tracts required quantification using a thresholding-based area analysis as previously described (Hanlon et al., 2016; Ng et al., 2012). Quantification was performed by co-authors (LAH, JWH) blinded to both injury and treatment status.
Spatial Learning and Memory Assessment
Spatial learning was assessed on days 10–13 after sham- or brain injury using the Morris water maze as previously described (Hanlon et al., 2016; Huh et al., 2008). On day 14 post-injury, animals underwent probe trials (retention) and a visible platform trial (vision deficit testing) as previously described (Hanlon et al., 2016). Animals that had problems completing the learning trials were excluded from the study and are identified in Table 2.
Statistical Analysis
All statistics were performed using Statistica 7 (StatSoft, Tulsa OK). All data are presented as mean ± standard error of the mean. As previously observed (Hanlon et al., 2016), sham-injured animals that received vehicle injections were not statistically different from those that were treated with minocycline in any of the various histologic and behavioral outcome measures and were therefore combined for statistical purposes. Outcome measures were compared across groups (sham, injured + vehicle, injured + minocycline) as a function of time (when applicable) and sex using appropriate analyses of variance (ANOVA). When necessary, post-hoc analyses were performed using the Newman-Keuls test and a value of p≤0.05 was considered significant.
Results
Acute responses to the injury
Impact to the intact skull of 11-day-old rats resulted in a skull fracture and hematoma in brain-injured animals that were designated to receive either minocycline or vehicle. Chi-square analysis revealed that the severity of hematoma and herniation did not differ between brain-injured animals that went on to receive vehicle and those that were treated with minocycline (X2(2)=2.82, p=0.24; Table 1). Hematoma severity did not differ between brain-injured male and female animals (X2(2)=1.52, p=0.47; Table 1). Brain trauma resulted in a brief period of apnea that was similar in male and female animals and did not differ between the groups that were designated for treatment with minocycline and those that were to receive vehicle injections (two-way ANOVA, F1,91=0.53, p=0.47; Table 1). Brain-injured animals were unable to right themselves from a prone position immediately after the impact and a two-way ANOVA of times to regain righting revealed a main effect of group (F2,126=4.85, p<0.01; Table 1), but no interaction between group and sex (F2,126=0.34, p=0.71); post-hoc analysis indicated that both groups of brain-injured animals took significantly longer to right themselves compared to sham-injured animals (p<0.05). No animals died as a result of anesthesia and/or injury, but in study 1, one brain-injured animal that was treated with minocycline died at the end of 3 days (Table 2).
Study 1: Effect of minocycline in the cortex
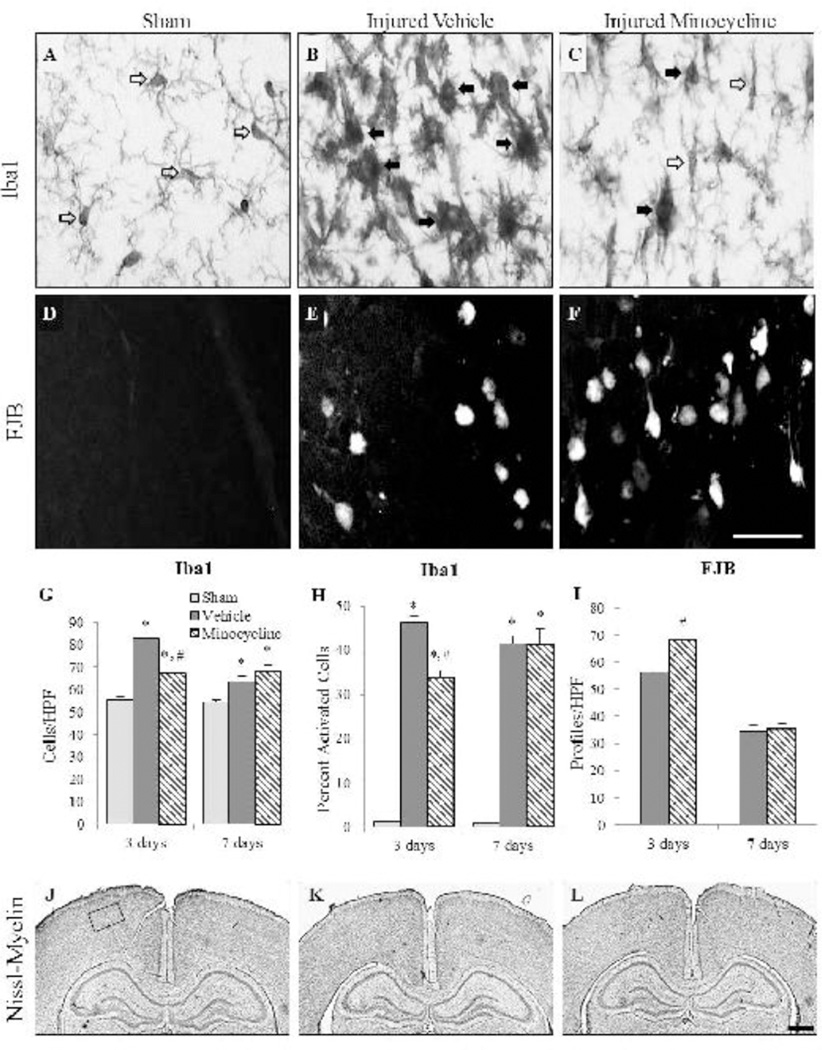
In sham-injured animals and in brain-injured minocycline-treated animals, Iba1-positive microglia displayed processes suggestive of a “resting” phenotype (Fig. 1A and 1C, open arrows). Brain-injured animals that received either vehicle or minocycline injections contained “activated” microglia, in which processes were fewer and/or shorter and cell bodies were larger (Fig. 1B and 1C, filled arrows), in the retrosplenial cortex and through all layers of the motor and somatosensory cortices. Quantification of the number of Iba1(+) microglia in the cortex at both 3 and 7 days post-injury revealed an interaction effect between group and time (F2,20=13.73, p<0.001; Fig. 1G) with a post-hoc analysis indicating that both brain-injured groups contained more microglia compared to the time-matched sham-injured animals, and that minocycline-treated, brain-injured animals had significantly fewer total microglia than brain-injured animals that received vehicle; the latter effect was only noted at 3 days post-injury (p<0.001), but not at 7 days post-injury (p=0.39; Fig. 1G). Sex, when used as an independent variable, demonstrated no interaction with group or time (F2,20=0.06, p=0.94). Similarly, quantitative analyses of activated microglia revealed an interaction effect between group and time (F2,20=4.88, p<0.05; Fig. 1H) with the post-hoc analysis indicating that minocycline treatment was only effective in decreasing the proportion of activated microglia in brain-injured animals at 3 days post-injury (p<0.01); sex of the animals did not influence the interaction between group and time (F2,20=0.01, p=0.99). In cortical areas that contained reactive microglia, FJB-labeled degenerating neurons were observed (Fig. 1E and 1F); there were no FJB(+) profiles in the sham-injured animals (Fig. 1D). A three-way ANOVA revealed an interaction effect between treatment status and time (F1,17=14.47, p<0.01; Fig. 1I) and minocycline-treated, brain-injured animals had significantly more FJB+ profiles than brain-injured animals (p<0.001) at 3 days post-injury but not at 7 days post-injury (p=0.61); sex of the animals did not affect FJB reactivity in the cortex (F1,17=0.79, p=0.39). Impact to the intact skull of either the neonate male or the female rat did not result in an overt lesion or cavitation of the underlying cortex (Fig. 1K and 1L). Cortical layers in sham-injured (Fig. 1J) and in brain-injured animals that received vehicle (Fig. 1K) or minocycline (Fig. 1L) demonstrated typical cellular labeling.
FIGURE 1. Effect of short-term minocycline administration in the cortex.
Representative photomicrographs illustrate Iba1(+) microglia (A–C) and FJB(+) profiles (D–F) in sham-injured animals (A,D), brain-injured animals that received vehicle (B,E), and brain-injured, minocycline-treated animals (C,F). Open arrows denote resting microglia while the closed arrows denote an activated phenotype. Graphs illustrate counts of Iba1(+) cells (G), the proportion of activated Iba1(+) cells (H), and counts of FJB(+) profiles (I). Representative photomicrographs of Nissl-myelin stained sections from sham-injured (J), brain-injured animals injected with vehicle (K), and brain-injured, minocycline-treated animals (L) at 3 days post-injury. As described in the results, there was no effect of sex so values for male and female rats were combined for graphical representation. *, p ≤ 0.05 compared to sham-injured values; #, p ≤ 0.05 compared to animals that received vehicle. HPF, high power field. Scale bar for panels A-F in panel F=50µm; scale bar for panels J-L in panel L=500µm. Box in panel J indicates the area of the cortex represented in panels A-F.
Study 1: Effect of minocycline in the hippocampus
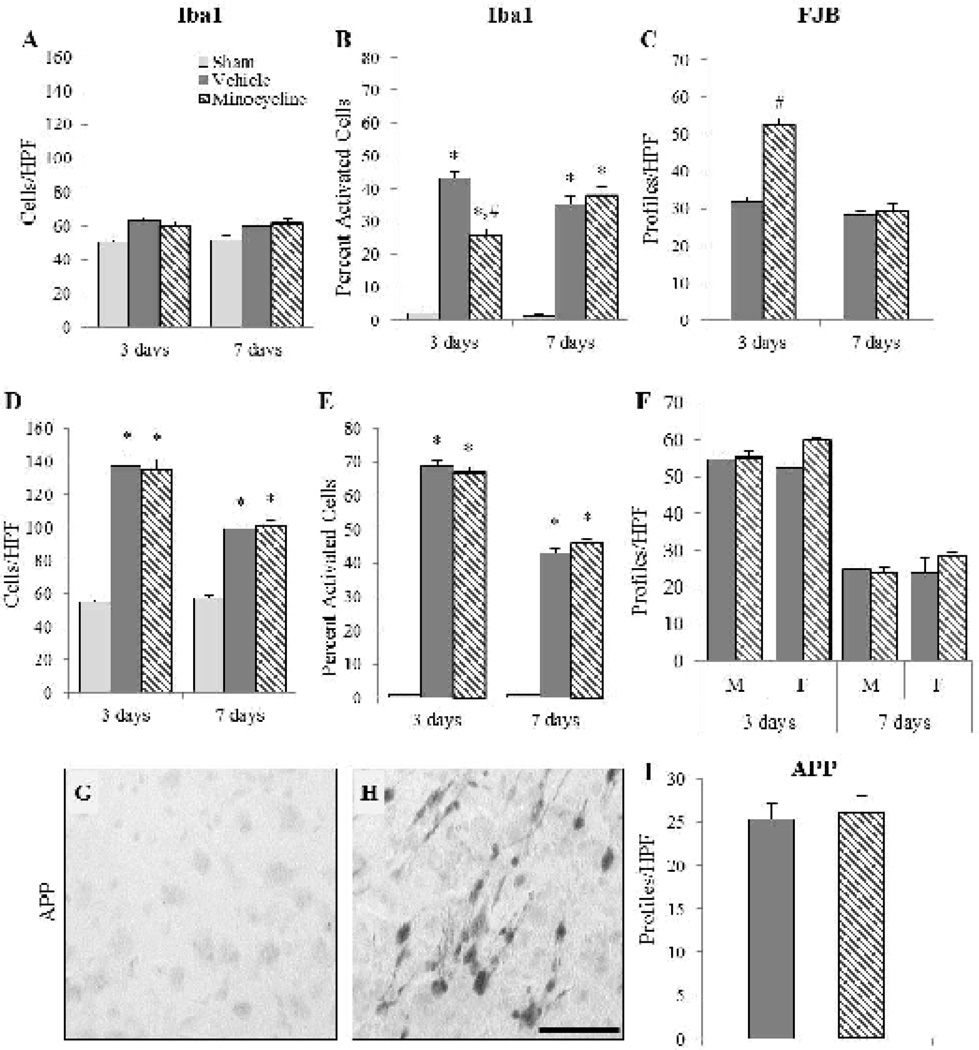
Closed head impact in the neonate rat resulted in neurodegeneration and microglial reactivity in the hippocampus and was restricted to the dorsal aspect of the subiculum. As observed in the cortex, most Iba1(+) microglia in the sham-injured animals exhibited the typical “resting” morphology, whereas in the brain-injured animals activated microglia were also observed (not shown). Quantification of Iba1(+) microglia only revealed a group effect (F2,20=8.44, p<0.01; Fig. 2A), with more microglia in brain-injured animals compared to sham-injured animals (p<0.001); however there was no difference between the two brain-injured groups (p=0.77). By contrast, analysis of the proportion of activated microglia indicated an interaction effect between group and time (F2,20=9.45, p<0.01; Fig. 2B). Brain-injured animals had a significantly higher proportion of activated microglia than sham-injured animals (p<0.001) and minocycline-treated brain-injured animals had a lower percent of activated microglia than brain-injured animals that were injected with vehicle (p<0.001). As was observed in the cortex, minocycline treatment only decreased the proportion of activated microglia in brain-injured animals at 3 days post-injury (p<0.001) and not at 7 days post-injury (p=0.47). The sex of the animals did not affect either the total amount of Iba1 (+) microglia (F2,20=0.30, p=0.74) or the proportion of activated microglia (F2,20=0.34, p=0.72) in the subiculum. Quantification of FJB(+) profiles revealed an effect between treatment status and time (F1,17=44.45, p<0.001; Fig. 2C) with minocycline treatment increasing the number of FJB(+) cells at 3 days (p<0.001) but not at 7 days post-injury (p=0.48; Fig. 2C). Sex of the animals did not influence FJB reactivity in the subiculum (F1,17=1.43, p=0.25).
FIGURE 2. Effect of short-term minocycline administration in the hippocampus and thalamus.
Graphs illustrate counts of Iba1(+) cells (A,D), the proportion of activated Iba1(+) cells (B,E), and counts of FJB(+) profiles (C,F) in the hippocampus (A–C) and the thalamus (D–F). Representative photomicrographs illustrating intra-axonal accumulation of amyloid precursor protein (APP) in the thalamus of a brain-injured animal injected with vehicle (H); note the lack of labeling in the sham-injured animal (G). (I) Graph illustrating counts of APP(+) profiles. In panels A–E, there was no effect of sex so values for male and female rats were combined for graphical representation. *, p ≤ 0.05 compared to sham-injured values; #, p ≤ 0.05 compared to brain-injured animals that received vehicle. HPF, high power field. Scale bar=50µm.
Study 1: Effect of minocycline in the thalamus
In the thalamus of brain-injured animals, reactive microglia (not shown), fluoro-Jade B(+) profiles (not shown) and evidence of TAI (Fig. 2H) were observed within the dorsolateral and lateral geniculate nuclei. Brain-injured animals, irrespective of treatment condition, had significantly more Iba(+) microglia (F2,20=5.55, p<0.05; Fig. 2D) and a significantly greater proportion of activated Iba1(+) microglia (F2,20=18.44, p<0.001; Fig. 2E) than sham-injured animals. Brain-injured animals treated with minocycline had similar numbers of microglia and a similar proportion of activated microglia compared with brain-injured animals that received vehicle at both 3 and 7 days post-injury (total: 3 days, p=0.80; 7 days, p=0.79; proportion: 3 days, p=0.47; 7 days, p=0.35; Fig. 2D and 2E). Neither the total Iba1(+) microglia (F2,20=0.10, p=0.90) nor the proportion of activated Iba1(+) microglia (F2,20=0.79, p=0.47) was affected by the sex of the brain-injured animals. Quantification of FJB+ profiles revealed a significant effect of treatment status (F1,17=4.45, p<0.05) that indicated that minocycline-treated brain-injured animals had significantly more FJB+ profiles compared to brain-injured animals that received vehicle irrespective of sex or time (p<0.05; Fig. 2F). There was, however, an interaction effect between sex and treatment status (F1,17=6.143, p<0.05) that demonstrated that female brain-injured animals that received the vehicle had fewer FJB+ reactivity than male animals that received the vehicle and female minocycline-treated animals (p<0.05, Fig. 2F). Evidence of traumatic axonal injury (TAI) as revealed by the presence of amyloid precursor protein (APP) accumulation within axons was observed only at 3 days post-injury (Fig. 2H) and not in sham-injured animals (Fig. 2G). Analysis of the number of APP(+) profiles indicated no effect of treatment status or sex (F1,5=2.90, p=0.15, Fig. 2I).
Study 1: Effect of minocycline in white matter tracts
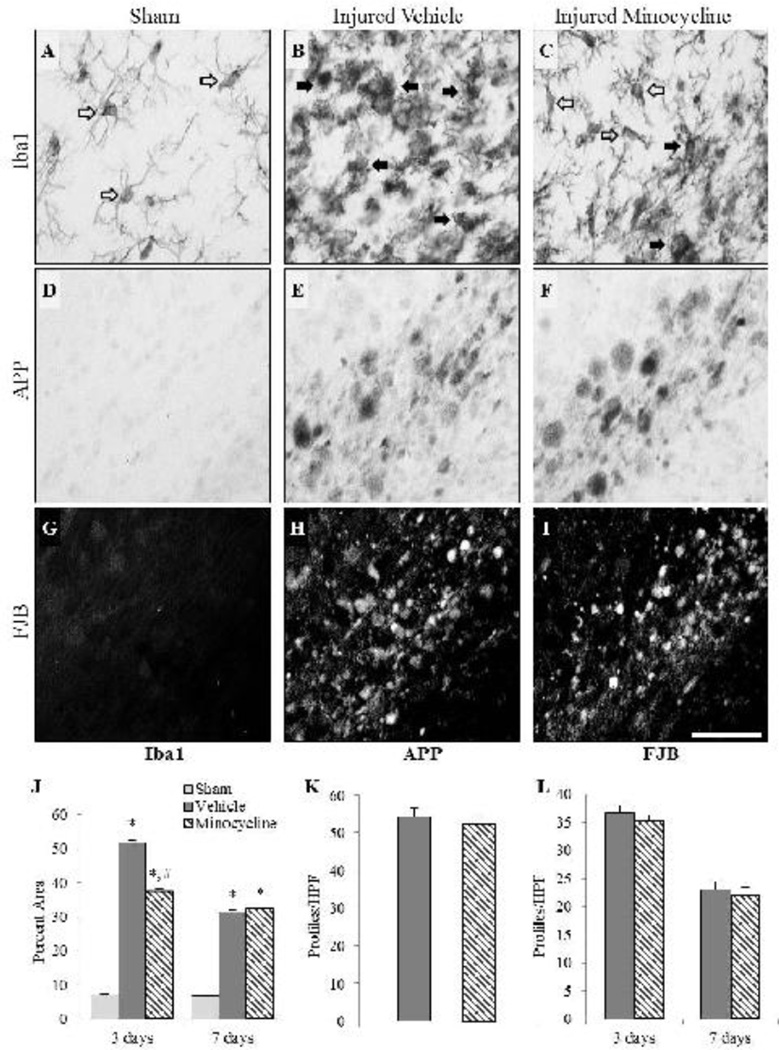
In sham-injured animals, Iba1-labeled microglia in the corpus callosum, cingulum and white matter tracts below the site of impact were sparse and displayed a large number of processes (Fig. 3A, open arrows). At 3 days post-injury, reactive microglia (Fig. 3B and 3C, filled arrows) were observed as extremely dense labeling, although some Iba1(+) cells in minocycline-treated, brain-injured animals, exhibited visible processes (Fig. 3C, open arrows). Regions containing reactive microglia also demonstrated injured axons exhibiting intra-axonal accumulation of APP (Fig. 3E and 3F) whereas sham-injured animals did not exhibit APP immunoreactivity (Fig. 3D). Fluoro-Jade B(+) profiles were observed at both 3 (Fig. 3H and 3I) and 7 days post-injury (not shown) in a diffuse and punctate pattern suggestive of axonal degeneration; no FJB+ labeling was observed in sham-injured animals (Fig. 3G). Quantitative analysis of Iba1(+) immunoreactivity revealed an interaction effect between group and time (F2,20=86.23, p<0.001), wherein a post-hoc analysis indicated that both brain-injured groups had a significantly larger Iba1 immunoreactive area than that in sham-injured animals (p<0.001) and that minocycline-treated brain-injured animals had significantly smaller labeled areas at 3 days post-injury (p<0.001) but not at 7 days post-injury (p=0.45) compared to brain-injured animals injected with vehicle (Fig. 3J). Despite this effect on Iba1 immunoreactivity, there was no effect of minocycline treatment on the number of APP+ profiles in brain-injured animals (F1,5=0.74, p=0.43; Fig. 3K). Additionally, minocycline treatment did not alter injury-induced FJB reactivity (F1,17=0.007, p=0.93; Fig. 3L). The sex of the animals failed to demonstrate any interaction effects with group or time with regards to Iba1 (F2,20=0.82, p=0.46), APP (F1,5=0.74, p=0.43) or FJB reactivity (F1,17=0.01, p=0.93) in the white matter tracts. The area of the white matter below the impact site in brain-injured animals was significantly smaller compared to that in sham-injured animals at both 3 and 7 days post-injury (F2,20=10.90, p<0.001) and was unaffected by treatment with minocycline at either 3 days (p=0.47) or 7 days post-injury (p=0.77) (data not shown) and sex (F2,20=0.09, p=0.91).
FIGURE 3. Effect of short-term minocycline administration in subcortical white matter tracts.
Representative photomicrographs illustrate Iba1(+) immunoreactivity (A–C), intra-axonal APP accumulation (D–F), and FJB(+) profiles (G–I) in sham-injured (A,D,G), brain-injured animals injected with vehicle (B,E,H), and brain-injured, minocycline-treated animals (C,F,I). Open arrows denote resting microglia while the closed arrows denote an activated phenotype. Graphs illustrate the area of Iba1 immunoreactivity (J), and the numbers of APP(+)(K) and FJB(+) profiles (L). Sex did not influence any of the outcomes and therefore, values for male and female rats were combined for graphical representation. *, p ≤ 0.05 compared to sham-injured values; #, p ≤ 0.05 compared to brain-injured animals receiving vehicle. HPF, high power field. Scale bar for all panels=50µm.
Study 1: Effect of minocycline on spatial learning and memory
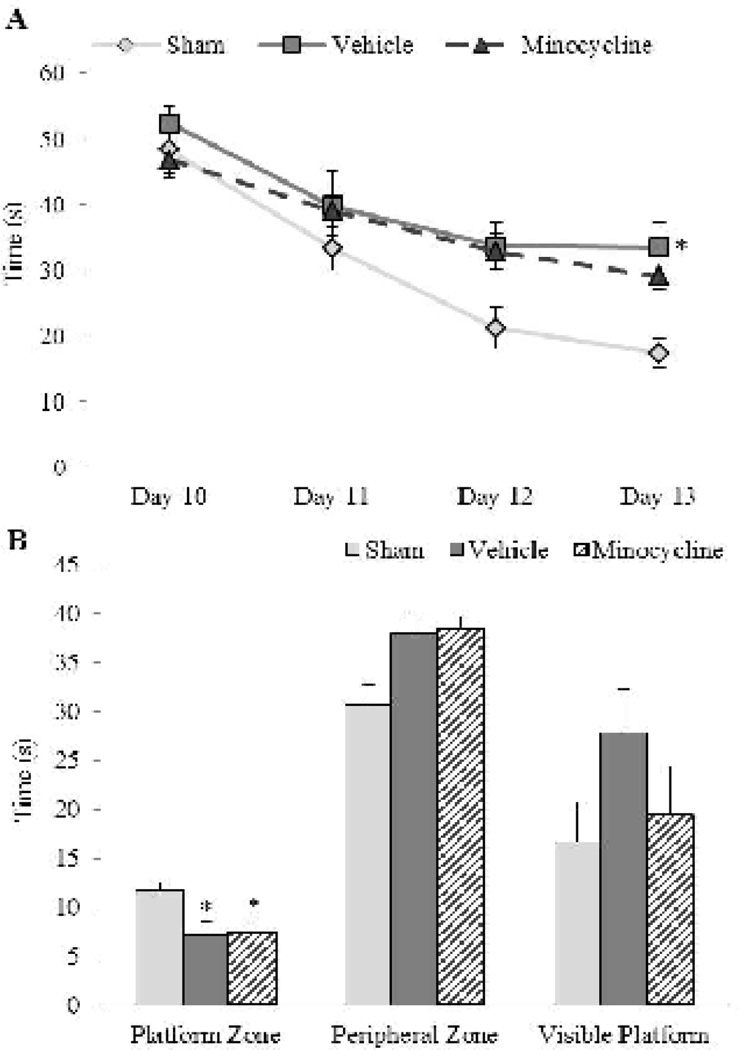
Brain injury resulted in a spatial learning deficit based on the observation that brain-injured animals exhibited an increased latency to locate the hidden platform compared to sham-injured animals (Fig. 4A). A repeated measures ANOVA revealed main effects of group (F2,84=3.14, p=0.05) and training day (F3,84=35.51, p<0.001), but no interaction effect between group and training day (F6,84=0.97, p=0.45) or between group, training day, and sex (F6,84=1.02, p=0.42; Fig. 4A). Post-hoc analysis indicated that sham-injured animals took a shorter amount of time to locate the hidden platform compared to brain-injured animals that received vehicle (p<0.05), but not those that received minocycline (p=0.06). However, both groups of brain-injured animals had similar latencies to the hidden platform across treatment days (p=0.42) indicating that minocycline treatment had no effect on injury-induced spatial learning deficits. The decreased efficiency in learning the location of the platform was associated with a retention deficit based on the observation that brain-injured animals spent less time in the platform zone (F2,28=3.41 p<0.05; Fig. 4B) that was not affected by treatment or sex (F2,28=0.31, p=0.73). There was no difference in the amount of time spent in the peripheral zone between brain- and sham-injured animals (F2,28=2.61, p=0.09) possibly because female rats, irrespective of injury/treatment status, spent significantly more time in the peripheral zone compared to male rats (F1,28=4.21, p<0.05, Fig. 4B). Importantly, the learning and memory deficits appeared not to be due to problems in vision based on the lack of either a group or sex effect in the visible platform trial (F2,28=1.67, p=0.21; Fig. 4B) or problems in motor function based on similar swim speeds in sham- and brain-injured male and female rats (sham-injured: 23 ± 0.84 cm/s; brain-injured vehicle: 24 ± 1.39 cm/s; brain-injured minocycline: 21 ± 0.88 cm/s; F2,28=0.49, p=0.62).
FIGURE 4. Effect of short-term minocycline administration on injury-induced spatial learning and memory deficits.
(A) Latency to the platform on each day represents the average of 4 trials. (B) Average times spent in the area surrounding the platform location and the periphery of the maze during the spatial retention (probe) trial. Also included is the time to the visible platform. Latencies to the platform and times spent in the various zones of the maze were unaffected by sex so values for male and female rats were combined for graphical representation. *, p ≤ 0.05 compared to sham-injured animals.
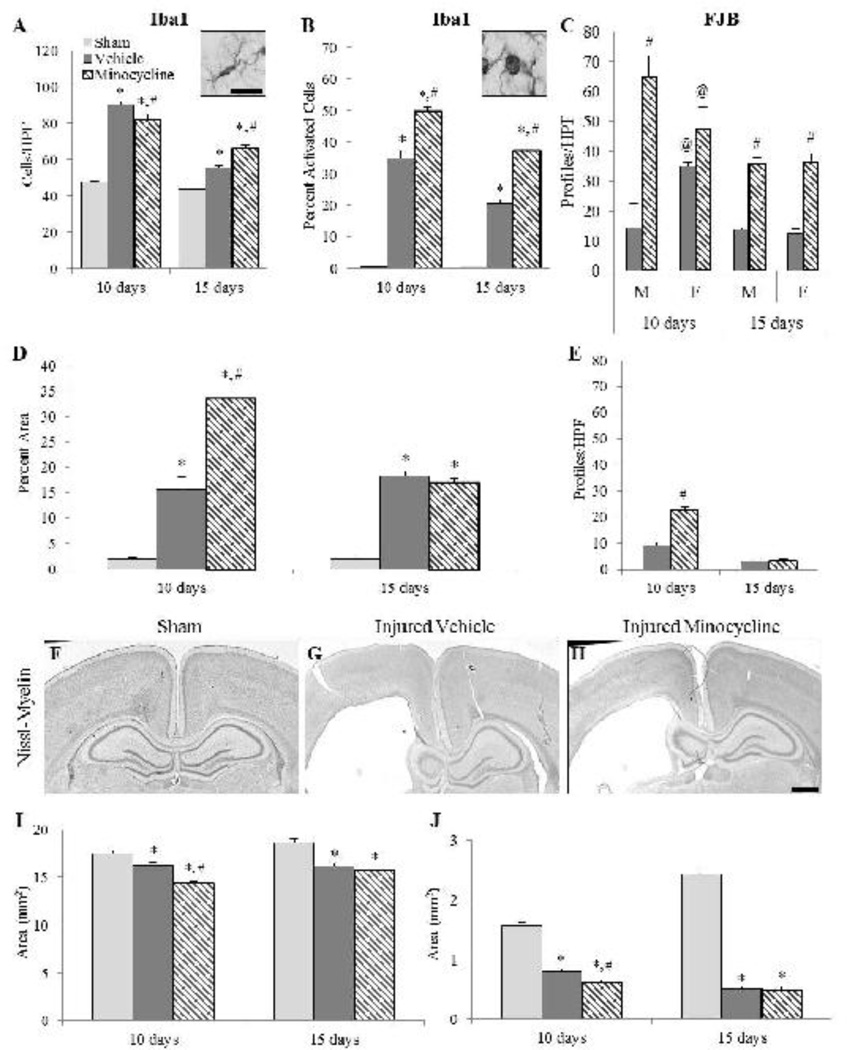
Study 2: Effect of minocycline in the cortex and white matter tracts
At 10 and 15 days post-injury, brain-injured animals demonstrated evidence of microglial activation and FJB reactivity in the same areas of the cortex and white matter tracts (Fig. 5) as those observed at 3 and 7 days post-injury. In sham-injured animals, resting Iba1 (+) microglia with elongated cell bodies and long processes predominated (Fig. 5A inset), whereas Iba1(+) microglia in brain-injured animals, were amoeboid in appearance (Fig. 5B inset). The effects of extending minocycline administration to 9 days following brain injury (study 2) on microglial reactivity and neurodegeneration were different from the effects of the short duration treatment paradigm used in study 1. In the cortex, a 3-way ANOVA revealed an interaction effect only between group and time (F2,23=32.13, p<0.001; Fig. 5A), and not between group, time and sex (F2,23=0.05, p=0.96); post-hoc analysis indicated that at 10 days post-injury, minocycline-treated, brain-injured animals had significantly fewer microglia compared to vehicle-treated brain-injured animals (p<0.01; Fig. 5A). In contrast, at the 15-day time point, the number of Iba1(+) cells in the minocycline group was significantly greater than that in the vehicle group (p<0.001; Fig. 5A). Analysis of the proportion of activated microglia also demonstrated an interaction effect between group and time (F2,23=10.11, p<0.001) with minocycline-treated, brain-injured animals exhibiting a significantly greater proportion compared to their counterparts that received vehicle at both 10 (p<0.001; Fig. 5B) and 15 days (p<0.001, Fig. 5B). Again, sex of the animal had no interaction effect with group or time (F2,23=0.15, p=0.87). Minocycline-treated, brain-injured animals contained significantly more FJB(+) profiles compared to brain-injured animals that received vehicle (F1,15=76.78, p<0.001; Fig. 5C). There was an interaction effect between group, sex and time (F1,15=10.29, p<0.01) that indicated that at 10 days post-injury, male minocycline-treated, brain-injured animals had significantly more FJB(+) profiles in the cortex compared to male brain-injured animals that received the vehicle (p<0.001; Fig. 5C). FJB reactivity was greater in the cortex of brain-injured females that received the vehicle compared to their male counterparts at 10 days (p<0.01), but female brain-injured minocycline-treated animals had significantly fewer FJB(+) profiles in the cortex compared to their male counterparts at 10 days (p<0.05). Female brain-injured animals demonstrated similar FJB reactivity irrespective of treatment at 10 days post-injury (p=0.24). At 15 days, however, both male and female brain-injured minocycline-treated animals had significantly more FJB(+) profiles than their brain-injured counterparts that received the vehicle (p<0.05).
FIGURE 5. Effect of extended minocycline administration in the cortex and subcortical white matter tracts.
Graphs illustrate counts of Iba1(+) cells (A), the proportion of activated Iba1(+) cells (B), and counts of FJB(+) profiles (C) in the cortex, area of Iba1 immunoreactivity (D), and the number of FJB(+) profiles (E) in the subcortical white matter. Inset images in panels A and B are representative of Iba1 (+) cells from sham-injured and brain-injured animals, respectively. Representative photomicrographs of Nissl-myelin stained sections from sham-injured (F), brain-injured injected with vehicle (G), and brain-injured, minocycline-treated animals (H) at 10 days post-injury. Graphs illustrate the area of the cortex (I) and the underlying white matter (J). The sex of the animals affected the values for FJB profile counts (C) but did not affect the quantification in other outcomes and areas. *, p ≤ 0.05 compared to sham-injured values; #, p ≤ 0.05 compared to brain-injured animals receiving vehicle; @, p ≤ 0.05 compared to corresponding males. HPF, high power field. Scale bar for insets in panels A and B in panel A=10µm; scale bar for panels F-H in panel H=500µm.
In the white matter tracts below the site of impact, area analysis of Iba1 labeling revealed an interaction effect between group and time (F2,23=71.6, p<0.001) with a post-hoc analysis indicating that minocycline-treated, brain-injured animals had significantly greater labeled area at 10 days post-injury (p<0.001), but not at 15 days (p=0.30), than brain-injured animals that received vehicle (Fig. 5D). There was an interaction effect between sex and condition that indicated that male brain-injured animals that received the vehicle had significantly decreased labeled white matter area compared to female brain-injured animals that received the vehicle (p<0.001) while brain-injured minocycline-treated males had significantly increased labeled white matter area compared to brain-injured minocycline-treated females (p<0.01). Furthermore, both brain-injured minocycline-treated males and females demonstrated increased white matter area compared to their counterparts that received the vehicle (males, p<0.001; females, p<0.01). Minocycline-treated, brain-injured animals had significantly more FJB+ profiles at 10 days (p<0.001), but not at 15 days (p=0.91) post-injury, than their counterparts that received vehicle (Fig. 5E) and this was not affected by the sex of the animals.
Compared to sham-injured animals (Fig. 5F), brain-injured animals exhibited a substantially enlarged lateral ventricle in the hemisphere ipsilateral to the impact site at 10 (Fig. 5G and 5H) and 15 days post-injury (not shown). Moreover, both the cortex and the underlying white matter tracts were visibly thinner in the brain-injured animals. Quantitative analysis of the area of the cortex revealed an interaction effect between group and time post-injury (F2,22=4.70, p<0.05; Fig. 5I); post-hoc analysis indicated that at 10 days post-injury both brain-injured groups exhibited smaller cortical areas compared to the sham-injured group (p<0.01). Importantly, the area of the cortex in the brain-injured group that was treated with minocycline was significantly less than that in brain-injured animals that received vehicle (p<0.001; Fig. 5I). At 15 days post-injury, the cortical areas in both brain-injured groups were significantly less than in sham-injured animals (p<0.001; Fig. 5I) but the minocycline-treated group was not different from the group that received vehicle (p=0.31). An interaction effect between group, sex, and time was observed (F2,22=3.42, p=0.05) that indicated that sham-injured males had significantly increased cortical area compared to sham-injured females (p<0.05) at 15 days post-injury. In sham-injured animals and in the hemisphere contralateral to the impact site in brain-injured animals, robust myelin labeling in the corpus callosum and lateral white matter was observed (Figures 5F–H). In brain-injured animals the white matter atrophy that was observed at both 3 and 7 days post-injury (vide supra) persisted to the 10 and 15 day time points. Quantitative analyses revealed an interaction effect between group and time (F2,23=57.23, p<0.001; Fig. 5J) and the post-hoc analysis indicated that brain-injured animals exhibited a significantly smaller area compared to sham-injured animals at both 10 and 15 days post-injury (p<0.001); importantly, minocycline-treated brain-injured animals had significantly smaller white matter areas than the brain-injured animals that received the vehicle at 10 days post-injury (p<0.05), but not at 15 days post-injury (p=0.93; Fig. 5J). An interaction effect between group, sex and time (F2,23=5.19, p<0.05) indicated that sham-injured males had significantly greater white matter areas than sham-injured females (p<0.001) at 15 days post-injury.
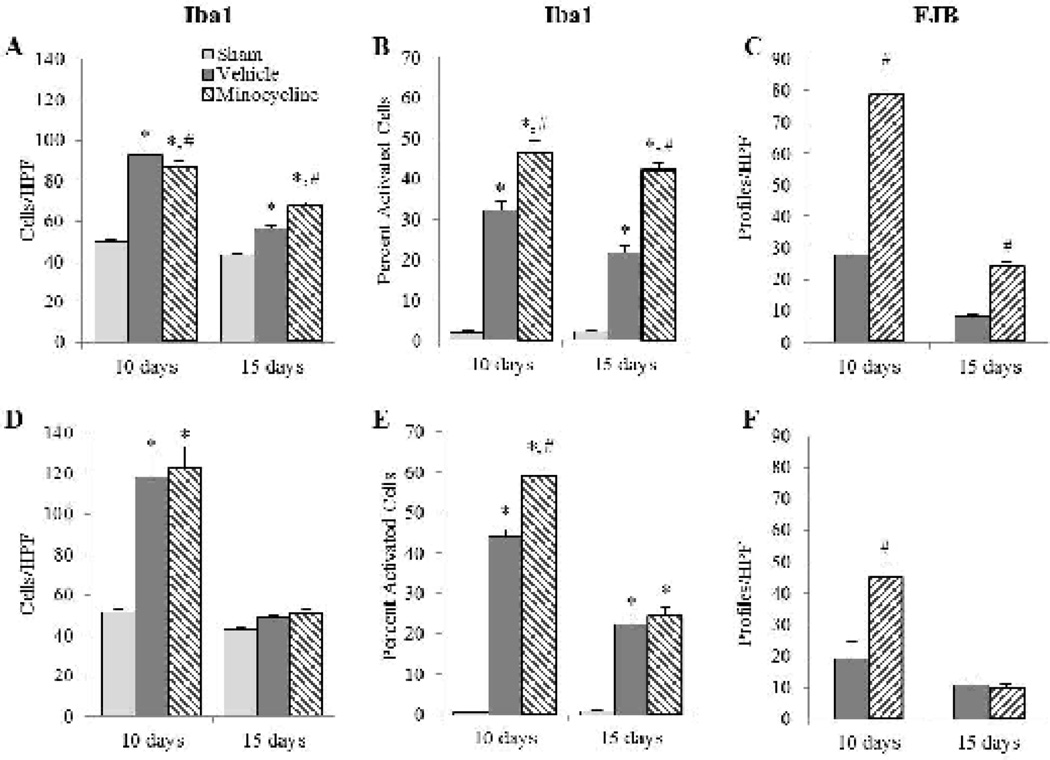
Study 2: Effect of minocycline in the hippocampus and thalamus
At 10 and 15 days post-injury, brain-injured animals demonstrated increased numbers of microglia and FJB reactivity in the hippocampus and thalamus compared to their sham-injured counterparts (Fig. 6). In the hippocampus, these alterations continued to be restricted to the dorsal aspect of the subiculum wherein a 3-way ANOVA revealed an interaction effect between group and time (F2,23=23.01, p<0.001; Fig. 6A), but no effect of sex (F2,23=0.01, p=0.99); post-hoc analysis indicated that at 10 days post-injury, minocycline-treated, brain-injured animals had significantly fewer microglia compared to their counterparts that received vehicle (p=0.05; Fig. 6A). At 15 days post-injury, however, minocycline-treated brain-injured animals had significantly more microglia than brain-injured animals that received vehicle (p<0.001). The proportion of activated microglia in the subiculum also demonstrated an interaction effect between group and time (F2,23=3.93, p<0.05; Fig. 6B) with no effect of sex (F2,23=2.59, p=0.10). There was, however, an increase in the proportion of activated microglia in minocycline-treated, brain-injured animals compared to those that received vehicle animals at both times post-injury (p<0.001; Fig. 6B). Minocycline-treated animals also contained greater numbers of FJB(+) profiles at both time points post-injury compared to brain-injured animals that received vehicle (F1,15=18.15, p<0.001; Fig. 6C). Similarly, there was no effect of sex with regards to FJB in the subiculum (F1,15=0.57, p=0.46).
FIGURE 6. Effect of extended minocycline administration in the hippocampus and thalamus.
Graphs illustrate counts of Iba1(+) (A, D), the proportion of activated Iba1(+) cells (B,E), and counts of FJB(+) profiles (C,F) in the hippocampus (A–C) and the thalamus (D–F). Sex had no influence on outcomes so values for male and female rats were combined for graphical representation. *, p ≤ 0.05 compared to sham-injured values; #,p ≤ 0.05 compared to brain-injured animals injected with vehicle. HPF, high power field.
In the thalamus, the regional distribution of activated microglia and FJB reactive profiles at 10 and 15 days post-injury was similar to that observed at 3 and 7 days post-injury (not shown). Analysis of the number of microglia revealed an interaction effect between group and time (F2,23=16.26, p<0.001; Fig. 6D) with the post-hoc test indicating that brain-injured animals had significantly more microglia compared to sham-injured animals at 10 days post-injury (p<0.001), but not at 15 days post-injury (vehicle group, p=0.48; minocycline group, p=0.59). Sex did not influence the interaction between group and time (F2,23=1.05, p=0.37). When the population of activated microglia was analyzed, an interaction effect between group and time (F2,23=53.46, p<0.001; Fig. 6E) was observed with a subsequent post-hoc analysis revealing that at 10 days post-injury, minocycline-treated, brain-injured animals had a higher percentage of activated cells compared to brain-injured animals that received vehicle (p<0.001), and that this effect was lost at 15 days post-injury (p=0.39); there was no effect of sex (F2,23=2.50, p=0.10). Minocycline-treated, brain-injured animals exhibited significantly more FJB+ profiles in the thalamus at 10 days post-injury (p<0.001), but not at 15 days post-injury (p=0.88), compared to brain-injured animals that received vehicle (Fig. 6F) and this was unaffected by sex (F1,15=0.79, p=0.39).
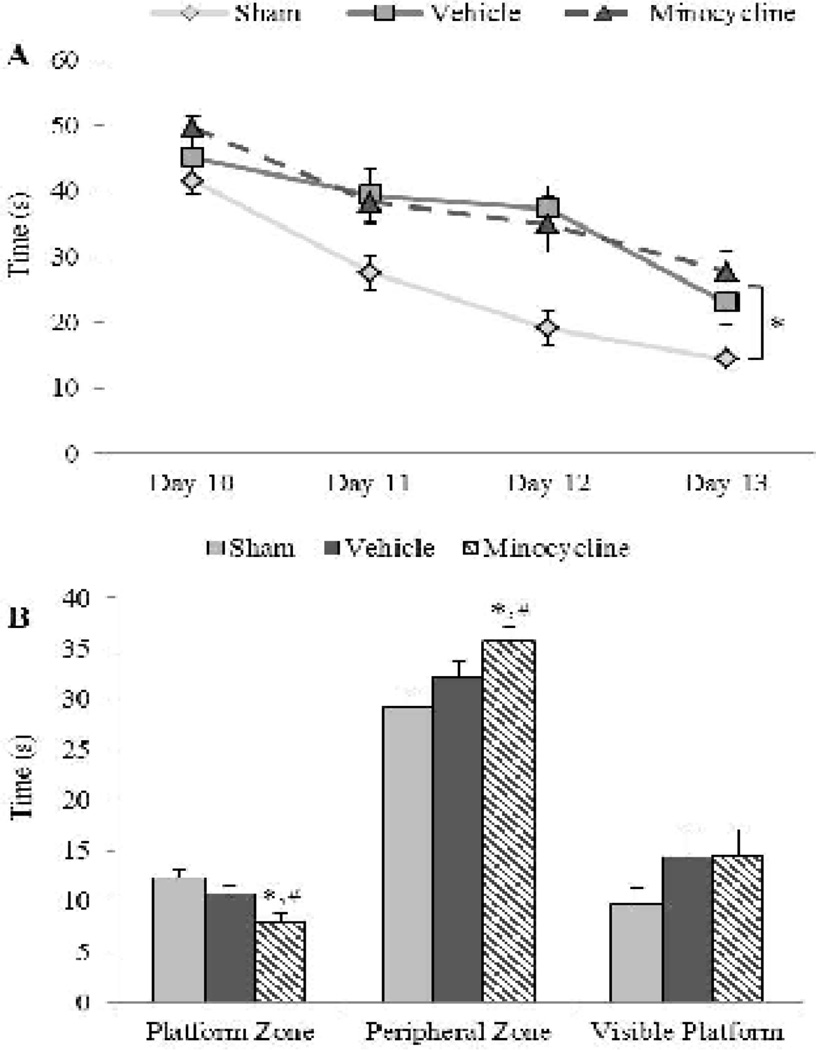
Study 2: Effect of minocycline on spatial learning and memory
As observed in study 1, brain-injured animals in study 2 were also impaired in their ability to learn and remember the location of the submerged platform (Fig. 7). Analysis of spatial learning patterns illustrated in Figure 7A revealed main effects of group (F2,105=9.16, p<0.001) and training day (F3,105=44.72, p<0.001), but no interaction (F6,105=1.41, p=0.22; Fig. 7A). Post-hoc analysis demonstrated that brain-injured animals took significantly longer to locate the hidden platform compared to sham-injured animals (p<0.01), and that there was no difference in latencies between the two brain-injured groups (p=0.65) indicating that prolonged minocycline treatment had no effect on injury-induced spatial learning deficits; there was no influence of sex of the animal on these observations (F6,105=0.62, p=0.71). A 2-way ANOVA for the time spent in the platform zones revealed a main effect of group (F2,35=7.33, p<0.01) and an interaction effect between group and sex (F2,35=3.31, p<0.05). Post-hoc analysis indicated that sham-injured animals spent more time in the platform zones than brain-injured animals treated with minocycline (p<0.01), but not with vehicle (p=0.18); however, brain-injured minocycline-treated animals spent significantly less time in the platform zone than their counterparts that received vehicle (p<0.05). Male sham-injured animals spent more time in the platform zone than their minocycline-treated, brain-injured counterparts (p=0.10), but this was not different from either brain-injured counterparts that received vehicle (p=0.10) or female sham-injured animals (p>0.05). Similarly, analysis of the time spent in the peripheral zone revealed a main effect of group (F2,35=6.24, p<0.01) and an interaction effect between group and sex (F2,35=4.15, p<0.05). Post-hoc analyses indicated that sham-injured animals spent significantly less time in the peripheral zone compared to brain-injured animals treated with minocycline (p<0.01), but not brain-injured animals that received vehicle (p=0.15; Fig. 7B); importantly, minocycline-treated brain-injured animals spent more time in the peripheral zones compared to their counterparts that received the vehicle (p=0.05). Male sham-injured animals spent less time in the peripheral zone compared to males from both brain-injured groups (vehicle, p<0.05; minocycline, p<0.01) and female sham-injured animals (p<0.05). There was no group or sex effect in the visible platform trial, indicating that observed spatial learning deficits in brain-injured animals were not due to a problem in visual function (F2,35=0.08 ,p=0.92; Fig. 7B). Additionally, deficits in learning and retention were not due to deficits in motor function as indicated by a lack of differences between groups or sexes in swim speed (sham-injured: 26 ± 0.43 cm/s; brain-injured vehicle: 24 ± 0.81 cm/s; brain-injured minocycline: 24 ± 0.82 cm/s; F2,35=1.52, p=0.23).
FIGURE 7. Effect of extended minocycline administration on injury-induced spatial learning and memory deficits.
(A) Latency to the platform on each day represents the average of 4 trials. (B) Average times spent in the area surrounding the platform location and the periphery of the maze during the spatial retention (probe) trial. Also included is the time to the visible platform. Sex had no effect on spatial learning and memory outcomes so values for male and female rats were combined for graphical representation. *, p ≤ 0.05 compared to sham-injured animals; #, p ≤ 0.05 compared to brain-injured animals injected with vehicle.
Discussion
The present study demonstrates that short-term minocycline administration initiated immediately following closed head injury in the neonate rat reduced the total number of microglia and microglial activation when evaluated upon termination of treatment. This reduction was accompanied by an increase in the extent of neurodegeneration and was not sustained to 4 days after treatment ended. Moreover, there was no attenuation of spatial learning and memory deficits that were tested in the second week post-injury. When the dosing of minocycline was extended to the second week post-injury, microglial activation, axonal degeneration and neurodegeneration were exacerbated in multiple brain regions and this effect was sustained in the cortex and hippocampus up to the third week post-injury; whereas spatial learning deficits were unaffected by treatment, retention of the learned task was mildly but significantly worsened in the minocycline-treated, brain-injured group. Collectively, these data suggest that acute minocycline treatment may not be a viable strategy for TBI in the neonate.
Short-term administration of minocycline (similar to the paradigm used in study 1) has been found effective in decreasing microglial activation following neonatal hypoxic ischemia (Cai et al., 2006; Cikla et al., 2016; Fan et al., 2006; Lechpammer et al., 2008), pediatric cardiac arrest (Tang et al., 2010), neonatal repetitive TBI (Hanlon et al., 2016), and adult TBI (Bye et al., 2007; Homsi et al., 2010; Siopi et al., 2011). The reduction in activated microglia was accompanied by an attenuation of neuronal damage (Cikla et al., 2016), a decrease in asphyxia-induced neurodegeneration (Tang et al., 2010), and a reduction of lesion volume following TBI (Homsi et al., 2010). However, the association between microglial activation and attendant neurodegeneration in brain injury is not quite clear-cut: minocycline treatment following TBI in the adult mouse decreased F4/80-labeled microglia that exhibited the activated phenotype without affecting lesion volume or TUNEL labeling (Bye et al., 2007) whereas in a model of neonatal stroke minocycline failed to reduce microglial activation, but still had a beneficial effect on lesion volume (Fox et al., 2005). In contrast to all the above studies, the decrease in acute microglial activation observed in the present study was in fact, associated with an increase in the number of FJB+ neurons suggestive of either a lack of clearance of degenerating neurons or that microglia may play a more active role in neuronal survival following injury to the immature brain (Potter et al., 2009). This latter possibility is supported by the fact that depletion of microglia following stroke in neonatal rats causes an increase in lesion size (Faustino et al., 2011). Microglial activation also occurs in white matter tracts following injury to either the neonate or the adult brain. In the neonate, HI-induced microglial activation and the concomitant oligodendrocyte cell death, myelin loss and decrease in white matter volume were attenuated by minocycline (Cai et al., 2006; Fan et al., 2006; Lechpammer et al., 2008). In the adult mouse, TBI-induced loss of corpus callosum volume was attenuated by minocycline treatment (Siopi et al., 2011). In contrast, short-term administration of minocycline in the present study did not affect axonal injury or degeneration, an observation that was similar to those in a model of repetitive brain trauma in the neonate rat (Hanlon et al., 2016) and closed head injury in the adult mouse (Homsi et al., 2010). In this regard, genetic ablation of microglia in the white matter did not reduce trauma-induced axonal injury (Bennett and Brody, 2014).
In study 1, a sustained effect on microglial activation and neurodegeneration was not observed once the treatment was terminated; moreover, there was no attenuation of injury-induced spatial learning and retention deficits. Consistent with these observations, minocycline did not affect motor deficits in brain-injured adult mice (Bye et al., 2007) or spatial learning and memory following HI in neonatal mice (Cikla et al., 2016) and TBI in adult rats (Kelso et al., 2011). This lack of sustained effect may be due to the fact that the half-life of minocycline in rodents is rather short (2–3 hours) (Andes and Craig, 2002). In contrast, a number of studies have demonstrated that minocycline reduced behavioral impairments days and weeks after treatment termination in HI-injured neonatal rats (Fan et al., 2006) and in adult mice following TBI (Homsi et al., 2010; Siopi et al., 2012). It is possible that these differences may relate to the variability in dose and dosing paradigms employed in the various studies.
Because sustained benefits with the short-term administration of minocycline were not observed, the dosing was extended into the second week post-injury and terminated immediately prior to behavioral analyses. Dosing minocycline for 6 days decreased microglial activation while simultaneously reducing the extent of HI-induced oligodendrocyte cell death and myelin loss (Carty et al., 2008; Wixey et al., 2011) and neurodegeneration (Leonardo et al., 2008). In contrast, a 7- or 14-day dosing paradigm that was effective in decreasing the number of activated microglia did not affect brain trauma-induced neurogenesis at 2 or 6 weeks post-injury (Ng et al., 2012). In a model of adult TBI, daily administration of minocycline for 7, 12 or 16 days reduced microglial activation and reversed spatial learning and memory deficits at 8 weeks post-injury but did not attenuate lesion volume (Lam et al., 2013). In the current study, extended minocycline administration resulted in an increase in the number of activated microglia in the multiple brain regions which was accompanied by an increase in the number of FJB(+) profiles. This observation is consistent with that in a mouse model of visual cortex ablation in which minocycline administration to injured metallothionein-deficient immature brain increased the number of activated microglia while simultaneously increasing the extent of neuronal loss which was associated with a minocycline-induced upregulation of pro-apoptotic genes (Potter et al., 2009). Moreover, the modest exacerbation of spatial memory deficits observed in the minocycline-treated animals may be related to increased neurodegeneration in the subiculum of the hippocampus, an area that has been implicated in spatial learning and memory function (O’Mara, 2005; O’Mara et al., 2009). In addition, the increased numbers of activated microglia may have led to pathologic increases in pro-inflammatory cytokines such as IL-1β which can inhibit both long-term potentiation and worsen acquisition and retention of a spatial memory task (Ross et al., 2003; Trofimov et al., 2012).
In addition to a change in morphology, microglial activation has also been characterized as an increase in total number of cells possibly due to a combination of proliferation and migration (Loane and Kumar, 2016; Potts et al., 2006). Following TBI in the adult mouse, short-term minocycline administration led to a sustained suppression of total microglial numbers at 3 months after the injury (Homsi et al., 2010; Siopi et al., 2011). Total microglia was decreased in minocycline-treated neonatal mice up to 9 days following hypoxic-ischemia (Cikla et al., 2016). These reports are consistent with those in the current study and support the need for including the two different aspects of the post-injury microglial response in the evaluation of interventions targeting these cells. All of our statistical analyses included sex as an independent variable and led to the observation that sex did not influence injury-induced acute neurologic responses, microglial reactivity, neurodegeneration or cognitive function. In this regard, a recent study reported that injury-induced increases in microglial number was not dependent on the sex of the injured neonate animal (Chhor, et al. 2016). However, injury-induced mitochondrial dysfunction (Robertson and Saraswati, 2015), changes in neuronal structure (Semple, et al., 2016) and social recognition (Semple et al., 2016) were more apparent in male rats, whereas injury-induced deficits in play behavior was predominantly observed in female rats (Mychasiuk, et al., 2014). In our study, sex did not influence the effects of minocycline in the various outcomes, an observation that is in contrast to the effect of progesterone which was effective in reversing mitochondrial dysfunction only in male rat pups (Robertson and Saraswati, 2015). These observations underscore the importance of utilizing sex as a variable in histologic and behavioral analyses when injury-induced alterations and efficacy of neuroprotective strategies are tested. It must be noted that insufficient information in clinical reports on pediatric moderate-severe TBI makes it difficult to ascertain whether sex is a contributing factor to acute and chronic outcomes.
The results described here have several limitations. First, although total microglia and microglial morphology were evaluated using two different dosing paradigms, only a single dose (45 mg/kg/injection) was utilized in each paradigm; the efficacy of this dose has been well-documented (Buller et al., 2009). Neonatal mice that received a lower daily dose (22.5 mg/kg) of minocycline for 6 days showed improvement in white matter pathology following hypoxia-ischemia (Carty et al., 2008), raising the possibility the 45 mg/kg dose for 9 days may be too high. Second, only spatial learning and memory in the Morris water maze was used to test cognitive function, although minocycline has been effective in reducing deficits in this measure of cognition (Fan et al., 2006; Lam et al., 2013). Nevertheless, future studies warrant the use of a wider range of functional outcomes that may allow researchers to better understand the differential effects of minocycline treatment following injury to the developing brain. Additional future investigations should investigate the effect of minocycline on microglial phenotype as it has been documented that minocycline can selectively target the pro-inflammatory M1 phenotype (Kobayashi et al., 2013) and similarly decrease harmful mediators associated with an M1 phenotype such as IL-1β and TNFα (Bye et al., 2007; Wang et al., 2005; Wasserman and Schlichter, 2007; Yrjanheikki et al., 1999).
Conclusions
The data presented here indicate that minocycline treatment in the acute period for 3 days following closed head injury in the neonate rat transiently inhibits microglial activation but does not reduce neurodegeneration, axonal injury or spatial learning deficits. Extending the dosing period of minocycline to 9 days exacerbates microglial activation and neurodegeneration and cognitive deficits. Collectively, these data underscore the complex nature of post-traumatic cellular pathology in the developing brain and further suggest that acute interventions targeting the inflammatory cascade may not be a viable strategy for pediatric TBI.
Highlights.
-
-
Short-term minocycline administration following closed head injury in the neonate rat reduced trauma-induced microglial activation but exacerbated neurodegeneration.
-
-
Short-term minocycline administration failed to attenuate injury-induced spatial learning or memory deficits.
-
-
Extending the time of minocycline administration following closed head injury in the neonate rat exacerbated trauma-induced microglial activation, neurodegeneration and axonal degeneration.
-
-
Extended minocycline administration failed to attenuate injury-induced spatial learning deficits but exacerbated memory deficits.
-
-
The effects of minocycline administration was not influenced by the sex of the brain-injured animal.
Acknowledgments
This study was funded, in part, by a grant from NICHD (HD 061963). The authors would like to thank Ms. Rupal Prasad and Mr. Douglas Fox for their technical assistance in performing animal surgeries/injuries and histologic analyses, respectively.
Abbreviations
- TBI
Traumatic brain injury
- TAI
traumatic axonal injury
- CSF
cerebrospinal fluid
- HI
hypoxic-ischemia
- eCCI
electronically driven controlled cortical impact
- PBS
phosphate-buffered saline
- FJB
fluoro-jade B
- Iba1
anti-ionized calcium-binding adaptor molecule 1
- APP
amyloid precursor protein
- HPF
high power field
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel Baki SG, Schwab B, Haber M, Fenton AA, Bergold PJ. Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PloS one. 2010;5:e12490. doi: 10.1371/journal.pone.0012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld JV. Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics. 2009;124:e1064–e1071. doi: 10.1542/peds.2009-0365. [DOI] [PubMed] [Google Scholar]
- Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: a critical review. International journal of antimicrobial agents. 2002;19:261–268. doi: 10.1016/s0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Annals of neurology. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- Babikian T, Merkley T, Savage RC, Giza CC, Levin H. Chronic Aspects of Pediatric Traumatic Brain Injury: Review of the Literature. Journal of neurotrauma. 2015;32:1849–1860. doi: 10.1089/neu.2015.3971. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Wisniewski SR, Whalen MJ, DeKosky ST. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. Journal of neurotrauma. 1997;14:451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- Bennett RE, Brody DL. Acute reduction of microglia does not alter axonal injury in a mouse model of repetitive concussive traumatic brain injury. Journal of neurotrauma. 2014;31:1647–1663. doi: 10.1089/neu.2013.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon SB, Walker FR. Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience. 2012;225:162–171. doi: 10.1016/j.neuroscience.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Buller KM, Carty ML, Reinebrant HE, Wixey JA. Minocycline: a neuroprotective agent for hypoxic-ischemic brain injury in the neonate? Journal of neuroscience research. 2009;87:599–608. doi: 10.1002/jnr.21890. [DOI] [PubMed] [Google Scholar]
- Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, Morganti-Kossmann MC. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Experimental neurology. 2007;204:220–233. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Cai Z, Lin S, Fan LW, Pang Y, Rhodes PG. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience. 2006;137:425–435. doi: 10.1016/j.neuroscience.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Carty ML, Wixey JA, Colditz PB, Buller KM. Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26:477–485. doi: 10.1016/j.ijdevneu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V, Ditchfield M, Coleman L. Using magnetic resonance imaging to predict new learning outcome at 5 years after childhood traumatic brain injury. Journal of child neurology. 2008;23:486–496. doi: 10.1177/0883073807309773. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Children’s attentional skills 5 years post-TBI. Journal of pediatric psychology. 2007;32:354–369. doi: 10.1093/jpepsy/jsl019. [DOI] [PubMed] [Google Scholar]
- Chhor V, Moretti R, Le Charpentier T, Sigaut S, Lebon S, Schwendimann L, Ore MV, Zuiani C, Milan V, Josserand J, Vontell R, Pansiot J, Degos V, Ikonomidou C, Titomanlio L, Hagberg H, Gressens P, Fleiss B. Role of microglia in a mouse model of paediatric traumatic brain injury. Brain, behavior, and immunity. 2016 doi: 10.1016/j.bbi.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikla U, Chanana V, Kintner DB, Covert L, Dewall T, Waldman A, Rowley P, Cengiz P, Ferrazzano P. Suppression of microglia activation after hypoxia-ischemia results in age-dependent improvements in neurologic injury. Journal of neuroimmunology. 2016;291:18–27. doi: 10.1016/j.jneuroim.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD Centers for Disease, C., Prevention. Surveillance for traumatic brain injury-related deaths--United States, 1997–2007. Morbidity and mortality weekly report. Surveillance summaries. 2011;60:1–32. [PubMed] [Google Scholar]
- Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. Journal of neurochemistry. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhaime AC, Raghupathi R. Age-specific therapy for traumatic injury of the immature brain: experimental approaches. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 1999;51:172–177. doi: 10.1016/S0940-2993(99)80091-8. [DOI] [PubMed] [Google Scholar]
- Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Kramer L, Cox CS, Jr, Baumgartner J, Fletcher S, Mendez D, Barnes M, Zhang X, Swank P. Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. Journal of neurosurgery. 2006;105:287–296. doi: 10.3171/ped.2006.105.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Landry SH, Kramer L, DeLeon R. Executive functions following traumatic brain injury in young children: a preliminary analysis. Developmental neuropsychology. 2004;26:487–512. doi: 10.1207/s15326942dn2601_7. [DOI] [PubMed] [Google Scholar]
- Fan LW, Lin S, Pang Y, Rhodes PG, Cai Z. Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat. The European journal of neuroscience. 2006;24:341–350. doi: 10.1111/j.1460-9568.2006.04918.x. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, Vexler ZS. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazzano P, Chanana V, Uluc K, Fidan E, Akture E, Kintner DB, Cengiz P, Sun D. Age-dependent microglial activation in immature brains after hypoxia- ischemia. CNS & neurological disorders drug targets. 2013;12:338–349. doi: 10.2174/1871527311312030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM, Vexler ZS. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. British journal of pharmacology. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta neuropathologica. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature neuroscience. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hanlon LA, Huh JW, Raghupathi R. Minocycline Transiently Reduces Microglia/Macrophage Activation but Exacerbates Cognitive Deficits Following Repetitive Traumatic Brain Injury in the Neonatal Rat. Journal of neuropathology and experimental neurology. 2016;75:214–226. doi: 10.1093/jnen/nlv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi S, Federico F, Croci N, Palmier B, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain research. 2009;1291:122–132. doi: 10.1016/j.brainres.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Homsi S, Piaggio T, Croci N, Noble F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. Journal of neurotrauma. 2010;27:911–921. doi: 10.1089/neu.2009.1223. [DOI] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. Basic science; repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: a preliminary report. Journal of neurotrauma. 2007;24:15–27. doi: 10.1089/neu.2006.0072. [DOI] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. Midline brain injury in the immature rat induces sustained cognitive deficits, bihemispheric axonal injury and neurodegeneration. Experimental neurology. 2008;213:84–92. doi: 10.1016/j.expneurol.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivacko JA, Sun R, Silverstein FS. Hypoxic-ischemic brain injury induces an acute microglial reaction in perinatal rats. Pediatric research. 1996;39:39–47. doi: 10.1203/00006450-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Scheff NN, Scheff SW, Pauly JR. Melatonin and minocycline for combinatorial therapy to improve functional and histopathological deficits following traumatic brain injury. Neuroscience letters. 2011;488:60–64. doi: 10.1016/j.neulet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behavioural brain research. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell death & disease. 2013;4:e525. doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi E, Kamnaksh A, Wingo D, Ahmed F, Grunberg NE, Long JB, Kasper CE, Agoston DV. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Frontiers in neurology. 2012;3:111. doi: 10.3389/fneur.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lam TI, Bingham D, Chang TJ, Lee CC, Shi J, Wang D, Massa S, Swanson RA, Liu J. Beneficial effects of minocycline and botulinum toxin-induced constraint physical therapy following experimental traumatic brain injury. Neurorehabilitation and neural repair. 2013;27:889–899. doi: 10.1177/1545968313491003. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. The Journal of head trauma rehabilitation. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Manning SM, Samonte F, Nelligan J, Sabo E, Talos DM, Volpe JJ, Jensen FE. Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leucomalacia. Neuropathology and applied neurobiology. 2008;34:379–393. doi: 10.1111/j.1365-2990.2007.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo CC, Eakin AK, Ajmo JM, Collier LA, Pennypacker KR, Strongin AY, Gottschall PE. Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat. Journal of neuroinflammation. 2008;5:34. doi: 10.1186/1742-2094-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Experimental neurology 275 Pt. 2016;3:316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae A, Gilland E, Bona E, Hagberg H. Microglia activation after neonatal hypoxic-ischemia. Brain research. Developmental brain research. 1995;84:245–252. doi: 10.1016/0165-3806(94)00177-2. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Farran A, Esser MJ. Mean girls: sex differences in the effects of mild traumatic brain injury on the social dynamics of juvenile rat play behaviour. Behavioural brain research. 2014;259:284–291. doi: 10.1016/j.bbr.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Newell E, Shellington DK, Simon DW, Bell MJ, Kochanek PM, Feldman K, Bayir H, Aneja RK, Carcillo JA, Clark RS. Cerebrospinal Fluid Markers of Macrophage and Lymphocyte Activation After Traumatic Brain Injury in Children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2015;16:549–557. doi: 10.1097/PCC.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SY, Semple BD, Morganti-Kossmann MC, Bye N. Attenuation of microglial activation with minocycline is not associated with changes in neurogenesis after focal traumatic brain injury in adult mice. Journal of neurotrauma. 2012;29:1410–1425. doi: 10.1089/neu.2011.2188. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- O’Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. Journal of anatomy. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara SM, Sanchez-Vives MV, Brotons-Mas JR, O’Hare E. Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:782–790. doi: 10.1016/j.pnpbp.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Archives of neurology. 2010;67:1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter EG, Cheng Y, Natale JE. Deleterious effects of minocycline after in vivo target deprivation of thalamocortical neurons in the immature, metallothionein-deficient mouse brain. Journal of neuroscience research. 2009;87:1356–1368. doi: 10.1002/jnr.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts MB, Koh SE, Whetstone WD, Walker BA, Yoneyama T, Claus CP, Manvelyan HM, Noble-Haeusslein LJ. Traumatic injury to the immature brain: inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2006;3:143–153. doi: 10.1016/j.nurx.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullela R, Raber J, Pfankuch T, Ferriero DM, Claus CP, Koh SE, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Developmental neuroscience. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- Raghupathi R, Huh JW. Diffuse brain injury in the immature rat: evidence for an age-at-injury effect on cognitive function and histopathologic damage. Journal of neurotrauma. 2007;24:1596–1608. doi: 10.1089/neu.2007.3790. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annual review of immunology. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Saraswati M. Progesterone protects mitochondrial function in a rat model of pediatric traumatic brain injury. Journal of bioenergetics and biomembranes. 2015;47:43–51. doi: 10.1007/s10863-014-9585-5. [DOI] [PubMed] [Google Scholar]
- Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. Journal of neuroimmunology. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–1399. doi: 10.1097/00006123-200106000-00051. discussion 1399-1401. [DOI] [PubMed] [Google Scholar]
- Semple BD, Dixit S, Shultz SR, Boon WC, O’Brien TJ. Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury. Behavioural brain research. 2016;319:48–62. doi: 10.1016/j.bbr.2016.10.045. [DOI] [PubMed] [Google Scholar]
- Siopi E, Cho AH, Homsi S, Croci N, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline restores sAPPalpha levels and reduces the late histopathological consequences of traumatic brain injury in mice. Journal of neurotrauma. 2011;28:2135–2143. doi: 10.1089/neu.2010.1738. [DOI] [PubMed] [Google Scholar]
- Siopi E, Llufriu-Daben G, Fanucchi F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: the effect of minocycline. Neuroscience letters. 2012;511:110–115. doi: 10.1016/j.neulet.2012.01.051. [DOI] [PubMed] [Google Scholar]
- Tang M, Alexander H, Clark RS, Kochanek PM, Kagan VE, Bayir H. Minocycline reduces neuronal death and attenuates microglial response after pediatric asphyxial cardiac arrest. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:119–129. doi: 10.1038/jcbfm.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong KA, Ashwal S, Holshouser BA, Nickerson JP, Wall CJ, Shutter LA, Osterdock RJ, Haacke EM, Kido D. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Annals of neurology. 2004;56:36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Experimental neurology. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- Trofimov AN, Zubareva OE, Simbirtsev AS, Klimenko VM. [The influence of neonatal interleukin-1beta increase on the formation of adult rats’ spatial memory]. Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova / Rossiiskaia akademiia nauk. 2012;98:782–792. [PubMed] [Google Scholar]
- Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Experimental neurology. 2004;189:58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Developmental neuroscience. 2009;31:378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Yu AC, Lau LT, Lee C, Wu le M, Zhu X, Tso MO. Minocycline inhibits LPS-induced retinal microglia activation. Neurochemistry international. 2005;47:152–158. doi: 10.1016/j.neuint.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Experimental neurology. 2007;207:227–237. doi: 10.1016/j.expneurol.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Kochanek PM, Wisniewski SR, Bell MJ, Clark RS, DeKosky ST, Marion DW, Adelson PD. Interleukin-8 is increased in cerebrospinal fluid of children with severe head injury. Critical care medicine. 2000;28:929–934. doi: 10.1097/00003246-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Wixey JA, Reinebrant HE, Spencer SJ, Buller KM. Efficacy of post-insult minocycline administration to alter long-term hypoxia-ischemia-induced damage to the serotonergic system in the immature rat brain. Neuroscience. 2011;182:184–192. doi: 10.1016/j.neuroscience.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Saraswati M, Koehler RC, Robertson C, Kannan S. A New Rabbit Model of Pediatric Traumatic Brain Injury. Journal of neurotrauma. 2015;32:1369–1379. doi: 10.1089/neu.2014.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]