Abstract
The criteria for differentiating symptomatic from asymptomatic HIV-associated neurocognitive disorder require evaluation of (1) cognitive impairment, (2) daily functioning declines, and (3) whether the functional declines are attributable to cognitive versus physical problems. Many providers rely only on self-report to evaluate these latter criteria. However, the accuracy of patient-provided information may be limited. This study evaluated the validity of self-assessment for HIV-associated neurocognitive disorder (HAND) diagnoses by comparing objective findings with self-report of criteria 2 and 3 above. Self-reports were used to stratify 277 cognitively impaired HIV+ individuals into functionally dependent (n = 159) and independent (n = 118) groups, followed by group comparisons of objective functional problems. The dependent group was then divided into those who self-attributed their functional dependence to only cognitive (n = 80) versus only physical (n = 79) causes, for further comparisons on objective findings. The functionally dependent group was significantly worse than the independent group on all objective disability characteristics except severity of cognitive impairment, while those who attributed their dependence to physical (versus cognitive) factors were similar on all objective physical, cognitive, and functioning variables. Of note, 28 % of physical attributors showed no physical abnormalities on neuromedical examinations. Results suggest that patient report is consistently associated with objective measures of functional loss; in contrast, patient identification of physical versus cognitive causes is poorly associated with objective criteria. These findings caution against relying solely on patient self-report to determine whether functional disability in cognitively impaired HIV+ individuals can be attributed to strictly physical causes.
Keywords: AIDS, Activities of daily living, Self-assessment, Cognitive disorders, Etiology
Introduction
Neurocognitive impairment affects up to 50 % of HIV-infected (HIV+) individuals (Heaton et al. 2010). Those with cognitive impairments often have greater difficulties completing tasks that are important for daily functioning (e.g., driving, adhering to medication regimens; Heaton et al. 2004a; Hinkin et al. 2002; Marcotte et al. 1999), as well as have worse health outcomes (McCutchan et al. 2012), poorer insight into their functioning (e.g., Casaletto et al. 2014), and a higher mortality rate (Tozzi et al. 2005) than comparable HIV+ individuals who are not cognitively impaired. Despite the increased risk of everyday difficulties, some HIV+ individuals with cognitive impairment remain functionally independent. This subgroup, classified as asymptomatic neurocognitive impairment (ANI), exhibits a profile of neurocognitive deficits identical to many of those with functional dependence (Heaton et al. 2010). However, those with ANI depart from cognitively normal HIV+ individuals in that they evidence incident functional difficulties faster than their cognitively unimpaired HIV+ counterparts (Grant et al. 2014).
According to the current international “Frascati” classification criteria for HIV-associated neurocognitive disorder (HAND; Antinori et al. 2007), ANI is assigned when individuals exhibit at least mild cognitive impairment in two or more cognitive domains but whose everyday functioning difficulties either are not present or are present but unrelated to their cognitive impairments. In contrast, mild neurocognitive disorder (MND) is distinguished by at least mild cognitive impairment that is found to be associated with daily functioning difficulties (Antinori et al. 2007). Finally, HIV-associated dementia (HAD) is the most severe subcategory of HAND and is assigned when a patient has substantial cognitive impairment that is associated with more severe daily functioning difficulties (Antinori et al. 2007). Therefore, the main diagnostic differentiations between the HAND subcategories involve assessing (1) the severity of cognitive impairment, (2) the presence and severity of daily functioning difficulties, and (3) if the individual’s functional difficulties can be attributed to their cognitive impairments. Differentiating these subcategories is helpful clinically both for determining how these impairments impact an individual’s daily life and for enhancing a clinician’s ability to predict the trajectory of a patient’s immediate needs. Therefore, the accuracy of current diagnostic practice warrants closer scrutiny.
Many clinicians and researchers rely on patient self-report to evaluate both the presence of functional dependence and whether this dependence can be attributed to their HIV-associated cognitive deficits instead of strictly physical causes. Self-assessment of common everyday functioning declines has proven to be valid (e.g., Scott et al. 2011; Vigil et al. 2008; Morgan et al. 2012). However, when the self-assessment relates to more complex concepts, such as general cognitive ability (e.g., “Am I unemployed for only physical reasons?” versus “Am I unemployed?”), inaccuracy is seen in up to 50 % of individuals with HIV (i.e., metacognitive deficit; Blackstone et al. 2012). In instances of meta-cognitive deficits, self-reported neurocognitive difficulties are often related to affective distress rather than objective performances across cognitive domains (Hinkin et al. 1996; van Gorp et al. 1991; Blackstone et al. 2012; Juengst et al. 2012). HIV-associated neurocognitive impairment increases the risk of these metacognitive disturbances (Casaletto et al. 2014; Juengst et al. 2012), even in cases of mild and asymptomatic neurocognitive impairment (Chiao et al. 2013). Of ecological relevance, such difficulties in awareness are linked to poorer everyday functioning outcomes among these individuals (Blackstone et al. 2012). While it is known that self-reported functional measures often relate to objective outcomes and cognitively impaired individuals are less accurate in assessing their own cognitive abilities, currently there is scant literature regarding patients’ abilities to accurately understand and differentiate the etiology (cognitive versus physical) of any daily functioning disabilities. Misclassification of HAND due to inadequate insight has important implications both on the patient (e.g., appropriate treatment/aids, prognostic recommendations) and public health concerns (e.g., driver’s license retention, HIV transmission risk behaviors).
The purpose of this study was to examine the validity of self-reported dependence in activities of daily living and of self-reported attribution of functional impairments (e.g., daily functioning dependence due to cognitive versus physical causes) in HIV+ individuals. In a large sample of cognitively impaired HIV+ adults, we evaluated the frequency and nature of reported functional dependence, the attribution of such dependence to cognitive versus physical causes, and the associations of these attributions to objective real-world, neuromedical, and cognitive findings. Affective distress could also be a cause of disability or functional decline (e.g., major depressive disorder) or instead could be a reaction to functional decline as disability (current depressive symptoms); therefore, depression and depressive symptoms also were evaluated. In comparisons between Activities of Daily Living (ADL) independent and dependent individuals, we hypothesized that the dependent group would demonstrate worse objective functional disability, physical problems, and possibly cognitive performance. Within the functionally dependent group, we evaluated the validity of self-reported etiology of functional dependence by comparing those who attributed their dependence to only physical problems and those who reported only cognitive contributions to their functioning dependence. If these self-reported attributions were accurate, we would expect the self-reported physical attribution group to have more physical problems identified in objective neuromedical examinations, but relatively better objective cognitive functioning than the cognitive attributors. The results of our study have significant implications concerning the weight clinicians can assign to patient self-report in the classification of symptomatic versus asymptomatic HAND.
Method
Participants
This study included 277 HIV-seropositive (HIV+) participants from the CNS Anti-Retroviral Therapy Effects Research (CHARTER) study cohort, which was funded by the National Institute of Mental Health (NIMH) and the National Institute of Neurological Disorders and Stroke (NINDS). CHARTER is a multi-site national study aimed at determining the prevalence and nature of HIV-related central nervous system complications in the era of combination anti-retroviral therapy (cART). The study cohort included HIV+ adults at varying stages of disease and with different histories of antiretroviral medication use (ART).
Because the purpose of the current study was to examine the process of assigning HAND subcategories, all participants included in this sample were first required to be eligible for a HAND diagnosis. The diagnostic criteria for HAND (DSM-IV guidelines and Frascati criteria; Antinori et al. 2007) require the presence of at least mild impairment in at least two of the seven cognitive domains (see below for a list of domains included) using standardized guidelines for clinical ratings to classify the presence and severity of the neurocognitive impairment (see Woods et al. 2004). In line with the international Frascati criteria for categorizing subtypes of HAND, if participants in this study experienced a loss of functional independence in addition to their cognitive impairment, we required that to be included in the analyses regarding cognitive versus physical attribution they had to have attributed the dependence to either only cognitive or only physical causes. Potential participants were not included if they failed to provide a definitive judgment regarding physical versus cognitive causation. Additionally, HAND requires the impairment be primarily due to HIV, therefore those with significant confounding conditions that could better explain the cognitive impairment (e.g., stroke) were excluded from this study.
Participants completed the study assessments at one of six university centers: John Hopkins University (Baltimore, MD), Icahn School of Medicine at Mount Sinai (New York, NY), University of California at San Diego (San Diego, CA), University of Texas Medical Branch (Galveston, TX), University of Washington (Seattle, WA), and Washington University (St. Louis, MO).Study procedures were approved by the Human Subjects Protection Committees of each participating university. All participants provided written informed consent prior to study participation.
Materials and procedures
Participants completed a comprehensive neuromedical examination, neurocognitive test battery, self-report questionnaires, and a structured psychiatric interview (see below and Heaton et al. 2010 for further details).
Demographics
On average, participants were 44.0 (SD = 7.7) years old. The sample was 75 % male and 48 % non-Hispanic white and completed an average of 12.7 (SD = 2.5) years of education.
Disease characteristics
All participants completed a standard medical examination. Blood and urine specimens were collected in order to evaluate disease status and recent substance use. HIV infection was diagnosed by the enzyme-linked immunosorbent assay (ELISA) followed by a Western blot test. Routine clinical chemistry panels, rapid plasma reagin, blood counts, hepatitis C virus (HCV) antibody, and CD4+ T cells (flow cytometry) were performed at each participating institution’s medical center laboratory. HIV RNA levels were measured in plasma and cerebrospinal fluid by reverse transcriptase-polymerase chain reaction (Roche Amplicor, v. 1.5, lower limit of quantitation 50 copies/mL). Nadir CD4 and antiretroviral medication (ART) regimen were collected during the medical history interview. Overall, 64 % of participants had a diagnosis of acquired immune deficiency syndrome (AIDS). The average nadir CD4 count was 186.6 (SD = 173.3).
Disability characteristics
Everyday functioning difficulties
Self-report assessment
Participants completed a modified version of the Lawton and Brody (1969) ADL scale (Heaton et al. 2004a, b; Woods et al. 2006). This ADL scale is a self-report questionnaire that rates participants’ current and best levels of independence on 16 functional domains (i.e., employment, planning and initiating social activities, comprehension of reading/television, housekeeping, home repairs, financial management, general shopping, buying groceries, laundry, cooking, managing transportation, medication management, telephone use, child care, bathing, and dressing). The 16 items describe the extent to which participants independently function on both basic (e.g., bathing, dressing) and instrumental (e.g., managing finances, cooking, housekeeping) activities of daily living. The ADL total score represents the total number of domains for which there is a decline in an individual’s completion of the task on their own now compared to their best previous level of functioning (e.g., previously versus now “I am able to dress myself and pick out my own clothes,” “I dress myself, but someone else must pick out my clothes for me,” “I need occasional assistance getting dressed or frequently make mistakes in choosing clothes,” “I need frequent assistance in getting dressed”), with a total score ranging from 0 (no functional declines) to 16 (decline toward dependence in all activities). Declines on two or more of the 16 domains were used as a cutoff for overall functional dependence, consistent with the Frascati criteria for symptomatic HAND (Antinori et al. 2007; Heaton et al. 2004a, b).
Objective assessment
Employment status was determined by a single-item response on the Patient’s Assessment of Own Functioning Inventory (PAOFI; Chelune et al. 1986; “Are you presently holding a job?”).
Causal attribution of functional impairment
Self-report assessment
Participants were asked to report whether the everyday functioning dependence endorsed on the ADL scale was due to physical difficulties (n = 79; e.g., the participant cannot shop because their peripheral neuropathy precludes them from walking around the store) or cognitive problems (n = 80; e.g., the participant has difficulties shopping because it is too difficult to remember which grocery items are needed).
Objective assessment
Cognitive performance
Participants were administered a comprehensive neurocognitive test battery that included tests in the following seven cognitive domains: speed of information processing, learning, delayed recall, executive function, verbal fluency, attention/working memory, and complex motor skills. See Heaton et al. (2010) for battery details. Raw test scores were converted to normally distributed and demographically corrected standard scores (T scores adjusted for age, education, sex, and race/ethnicity where available) using the best available normative standards (Heaton et al. 2002, 2004b; Norman et al. 2011). T scores on each of the neurocognitive measures were converted into a deficit score using a five-point scale (Carey et al. 2005). The average of the deficit scores from each test generated a global deficit score (GDS) for each study participant, which reflects the number and severity of impairments across all measures (Carey et al. 2005). Neuropsychological impairment was defined as a GDS of ≥0.5, a cutoff that provides the best balance between sensitivity and specificity (e.g., Carey et al. 2005).
Physical disability
The Karnofsky Performance Status Scale (Karnofsky scale; Karnofsky and Burchenal 1949) was used to provide a neuromedical clinician rating of health-related functioning. During the neuromedical examination and collection of medical history, clinicians assessed multiple areas of physical difficulties that are often associated with HIV (e.g., neuropathy), as well as the impact of disease on daily functioning. The Karnofsky index ranges from 0 (indicating death) to 100 (indicating normal functioning/no complaints or signs of disease).
During the neuromedical examination, clinicians administered standard neurologic tests evaluating distal-to-proximal gradients of reflex elicitation and sensation as well as assessments of other areas of physical disabilities. A committee of experts extracted 11 common HIV-associated physical outcomes from the medical examination to create a composite variable representing patients’ physical disability. These key variables included gait/balance disturbance, impaired hand coordination, involuntary movements (e.g., tremors, jerks), muscle weakness, myopathy, dysesthesias (e.g., burning, aching, shooting pain), parasthesias (e.g., tingling), loss of sensation, bladder control, weight loss, and diarrhea. Based on a participant’s performance or response on each area of function, clinicians assigned a severity rating from 0 (normal) to 4 (severe). Two composite scores were derived from the 11 physical disability variables. The Total Number of Abnormal Findings composite is a continuous variable that represents the number of physical disabilities that were rated at least moderately severe (≥2). The Sum of Severity Ratings composite is a continuous variable derived by summing raw scores from the 11 HIV-associated physical outcome variables to determine the overall severity of their physical disability.
Psychiatric interview
Current depressive symptoms were assessed with the Beck Depression Inventory II (BDI-II; Beck et al. 1996). The computer-assisted Composite International Diagnostic Interview (CIDI version 2.1 World Health Organization 1998) is a structured clinical interview that was used to diagnose current (last 30 days) and lifetime mood and substance use disorders using DSM-IV criteria.
Statistical analysis
To examine the validity of self-reported ADL dependence and causal attribution, two separate sets of group comparisons (functionally dependent versus functionally independent, and cognitive versus physical attributors) were completed utilizing chi-square and Student’s t tests (for normally distributed variables) or Wilcoxon Rank-Sum test (for non-normally distributed variables; i.e., education, premorbid verbal IQ estimate, duration of HIV, nadir CD4, current CD4, plasma viral load, severity of cognitive impairment, total number of abnormal findings on the neuromedical exam, sum of severity ratings for abnormal neuromedical findings, and BDI-II). In each analysis, we evaluated the consistency between self-reported versus objective levels of functioning/disability within the same construct (e.g., physical attribution with medically documented physical disabilities). The first set of group comparisons (Table 1) identified differences between the self-reported functionally dependent and independent groups. To evaluate the validity of these self-reported differences, we examined whether there was worse objective functional dependence (e.g., higher rates of unemployment) and disability ratings by the neuromedical clinician in the self-reported dependent group than the self-reported independent group and whether other objective performance indicators were worse in the dependent group (i.e., cognitive and physical findings). The second set of comparisons (Table 2) evaluated differences between those who self-reported the attribution of their functional dependence to physical versus cognitive causes. Because attributions represent the causes of functional dependence, only those in the dependent group were included in the second set of group comparisons. To evaluate the validity of causal attribution, we examined whether those who attributed their functional dependence to cognitive causes had worse objective neurocognitive performance than those who attributed their dependence to strictly physical causes and whether physical attributors had worse physical disabilities on the objective neuromedical examinations compared to those who attributed dependence to strictly cognitive problems. Because psychiatric variables robustly predict self-report, the differences in psychiatric variables (current depressive symptoms, and both current and lifetime depression and substance use disorders) between the aforementioned groups were also evaluated.
Table 1.
Comparison of dependent versus independent groups
| Independent n = 118 | Dependent n = 159 | Effect size
|
p | ||
|---|---|---|---|---|---|
| Cohen’s d | Odds ratio | ||||
| Demographics | |||||
| Age | 43.4 (7.6) | 44.4 (7.8) | .01 | .297 | |
| Gender (% male) | 73.7 % | 76.7 % | .96 | .567 | |
| Ethnicity (% white) | 47.5 % | 49.1 % | 1.46 | .792 | |
| Education | 12.8 (2.5) | 12.7 (2.5) | .04 | .951 | |
| Premorbid IQ estimate (WRAT-3) | 91.1 (16.2) | 90.0 (15.4) | .07 | .526 | |
| Disease characteristics | |||||
| AIDS | 61.0 % | 67.1 % | 1.28 | .298 | |
| Duration of HIV (years) | 9.4 (5.9) | 10.2 (6.6) | .13 | .351 | |
| On ART | 76.3 % | 74.8 % | 1.20 | .785 | |
| Nadir CD4 | 188.4 (171.2) | 185.2 (175.4) | .02 | .729 | |
| Current CD4 | 428.1 (237.6) | 464.9 (324.8) | .13 | .567 | |
| Plasma viral load | 2.6 (1.2) | 2.9 (1.3) | .24 | .384 | |
| Hepatitis C | 18.8 % | 21.7 % | .85 | .557 | |
| Psychiatric disability | |||||
| Beck Depression Inventory-II | 14.2 (9.3) | 18.1 (10.1) | .40 | <.001 | |
| Current major depression | 11.9 % | 24.1 % | 2.4 | .009 | |
| Current any substance use disorder | 1.7 % | 4.4 % | 2.7 | .189 | |
| Lifetime major depression | 47.5 % | 58.9 % | 1.6 | .060 | |
| Lifetime any substance use disorder | 68.6 % | 73.4 % | 1.3 | .386 | |
| Disability characteristics | |||||
| % Unemployed | 68.6 % | 82.4 % | 2.14 | .008 | |
| Severity of cognitive impairment (global deficit score) | .86 (.50) | .86 (.54) | 0.0 | .953 | |
| Cognitive domain T scores | |||||
| Speed of information processing | 45.3 (8.7) | 44.2 (7.5) | .14 | .271 | |
| Learning | 39.2 (6.9) | 38.8 (6.3) | .06 | .647 | |
| Delayed recall | 40.3 (7.3) | 40.8 (6.7) | .07 | .506 | |
| Executive function | 41.0 (7.7) | 41.5 (7.5) | .07 | .637 | |
| Verbal fluency | 45.7 (8.0) | 45.6 (8.4) | .01 | .858 | |
| Attention/working memory | 41.9 (7.3) | 42.3 (7.6) | .05 | .659 | |
| Complex motor skills | 42.0 (10.3) | 39.7 (9.7) | .23 | .064 | |
| Clinician ratings of functioning (Karnofsky) | 88.6 (9.9) | 82.0 (11.8) | .62 | <.001 | |
| Neuromedical examination | |||||
| Total no. of abnormal findings | 1.4 (1.7) | 2.2 (2.2) | .14 | .001 | |
| Sum of severity ratings | 2.0 (2.8) | 3.4 (3.8) | .42 | .002 | |
Note: Mean (SD) are represented for all continuous variables. Cohen’s d and odds ratios are used for continuous and categorical variables respectively. The dependent group included 159 participants in all comparisons, except where data were missing [i.e., AIDS (n = 158), plasma (n = 156), CD4 absolute (n = 157), hepatitis C (n = 152), major depression (n = 158), substance use disorders (n = 158), and complex motor skills domain (n = 158)]. The independent sample included 118 participants, except where data were missing [i.e., WRAT (n = 115), plasma (n = 117), CD4 absolute (n = 116), HCV (n = 117), and complex motor skills (n = 116)]
WRAT-3 Wide Range Achievement Test 3rd Edition, GDS global deficit score
Table 2.
Comparison of dependent participants with cognitive versus physical attribution of disability
| Cognitive | Physical | Effect size
|
p | ||
|---|---|---|---|---|---|
| n = 80 | n = 79 | Cohen’s d | Odds ratio | ||
| Demographics | |||||
| Age | 42.9 (7.8) | 46.0 (7.5) | .41 | .011 | |
| Gender (% male) | 73.8 % | 79.8 % | .71 | .370 | |
| Ethnicity (% white) | 53.8 % | 44.3 % | 1.46 | .233 | |
| Education | 12.6 (2.3) | 12.8 (2.6) | .08 | .632 | |
| Premorbid IQ estimate (WRAT-3) | 91.0 (13.7) | 88.9 (17.0) | .14 | .703 | |
| Disease characteristics | |||||
| AIDS | 58.8 % | 75.6 % | .46 | .023 | |
| Duration of HIV (years) | 9.2 (7.0) | 11.2 (6.0) | .31 | .033 | |
| On ART | 68.8 % | 81.0 % | 1.94 | .074 | |
| Nadir CD4 | 222.6 (194.2) | 147.3 (145.7) | .44 | .012 | |
| Current CD4 | 476.2 (284.5) | 453.4 (362.6) | .07 | .309 | |
| Plasma viral load | 2.8 (1.3) | 2.9 (1.3) | .08 | .993 | |
| Hepatitis C | 20.3 % | 23.3 % | .84 | .650 | |
| Psychiatric disability | |||||
| Beck Depression Inventory-II | 20.1 (10.3) | 16.1 (9.4) | .41 | .013 | |
| Current major depression | 26.3 % | 21.8 % | 1.28 | .512 | |
| Current any substance use disorder | 3.8 % | 5.1 % | .72 | .673 | |
| Lifetime major depression | 70.0 % | 47.4 % | 2.59 | .004 | |
| Lifetime any substance use disorder | 77.5 % | 69.2 % | 1.53 | .239 | |
| Disability characteristics | |||||
| % Unemployed | 81.3 % | 83.5 % | .85 | .704 | |
| Cognitive impairment severity (global deficit score) | .93 (.62) | .79 (.43) | .26 | .239 | |
| Cognitive domain T scores | |||||
| Speed of information processing | 43.7 (8.2) | 44.8 (6.7) | .15 | .346 | |
| Learning | 38.2 (6.6) | 39.5 (5.9) | .21 | .165 | |
| Delayed recall | 40.3 (6.8) | 41.3 (6.5) | .15 | .346 | |
| Executive function | 41.3 (8.2) | 41.6 (6.9) | .04 | .762 | |
| Verbal fluency | 44.4 (8.4) | 46.8 (8.2) | .29 | .070 | |
| Attention/working memory | 41.3 (8.2) | 43.2 (7.0) | .25 | .110 | |
| Complex motor skills | 39.3 (10.0) | 40.1 (9.4) | .08 | .570 | |
| Clinician ratings of functioning (Karnofsky) | 82.9 (12.1) | 81.1 (11.5) | .15 | .422 | |
| Neuromedical examination | |||||
| Total no. of abnormal findings | 2.0 (2.0) | 2.5 (2.4) | .23 | .236 | |
| Sum of severity ratings | 2.9 (3.2) | 3.9 (4.3) | .26 | .180 | |
Note: Mean (SD) are represented for all continuous variables. Cohen’s d and odds ratios are used for continuous and categorical variables, respectively. This sample includes only those who reported they were functionally dependent. The cognitive attribution group included 80 participants in all comparisons, except where data were missing [i.e., plasma (n = 79), CD4 absolute (n = 79), and HCV (n = 79)]. The physical attribution group included 79 participants, except where data were missing [i.e., AIDS (n = 79), plasma (n = 77), CD4 absolute (n = 78), hepatitis C (n = 73), major depression (n = 78), substance use disorder (n = 78), and complex motor skills (n = 78)]
WRAT-3 Wide Range Achievement Test 3rd Edition
Nature of disability in HIV infection
To understand which daily activities were identified as most problematic to participants, the prevalence of declines on each item of the ADL scale was compared across the above-mentioned groups (i.e., functionally dependent versus functionally independent and cognitive versus physical attributors).
Results
Of the 277 non-confounded HIV+ individuals with cognitive impairment in the CHARTER multi-site US study, 159 (57 %) reported decreased independence in at least two ADLs surveyed by the modified Lawton and Brody Scale (i.e., “functionally dependent”). A significance alpha level of .05 was used for all analyses.
ADL-dependent versus ADL-independent group comparisons
Demographics and disease characteristics
Table 1 shows differences between those who were functionally dependent (n = 159) and independent (n = 118). Compared to those who reported ADL independence, the dependent participants were demographically similar (i.e., age, gender, ethnicity, education, and reading-based estimate of premorbid intelligence) and had similar current HIV health and treatment status (p > .05).
Disability characteristics
Compared to those who reported ADL independence, dependent participants had a similar prevalence of current and lifetime substance use disorder, severity of cognitive impairment, and demographically corrected cognitive domain T scores (p > .05) but were significantly worse on all other disability variables including clinician-rated functional disability (Karnofsky), current depressive symptoms (BDI-II), higher prevalence of current and lifetime depression, higher rates of unemployment, and significantly worse findings on all objective physical examination variables (p < .05).
Nature of ADL declines in HIV infection
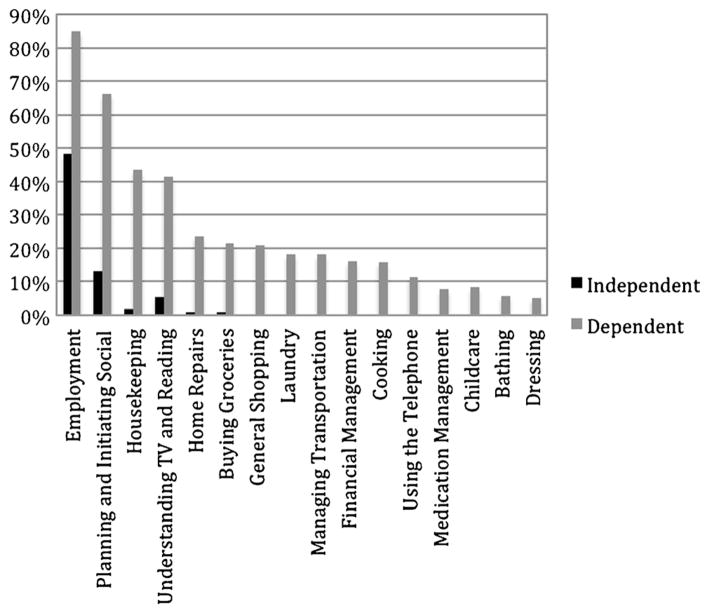
For dependent individuals, we next considered which ADLs they were most likely to need help with in their everyday lives (Fig. 1). Compared to the independent participants, the dependent participants showed higher frequencies of dependence on all 16 ADL items. The most frequently endorsed areas of difficulty were employment, planning and initiating social activities, housekeeping, and understanding TV programs and reading materials (all above 40 % in the dependent group).
Fig. 1.
Frequency of items endorsed by the functionally dependent and independent groups. Note. All items significantly differed (p < .001) between dependent and independent groups
Cognitive versus physical attributions for ADL declines
Within the functionally dependent cohort (n = 159), Table 2 shows the differences between those who attributed their functional difficulties to cognitive (n = 80; 50 %) versus strictly physical (n = 79; 50 %) problems.
Demographics and disease characteristics
Compared to the participants with cognitive attribution of their functional disability, those with a strictly physical attribution were similar on most demographic and disease characteristics. However, the physical attribution group was slightly older (46.0 versus 42.9) and had significantly longer duration of HIV, lower nadir CD4, and higher rates of AIDS (p < .05).
Disability characteristics
Compared to the cognitive attribution group, the physical attributors did not differ on current major depressive disorder, current or lifetime substance use disorders, or any of the objective findings including everyday functioning (employment), severity of cognitive impairment, demographically corrected cognitive domain T scores, or physical problems on examination, or neuromedical clinician rating of functioning (Karnofsky; p > .05). Also, 28 % of physical attributors showed no physical abnormalities on examination. The only characteristics that did differ were that the cognitive attribution group reported a greater number of depressive symptoms and higher prevalence of lifetime major depressive disorder compared to the physical attribution group (p < .05).
Nature of disability attribution in HIV infection
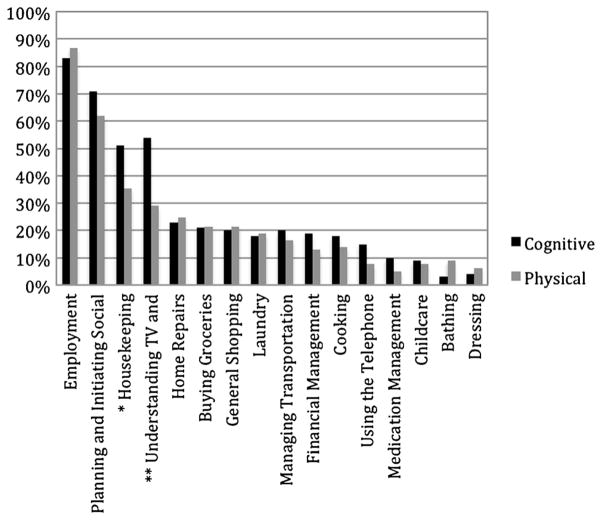
Of the 16 domains of daily functioning activities queried in the modified Lawton and Brody ADL form (Fig. 2), only two were reported as less problematic by participants who attributed their dependence to strictly physical causes (housekeeping and understanding reading and television material; p < .05). Although understanding reading and television materials has primarily cognitive requirements on face value, almost one third (29 %) of participants with strictly physical attributions reported needing help on this task and a relatively high percentage of participants who thought they had strictly physical disabilities reported needs for help with other cognitively demanding activities such as planning and initiating social activities (62 %) and financial management (13 %). No group differences were noted on the other 14 daily activity items.
Fig. 2.
Frequency of functional dependence items endorsed by cognitive and physical attributors in the dependent group. Note. Double asterisks indicate significant differences in frequency of endorsement between the two attribution groups at the significance level of p < .01; single asterisk indicates a significance level of p < .05
Discussion
The purpose of this study was to determine whether, in cognitively impaired HIV+ individuals, self-reported dependence in daily functioning and self-reported causal attribution of this dependence are associated with relevant/ supporting objective variables and, as such, whether such self-reports are valid stand-alone tools for diagnosing “symptomatic” HAND subtypes. As hypothesized, reports of ADL dependence were consistently associated with objective evidence of worse disability and more impaired everyday functioning. Dependent participants did not have more severe cognitive performance (either at the domain level or when measured with a global cognitive score), which is consistent with prior findings that the HAND subtypes of “Asymptomatic Neurocognitive Impairment” (ANI) and “Mild Neurocognitive Disorder” (MND) have quite similar neurocognitive profiles, as noted above; however, ANI does confer a significantly increased risk for transitioning to symptomatic HAND over time (Grant et al. 2014). It was anticipated that, if attributions of the cause of ADL dependence were valid, participants with strictly physical causes would have worse objective neuromedical physical findings and those who indicated cognitive causes would show worse performance on neuropsychological testing. By and large, this is not what the data showed. Self-assessed cognitive attribution of functional dependence was consistently associated with other measures of psychiatric disabilities, but was not consistently associated with any objective measures [cognitive (both global and domain scores), neuromedical, or daily functioning]. Additionally, cognitive and physical attributors did not report consistent differences in their ability to complete tasks that were either more cognitively (e.g., manage finances or medications) or physically (e.g., home repairs, laundry, or bathing) demanding. In fact, the only specific daily functioning tasks that cognitive and physical attributors differed on were housekeeping and understanding television and reading materials. While understanding reading and television materials requires primarily cognitive skills, one third of physical attributors reported significant difficulties of this nature, and while housekeeping may be more physically than cognitively demanding, cognitive attributors reported significantly higher rates of difficulty on this task than physical attributors (51 versus 35 %). Relevant to potential insight problems, more than a quarter of physical attributors evidenced no current physical abnormalities on neuromedical examinations. Considered together, these results suggest that HIV+ individuals may be able to accurately assess the presence of functional dependence, but struggle to make accurate assessments about the cause of their functional declines. Consistent with previous studies (e.g., Blackstone et al. 2012), our results indicate that self-reported attribution (especially of cognitive-based difficulties) may be more closely related to affective distress than objective or real-world indicators of causation.
One possible factor contributing to limited accuracy in attribution of causation among functionally dependent HIV+ individuals may be poor metacognition. Metacognition involves the conscious knowledge and monitoring of one’s own cognitive processes (Toglia and Kirk 2000) and is commonly conceptualized as part of “executive functioning” processes that involve the medial prefrontal brain systems (e.g., Brodmann’s area 10, Johnson et al. 2006; Stuss 2011). Prior literature suggests that up to 50 % of HIV+ individuals evidence a metacognitive deficit and that metacognitive inaccuracies are associated with poorer neurocognitive functioning (e.g., Hinkin et al. 1996; Casaletto et al. 2014) and worse self-reported predictions of cognitive performance (Casaletto et al. 2014). These metacognitive deficits extend beyond the HIV+ population. Individuals with non-HIV-associated neurodevelopmental disorders (e.g., attention-deficit/hyperactivity disorder) evidence similar difficulties in self-assessing their disabilities (e.g., driving safety self-report compared to actual performance; Knouse et al. 2005). In fact, in other populations known to have reduced cognitive (e.g., traumatic brain injury) and specifically executive function (e.g., schizophrenia) abilities, not only is poor metacognition observed (Hart et al. 1998; O’Keeffe et al. 2007; Gould et al. 2015), but metacognitive impairments are predictive of real-world functioning above and beyond cognitive and functional capacity testing (Gould et al. 2015). Given the complex metacognitive abstraction required in order to accurately identify the nature and, especially, etiology of functional problems and the prefrontal neural and cognitive systems often impacted by HIV infection, it may not be surprising that HIV+ participants evidenced difficulties with such granular self-reflective questions. While these studies provide a framework for understanding poor self-assessment in HIV, future studies should evaluate the direct link between metacognitive deficits and self-reported causal attribution inaccuracies to confirm this possible mechanistic explanation of poor attributional self-report.
Additionally, it is likely that affective distress may also contribute to these self-report discrepancies. Depression often occurs in the context of HIV infection (Catz et al. 2002; Kelly et al. 1993) and is associated with more rapid HIV disease progression, higher rates of mortality (Ickovics et al. 2001), and worse everyday functioning difficulties (e.g., Ammassari et al. 2004). In fact, ADL-dependent individuals reported very high rates of depressive symptoms on the BDI-II and almost a quarter of them met criteria for current major depressive disorder (Table 1). While everyday functioning difficulties are elevated in depressed individuals, the subjective perception of both physical disability severity (Severeijns et al. 2001) and cognitive deficits (Farrin et al. 2003) can also be significantly influenced by psychological distress. Across the literature, poor mood is a robust predictor of self-reported quality of life (e.g., Pompili et al. 2013), as well as less accurate self-awareness (e.g., Juengst et al. 2012), and increased complaints of cognitive difficulties (e.g., van Gorp et al. 1991). Therefore, it is no surprise that our results showed that self-reported cognitive attribution was primarily associated with current mood and lifetime depression, but not with objective measures of physical and cognitive impairments. Blackstone et al. (2012) identified that current mood influenced self-reports of daily functioning in HIV+ participants and, when relying solely on self-report to diagnose HAND, a greater number of patients were categorized as having HAD than when using more objective assessments. Further, by augmenting self-report assessment by also including performance-based evaluation of functioning, this depression bias was reduced and the specificity of differentiating asymptomatic versus symptomatic HAND improved (Blackstone et al. 2012).
Limitations
While the present study provides insight into the validity of self-report measures for differential diagnosis of HAND subtypes, some limitations exist. Some of the objective measures used here were at least partly influenced by participant responses. For example, while the Karnofsky scale represents a clinician’s rating of the patient’s performance on a physical examination, clinicians use information gathered from the patient during the medical history to inform their assessment. Additionally, there were no direct evaluations of the functional domains on which patients reported dependence (e.g., using the telephone). Although employment was used as one representation of objective everyday functioning performance, we recognize that employment may be influenced by factors outside of everyday functioning abilities (e.g., availability of jobs, qualifications for available jobs, motivation to obtain employment) and therefore is an imperfect representation of objective daily functioning. Furthermore, this study only captured the dichotomous response of whether participants were or were not employed. However, the level of occupational functioning in their current position as well as whether their occupational responsibilities, type of work, pay, or full-time status were decreased as a result of functional decline were not evaluated among those employed. Future studies would benefit from more refined real-world functional assessment. Despite the alternative explanations for unemployment and possible functional declines for those who retained employment, unemployment is consistently a robust predictor of other everyday functioning measures throughout the literature (e.g., Kalechstein et al. 2003; Heaton et al. 2004a, b) and likely provides a reasonably adequate proxy for more detailed employment information for the purposes of the current study. While a panel of experts in the neurological effects of HIV designed the physical composite variable, its psychometric properties remain unknown as this is the first study to use the composite. Finally, the cross-sectional nature of this sample precludes us from evaluating the change in attribution over time. It is possible that over time patients’ objective findings and subjective reports may become more convergent as their disease progresses and their cognitive versus physical declines become more apparent.
Clinical implications
These findings have significant implications for the weight we assign to a patient’s own attribution of everyday functioning dependence. Especially when these determinations guide our diagnostic categorizations, as in HAND, accuracy of the causative attribution of reduced functioning is important. A misdiagnosis of asymptomatic HAND could result in delayed interventions (e.g., a patient may not be provided with oversight of or cognitive tools for improving medication management because the cause of mismanagement is not attributed to forgetfulness), more rapid disease progression (e.g., if medications are mismanaged by the patient, viral suppression may be lost), imprecise clinical recommendations (e.g., patients, family members, and clinicians may not monitor a patient’s needs/concerns as closely if the patient is deemed functionally intact regarding cognitive abilities), and an increased risk of public health concerns (e.g., driving privileges may continue unchecked despite decreased driving ability due to impaired cognition). To enhance the accuracy of attributions, clinicians may be able to add short everyday functioning measures (e.g., UCSD Performance-Based Skills Test, which takes less than 10 min to complete; Mausbach et al. 2008) to evaluate the cognitive capacity associated with everyday functioning tasks, screen for depressive symptoms to be more alert to possible affective bias, possibly refer participants for more thorough neuropsychological testing and/ or neuromedical assessments, and maybe even obtain corroborating information from observers in order to differentiate the cognitive versus physical causes of known functional decline with more objective information. In instances where self-report of attribution does not correspond with objective performance/information, clinical judgment may help weigh the objective evidence more than subjective self-report to provide a more accurate differential diagnosis for HAND. In any event, whenever there is clear evidence of significant cognitive impairment, one should be wary about attaching the “asymptomatic” label based only upon self-report of non-cognitive attribution of causes of dependence.
Acknowledgments
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER; https://www.charterresource.ucsd.edu) is supported by awards N01 MH22005, HHSN271201000036C, and HHSN271201000030C from the National Institutes of Health. The CNS HIVAnti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; and Washington University, St. Louis, and is headquartered at the University of California, San Diego, and includes Director Igor Grant, M.D.; Co-directors Scott L. Letendre, M.D., Ronald J. Ellis, M.D., Ph.D., and Thomas D. Marcotte, Ph.D.; Center Manager Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), and J. Allen McCutchan, M.D.; Laboratory and Virology Component: Scott Letendre, M.D. (Co-P.I.), and Davey M. Smith, M.D. (Co-P.I.).; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., and Matthew Dawson; Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Michael J Taylor, Ph.D., and Rebecca Theilmann, Ph.D.; Data Management Component: Anthony C. Gamst, Ph.D. (P.I.), and Clint Cushman; Statistics Component: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., and Reena Deutsch, Ph.D.; Johns Hopkins University Site: Ned Sacktor (P.I.) and Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (Co-P.I.), David Simpson, M.D. (Co-P.I.), and Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), and Kaori Phillips, B.S.N.; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.), Christina Marra, M.D. (Co-P.I.), and Sher Storey, PA-C.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), and Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., and Mengesha Teshome, M.D. The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Government.
Footnotes
Compliance with ethical standards Study procedures were approved by the Human Subjects Protection Committees of each participating university. All participants provided written informed consent prior to study participation.
Conflict of interest Ms. Obermeit is funded by a National Institute on Drug Abuse training grant DA031098. Ms. Casaletto is funded by a National Institute of Health grant F31DA035708. Mr. Franklin receives support from National Institute of Health grants HHSN271201000030C and HHSN271201000036C. Dr. Letendre receives support from National Institute of Health grants HHSN271201000036C, R01MH58076, R01MH92225, P50DA26306, and P30MH62512 and has received support for research projects from Abbott, Merck, Tibotec, and GlaxoSmithKline; has consulted for Gilead Sciences, GlaxoSmithKline, Merck, and Tibotec; and has received lecture honoraria from Abbott and Boehringer-Ingelheim. Dr. Ellis received National Institute of Health grants R01MH058076, U01MH83506, P30MH62512, R01MH83552, P50DA26306 , R01MH095621 , 2U01NS32228 , and HHSN271201000036C and consultant fees from NeurogesX. Dr. Fennema-Notestine has received research support from National Institute of Health grants R01NS080655, R21DA037667, R01DA039775, R01AG048650, R03MH103995, and R01MH107345; R01 AG022381, P30 M H062512, and P50 DA026306; R01MH084796; and HHSN271201000036C. Dr. Vaida receives research support from National Institute of Health grants P30 MH62512, P50 DA26306, R01 MH083552, R01 AI47033, U01 AI74521, R01 MH085608, HHSN271201000030C, and HHSN271201000036C and Precision Photonics Corporation grant AI068543 and has served on a data safety and management board for Ardea Biosciences, Inc. Dr. Collier is supported by National Institute of Health grants MH22005, MH107345, AI069481, AI068636, AI120176, AI111806, NS082120, AI057005, DA037979, AI27757, and AI27767; had past research support from Bristol-Myers Squibb, Merck & Company, and Roche Molecular Systems; and is a member of a Data, Safety, and Monitoring Board for Merck-sponsored studies. Dr. Marra receives research support from National Institute of Health grants R01NS082120, R01NS34235, and R01MH107345 and receives royalties from Lippincott Williams and Wilkins and from UptoDate. Dr. Clifford is supported by National Institute of Health and Alzheimer Association grants NS077384, AI69495, NR012907, NR014449, NR012657, and UL1 TR000448; receives research support from Lilly and Roche; and has provided scientific advisory or consulting to Amgen, Biogen, Inhibikase, Genzyme/Sanofi, Takeda/Millennium, Roche/Genentech, Novartis, GSK, BMS, Pfizer, Quintiles, and Drinker Biddle & Reath (PML Consortium Scientific Advisory Board). Dr. Gelman receives support for National Institute of Health grants R01MH101017, R01MH107345, R01MH104134, R01NS072005, R01NS079166, R01DA036165, R56HL129881, and U24MH10093001. Dr. Sacktor receives support from National Institute of Health grants U01AI035042 , RO1AG034852 , HHSN271201000036C, RO1NS081196, RO1AG042165, P30MH075673, and RO1MH099733. Dr. Morgello receives support from National Institute of Health grants U24MH100931, R25MH080663, RO1MH107345, R21NR015009, R21DK105917, and RO1DA037611. Dr. Simpson receives research support from National Institute of Health grants U01MH083501, HHSN271201000027C, and UL1TR000067 and provided consultancy to GlaxoSmithKline and Gilead. Dr. McCutchan receives support from National Institute of Health grants P30MH62512, U01MH83506, U01AI69432, HHSN271201000036C, K30 RR22681, R01MH58076, and U13MH81676 and National Institute of Health/Centers for Disease Control and Prevention (CDC) grant U2G PS00623 and authors chapters on HIV for the Merck Manual. Dr. Grant receives support from National Institute of Health grants P30MH62512, P50DA26306, R01MH107345, 2RF1AG15301, R21DA036608, and R01MH094159. Dr. Heaton receives ongoing research support from National Institute of Health grants R01MH92225, P50DA26306, P30MH62512, R01MH60720, R01MH58076, R01MH78737, U01MH83506, R01MH83552, R01MH80150, and HHSN271201000036C.
References
- Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, Monforte ADA, Wu AW, Starace F. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45:394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price W, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory: second edition manual. San Antonio: 1996. [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Woods SP, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Rivera-Mindt M, Deutsch R, Ellis RJ, Hampton Atkinson J, Grant I CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Group. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18:79–88. doi: 10.1017/s135561771100141x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C, Woods S, Gonzalez R, Conover E, Marcotte T, Grant I, Heaton R. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2005;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Doyle KL, Weber E, Woods SP. Self-predictions of prospective memory in HIV-associated neurocognitive disorders: evidence of a metamemory deficit. Arch Clin Neuropsychol. 2014;29:818–827. doi: 10.1093/arclin/acu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz SL, Gore-Felton C, McClure JB. Psychological distress among minority and low-income women living with HIV. Behav Med. 2002;28:53–60. doi: 10.1080/08964280209596398. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability. In: Tarter RE, Goldstein G, editors. Adv Clin Neuropsychol. Vol. 3. 1986. pp. 95–126. [DOI] [Google Scholar]
- Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, Miller B, Valcour V. Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Res Hum Retro. 2013;29:949–956. doi: 10.1089/aid.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrin L, Hull L, Unwin C, Wykes T, David A. Effects of depressed mood on objective and subjective measures of attention. J Neuropsychiatry Clin Neurosci. 2003;15:98–104. doi: 10.1176/jnp.15.1.98. [DOI] [PubMed] [Google Scholar]
- Gould F, McGuire LS, Durand D, Sabbag S, Larrauri C, Patterson TL, Twamley EW, Harvey PD. Self-assessment in schizophrenia: accuracy of evaluation of cognition and everyday functioning. Neuropsychology. 2015;29:675–682. doi: 10.1037/neu0000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Heaton RK CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Group. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82:2055–2062. doi: 10.1212/wnl.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T, Giovannetti T, Montgomery MW, Schwartz MF. Awareness of errors in naturalistic action after traumatic brain injury. J Head Trauma Rehabil. 1998;13:16–28. doi: 10.1097/00001199-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Taylor MJ, Manly JJ. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky D, Saklofske D, Chelune GJ, Heaton RK, Ivnik RJ, Bornstein R, Prifitera A, Ledbetter MF, editors. Clinical interpretation of the WAIS-III and WMS-III. San Diego, California: 2002. [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I HIV Neurobehavioral Research Center (HNRC) Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004a;10:317–331. doi: 10.1017/s1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian adults scoring program. Odessa: 2004b. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/wnl.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, van Gorp WG, Satz P, Marcotte T, Durvasula RS, Wood S, Campbell L, Baluda MR. Actual versus self-reported cognitive dysfunction in HIV-1 infection: memory-metamemory dissociations. J Clin Exp Neuropsychol. 1996;18:431–443. doi: 10.1080/01688639608408999. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Thrasher D, Goetz MB, Stefaniak M. Medication adherence among HIV+ adults effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J HIV Epidemiology Research Study Group. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. J Am Med Assoc. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst S, Skidmore E, Pramuka M, McCue M, Becker J. Factors contributing to impaired self-awareness of cognitive functioning in an HIV positive and at-risk population. Disabil Rehabil. 2012;34:19–25. doi: 10.3109/09638288.2011.587088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, van Gorp WG. Neurocognitive functioning is associated with employment status: a quantitative review. J Clin Exp Neuropsychol. 2003;25:1186–1191. doi: 10.1076/jcen.25.8.1186.16723. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemo-therapeutic agents in cancer. New York: 1949. [Google Scholar]
- Kelly JA, Murphy DA, Bahr GR, Koob JJ, Morgan MG, Kalichman SC, Stevenson LY, Brasfield TL, Bernstein BM, St Lawrence JS. Factors associated with severity of depression and high-risk sexual behavior among persons diagnosed with human immunodeficiency virus (HIV) infection. Health Psychol. 1993;12:215–219. doi: 10.1037/0278-6133.12.3.215. [DOI] [PubMed] [Google Scholar]
- Knouse LE, Bagwell CL, Barkley RA, Murphy KR. Accuracy of self-evaluation in adults with ADHD: evidence form a driving study. J Atten Disord. 2005;8:221–234. doi: 10.1177/1087054705280159. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontol. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhassoon O, Arfaa K, Grant I. The impact of HIV-related neuropsychological dysfunction on driving behavior. J Int Neuropsychol Soc. 1999;5:579–592. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Bowie CR, Harvey PD, Twamley EW, Goldman SR, Jeste DV, Patterson TL. Usefulness of the UCSD performance-based skills assessment (UPSA) for predicting residential independence in patients with chronic schizophrenia. J Psychiatr Res. 2008;42:320–327. doi: 10.1016/j.jpsychires.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Heaton RK, Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, Ellis R, Grant I, Woods SP HIV Neurobehavioral Research Program (HNRP) Group. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012;61:341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D, Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK The HNRC Group. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-revised, brief visuospatial memory test-revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-card version. J Clin Exp Neuropsychol. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe F, Dockree P, Moloney P, Carton S, Robertson I. Characterising error-awareness of attentional lapses and inhibitory control failures in patients with traumatic brain injury. Exp Brain Res. 2007;180:59–67. doi: 10.1007/s00221-006-0832-9. [DOI] [PubMed] [Google Scholar]
- Pompili M, Pennica A, Serafini G, Battuello M, Innamorati M, Teti E, Girardi N, Amore M, Lamis DA, Aceti A, Girardi P. Depression and affective temperaments are associated with poor health-related quality of life in patients with HIV infection. J Psychiatr Pract. 2013;19:109–117. doi: 10.1097/01.pra.0000428557.56211.cf. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, Heaton RK, Schweinsburg BC, Ellis RJ, Grant I, Marcotte TD San Diego HIV Neurobehavioral Research Center (HNRC) Group. A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology. 2011;25:511. doi: 10.1037/a0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17:165–172. doi: 10.1097/00002508-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. J Int Neuropsychol Soc. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Toglia J, Kirk U. Understanding awareness deficits following brain injury. NeuroRehabilitation. 2000;15(1):57–70. [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, Piselli P, Lorenzini P, Visco-Comandini U, Vlassi C, Quartuccio ME, Giulianelli M. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retrovir. 2005;21:706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Satz P, Hinkin C, Selnes O, Miller EN, McArthur J, Cohen B, Paz D. Metacognition in HIV-1 seropositive asymptomatic individuals: self-ratings versus objective neuropsychological performance. J Clin Exp Neuropsychol. 1991;13:812–819. doi: 10.1080/01688639108401091. [DOI] [PubMed] [Google Scholar]
- Vigil O, Posada C, Woods SP, Hampton Atkinson J, Heaton RK, Perry W, Hassanein TI, Grant I, Letendre SL HIV Neurobehavioral Research Center (HNRC) Group. Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. J Clin Exp Neuropsychol. 2008;30:805–815. doi: 10.1080/13803390701802354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Dawson M, Scott JC, Grant I HIV Neurobehavioral Research Center (HNRC) Group. Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. J Clin Exp Neuropsychol. 2006;28:1030–1042. doi: 10.1080/13803390500350985. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Composite international diagnostic interview (CIDI, version 2.1) Geneva: 1998. [Google Scholar]