Abstract
Introduction
Strain elastography that uses the body’s cardiorespiratory pulsations to determine tissue stiffness (referred to here as “ambient strain elastography”) has not been previously described for the assessment of carpal tunnel syndrome. The objective of this study is to assess the reliability of ultrasonographic ambient strain elastography in the evaluation of carpal tunnel syndrome and compare median nerve stiffness between patients and healthy controls.
Methods
Ambient strain elastography was used to examine 17 patient and 26 control wrists in cross-sectional and longitudinal views, twice by two observers. The strain ratio between the median nerve and nearby tendons was obtained and tested for intra- and inter-rater reliability and differences between patients and healthy controls.
Results
Intra- and inter-rater reliabilities were strong, even for the less experienced rater (lowest r=0.566, highest r=0.905; p<0.001 for all comparisons). No significant difference in strain ratio between those with carpal tunnel syndrome and controls was detected (cross-sectional image p=0.32; longitudinal image p=0.20). Strain ratio did not correlate significantly with traditional ultrasound measures of carpal tunnel syndrome (lowest p=0.26), but did correlate significantly with body mass index if obtained from cross-sectional images (r=0.346; p=0.02).
Conclusions
This strain elastography method is reliable but does not show changes in median nerve stiffness with carpal tunnel syndrome. Body mass index may influence elastography and further studies should be conducted to clarify this relationship.
Keywords: carpal tunnel syndrome, strain elastography, median nerve, ultrasound, tendons, neuropathy
Carpal tunnel syndrome is a relatively common neuropathy caused by compression of the median nerve as it travels through the carpal tunnel in the wrist. This compression causes pain, paresthesia, and weakness in the wrist and hand. It is currently diagnosed based on clinical findings and nerve conduction studies (Newington et. al., 2015).
Neuromuscular ultrasound for the evaluation of carpal tunnel syndrome (CTS) was first described over two decades ago (Molitor, 1988), and since then has clarified that the median nerve enlarges proximal to the transverse carpal ligament in CTS and the nerve develops increased vascularity, decreased echogenicity, and decreased mobility (Buchberger et. al., 1991; Wiesler et. al., 2006; Klauser et. al., 2009). These ultrasonographic findings have both increased diagnostic capabilities and improved understanding of the pathophysiology of CTS. Since 2013, a small number of studies have been conducted to assess a newer ultrasonographic property of nerves, termed elastography. Elastography, which can be conducted using ultrasound or magnetic resonance imaging, allows for the quantification of tissue stiffness. Two main forms of elastography exist: strain elastography and shear wave elastography. Strain elastography measures tissue distortion due to pressure in order to determine the stiffness of visible objects, while shear wave elastography measures the speed of waves moving perpendicular to the probe to determine the stiffness of the medium (Shiina et. al., 2015). Of the studies performed on the median nerve in CTS, eight studies of the median nerve in have used strain elastography (Orman et. al., 2013; Miyamoto et. al., 2014a; Miyamoto et. al., 2014b; Yoshii et. al., 2014; Ghajarzadeh et. al., 2015; Liao et. al., 2015; Ogur et. al., 2015; Yoshii et. al., 2015) and one shear wave elastography (Kantarci et. al., 2014); many of these studies have found that patients with CTS have stiffer median nerves (Orman et. al., 2013; Kantarci et. al., 2014; Miyamoto et. al., 2014a; Yoshii et. al., 2014; Ghajarzadeh et. al., 2015).
This study was conducted to assess strain elastography of the median nerve using a technique not previously described for this purpose. This form of strain elastography relies on small oscillations in tissue – such as those caused by vascular pulsations – to distort the tissues, then compares two areas to each other to determine a strain ratio. This will be referred to as “ambient strain elastography.” The advantages of this technique include greater availability and better resolution of small objects than shear wave elastography (Shiina et. al., 2015), and previous studies have used it to evaluate tissues for which the application of pressure is problematic, such as the liver (Morikawa et. al., 2011). Furthermore, it is less reliant on the ultrasonographer as the pressure applied by the probe is not involved in calculating the strain ratio. The goal of this study is to assess the reliability of this form of strain elastography and the capability of this technique to differentiate the nerves of those with CTS from healthy controls, which was achieved.
Materials and Methods
Participants in this study were recruited in the Diagnostic Neurology Laboratory at Wake Forest School of Medicine over a 3 month period of time. The study was approved by the Wake Forest Institutional Review Board and all participants signed written informed consent. Eleven patients with consistent symptoms and meeting published electrodiagnostic criteria for CTS were enrolled, 7 of whom had bilateral disease (Jablecki et. al., 2002). Ultrasound images were captured as described below only on symptomatic wrists, resulting in 18 sets of images. As the median nerves in the asymptomatic wrists of patients with CTS are frequently found to have abnormal properties, fourteen asymptomatic volunteers served as controls (Bodofsky et. al., 2001). Ultrasound images for the controls were captured bilaterally, resulting in 28 sets of images. The study did not exclude participants with bifid median nerves or persistent median arteries; all participants were right-handed. The race of each patient was classified by the ultrasonographer (MSC). The effect of race, if any, on nerve elastography is not known, so this variable was recorded to assess for any effect. None of the participants in this study had known diabetes, renal failure, or inflammatory conditions.
Ultrasound imaging was performed by an experienced neuromuscular ultrasonographer (MSC) using a Philips iU22 Ultrasound device with a 12-5 MHz linear array transducer, designed for ambient strain elastography; this product is not currently approved for this use by the FDA. All images were obtained in the traditional position with the hand relaxed, palm-up, on a solid surface, facing the ultrasonographer (Wiesler et. al., 2006, Cartwright et. al., 2015). The depth of image capture was set at 3 cm so that all contents of the anterior wrist and forearm were visualized. Overall gain was adjusted to optimize the image and the time-gain compensation was set in the middle for all levels. The focal zone was adjusted to the level of the median nerve and the length of the focal adjusted to cover all aspects of the median nerve. Routine B-mode images were obtained first and then the elastography overlay was turned on to capture a brief video. For each wrist, median nerve cross-sectional areas were obtained at the wrist and forearm, along with a subjective assessment of median nerve echogenicity, mobility, and vascularity (Cartwright et. al., 2015). The echogenicity was described as “normal” if multiple fascicles could be identified, “slightly reduced” if there were 2 or fewer fascicles, and “decreased” if there were no fascicles. The mobility was described as “normal” if the nerve moved deep with finger and wrist flexion, “slightly reduced” if the nerve moved only side-to-side, and “decreased” if it remained in place. The vascularity was described as “normal” if there were no areas of increased flow, “slightly increased” if there were 1–2 areas of increased flow, and “increased” if there were multiple areas of increased flow; flow was detected using power Doppler.
For the elastography portion of the study, measures of tissue strain were obtained from 5 second videos taken from two views: (1) a cross-sectional view at either the nerve’s largest point (CTS patients) or the distal wrist crease (controls), and (2) a longitudinal view of the median nerve centered over the transverse carpal ligament. Measures of tissue strain were displayed as a translucent color map that could be laid over the ultrasound image in real time (Figure 1).
Fig 1. Sample Cross-Sectional and Longitudinal Images with ROIs and Strain Color Map.
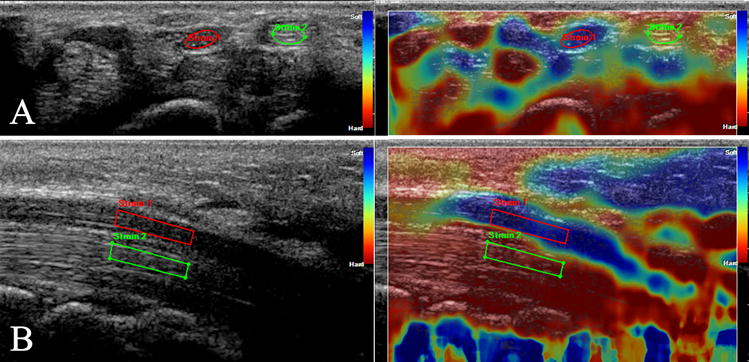
Raw ultrasound images on the left with sample overlaid color map of strain on the right, which was not used for ROI placement. Panel A is a cross-sectional ultrasound of a subject’s wrist at the distal wrist crease. Elliptical regions of interest are drawn within the median nerve (“strain 1”) and flexor carpi radialis tendon (“strain 2”). Panel B is a longitudinal ultrasound of a subject’s wrist centered on the transverse carpal ligament. A simple polygonal region of interest is drawn within a homogenous section of the median nerve near the center of the image (“strain 1”), and copied onto a representative portion of the flexor digitorum tendons directly below it (“strain 2”).
The videos were transferred to a computer installed with Philips QLab 9.0, and analyzed in the Elastography Quantification Module. This module is a proprietary Philips software package that allows for the analysis of still images and video. By selecting two areas of interest the strain ratio between the area can be calculated. For each video, regions of interest (ROIs) were drawn over nerve and tendon (as detailed below), without reference to the strain color map, using the first frame of the video. Repeat images were not used in the study. One control subject was dropped due to poor image quality.
For each cross-sectional video, elliptical ROIs were drawn within the median nerve and again within the flexor carpi radialis tendon, which was chosen as a reference tissue as it is outside the carpal tunnel. The ROI was drawn to be the largest ellipse that would fit entirely within the structure (Figure 1 A). Septate and bifid nerves with minimal intervening tissue were treated as single nerves, with a single ellipse drawn across both segments. For bifid nerves with a significant amount of intervening tissue, the segment closest to the center of the image was measured alone.
For each longitudinal video, a 4–6 vertex polygonal ROI was drawn within a homogenous segment of the median nerve near the center of the image (Figure 1 B). This ROI was then copied and placed over a representative portion of the flexor digitorum tendons below the nerve in the image; the size and shape were not adjusted unless necessary to fit the ROI within the confines of the tendons.
The placement of the ROIs was repeated four times to account for intra- and inter-observer variability: twice by an experienced neurologist (MSC) and twice by a medical student (MJM), with at least 48 hours between repetitions. For each video, QLab printed the nerve-to-tendon strain ratio for each frame to a file, from which a mean and standard deviation were calculated.
Intra-rater variability was measured by comparing the first and second set of observations made by each observer, while inter-rater variability was measured by comparing the observers based on their second set of observations alone. All comparisons were calculated using Pearson’s correlation coefficient and performed separately for the subset of cross-sectional images, the subset of longitudinal images, and all images combined.
The demographics of the control and experimental groups were then compared to test for signficant differences. Age and body mass index (BMI) were shown to be normally distributed in both groups using a probability-probability plot (p-p plot) and were compared using two-sample two-tailed t-tests assuming unequal variances. Ethnicity and sex were compared using a Fisher’s Exact Test.
Standard ultrasound information regarding the median nerve was then compared between the control and patient group. The subjective echogenicity, mobility, and vascularity were compared using a Fisher’s Exact Test. The wrist cross-sectional area, forearm cross-sectional area, and the wrist/forearm ratio were demonstrated to have normal distributions in both the control and patient groups using p-p plots, after which they were compared between the contol and patient groups using two-sample two-tailed t-tests assuming unequal variances.
The second set of observations made by the more experienced observer was then chosen as a representative sample of the data for further analyses. Strain ratio was shown to not follow a normal distribution using a p-p plot and was log-transformed before being analyzed. The strain ratio was compared between both wrists in those with bilateral CTS and all of the controls, and no significant correlation was detected, indicating statistical independence between wrists. All analyses using strain ratio were performed separately on the cross-sectional and longitudinal images due to the use of different tendons and to avoid including non-independent observations in the data. The strain ratio distributions were compared between the control and patient groups using a Kolmogorov-Smirnov test and not found to be significantly different in either the cross-sectional images (p=0.93) or the longitudinal images (p=0.247). Finally, the strain ratio was compared between the control and patient groups using a multi-variable linear regression to control for subject BMI.
The strain ratio was then analyzed for relationships to other data, independent of the presence or absence of CTS. First, echogenicity, mobility, and vascularity were separately compared to the strain ratio by interpreting them as dummy variables using a multivariable regression that controlled for BMI. The median nerve cross-sectional areas at the wrist, forearm, and the ratio thereof were also separately compared to the strain ratio using a multivariable regression that controlled for BMI. Finally, BMI itself was compared to the strain ratio using linear regression to obtain Pearson’s correlation coefficient.
Results
Intra and inter-rater reliability were found to be strong (Table 1). Statistically significant correlations between the first and second observations for both the neurologist and the medical student were detected, although the reliability for the neurologist was stronger. There were also significant correlations between both observers’ measurements of the cross-sectional images and the longitudinal images, although the reliability for the longitudinal images was stronger (Table 1).
Table 1.
Intra- and Inter-rater Reliability for Elastographic Strain Ratio Measured as Pearson’s Correlation Coefficient
| Cross-Sectional Images | Longitudinal Images | All Images | |
|---|---|---|---|
| Neurologist Intra-Rater | r = 0.828 | r = 0.853 | r = 0.813 |
| Medical Student Intra-Rater | r = 0.631 | r = 0.806 | r = 0.604 |
| Inter-Rater | r = 0.566 | r = 0.905 | r = 0.590 |
All p-values for Pearson product-moment correlation coefficients in this table are significant with p-values < 0.001.
The only significant demographic difference between the two groups was BMI (p=0.01), which was controlled throughout the rest of the analyses. There was not a significant difference in age, ethnicity, or sex (Table 2).
Table 2.
Comparisons Between Healthy Controls and Patients with Carpal Tunnel Syndrome (CTS)
| Variable | Controls | CTS | P-value |
|---|---|---|---|
| n = 13 subjects, 26 wrists | n = 11 subjects, 18 wrists | ||
| Age* | 45 (8.4) years | 57 (19) years | 0.05 |
| BMI* | 26.2 (3.82) kg/m2 | 35.5 (9.37) kg/m2 | 0.01 |
| Ethnicity† | 8 W, 4 B, 2 A | 10 W, 1 B, 0 A | 0.27 |
| Sex† | 2 male, 12 female | 2 male, 9 female | 0.99 |
| Cross-Sectional Strain Ratio‡ | 1.83 (1.47) | 3.45 (5.68) | 0.32 |
| Longitudinal Strain Ratio‡ | 1.46 (0.981) | 1.00 (0.435) | 0.20 |
| Echogenicity† | 26 N, 0 SR, 0 D | 0 N, 12 SR, 6 D | < 0.001 |
| Mobility† | 19 N, 7 SR, 1 D | 0 N, 8 SR, 10 D | < 0.001 |
| Vascularity† | 24 N, 2 SI, 0 I | 10 N, 7 SI, 1 I | 0.01 |
| Wrist area* | 9.77 (1.65) mm2 | 18.2 (6.07) mm2 | < 0.001 |
| Forearm area* | 6.91 (1.19) mm2 | 7.61 (1.29) mm2 | 0.08 |
| Wrist/Forearm ratio* | 1.44 (0.260) | 2.47 (0.909) | < 0.001 |
Results are reported as means (standard deviations) except for ethnicity, sex, echogenicity, mobility, and vascularity, which are reported as the number of wrists in each category (see below for explanation of categories).
Compared using a two-sample two-tailed t-test assuming unequal variances
Compared using Fisher’s Exact Test
Compared using Multivariable Regression with respect to BMI
Ethnicity categories: Asian (A), Black (B), White (W)
Echogenicity scale: Normal (N), Slightly Reduced (SR), Decreased (D)
Mobility scale: Normal (N), Slightly Reduced (SR), Decreased (D)
Vascularity: Normal (N), Slightly Increased (SI), Increased (I)
All three assessments of subjective echogenicity, mobility, and vascularity were significantly different between the two groups, as were the measurements of cross-sectional area at the wrist, forearm, and the ratio thereof (Table 2). However, none of these variables independently correlated with strain ratio when the data was controlled for BMI (Table 3).
Table 3.
Correlations Between Strain Ratio and Other Parameters
| Variable | Correlation with Cross-Sectional Strain Ratio | Correlation with Longitudinal Strain Ratio | ||
|---|---|---|---|---|
| Echogenicity | r = 0.384 | p = 0.25 | r = 0.022 | p = 0.52 |
| Mobility | r = 0.366 | p = 0.42 | r = 0.175 | p = 0.38 |
| Vascularity | r = 0.352 | p = 0.67 | r = 0.206 | p = 0.26 |
| Area at Wrist | r = 0.348 | p = 0.82 | r = 0.134 | p = 0.60 |
| Area at Forearm | r = 0.346 | p = 0.91 | r = 0.157 | p = 0.46 |
| Wrist/Forearm Ratio | r = 0.354 | p = 0.60 | r = 0.149 | p = 0.51 |
| BMI* | r = 0.346 | p = 0.02 | r = 0.105 | p = 0.50 |
| Age | r = 0.421 | r = 0.10 | r = 0.105 | p = 0.96 |
Variable not controlled for BMI; all other comparisons controlled for BMI.
No significant differences in strain ratio were detected between the control and patient groups after controlling for BMI. This was true for both the cross-sectional (p=0.32) and longitudinal (p=0.20) images (Table 2). However, BMI was signficantly related to strain ratio in cross-sectional images. This remained true both when the data was controlled for the presence of disease (p=0.01) and independently (p=0.02). However, it was not related to strain ratio in longitudinal images when the data was controlled for disease (p=0.79) nor independently (p=0.50).
Discussion
The strong inter and intra-observer correlations indicate that this method of analyzing elastography data is reliable. The highest intra-observer correlations were found for the more experienced observer (MSC), although those found for the medical student (MJM) were still signficant. The highest inter-observer correlation was with the longitudinal images. The tissues studied have more visible area when examined longitudinally than in cross-section, which may increase the accuracy of strain mapping.
There are several possible explanations for the lack of significant difference in strain ratio between the control and patient groups. The first relates to assumptions inherent in the methods used. Strain elastography is based on measuring the distortion (“strain”) of tissues due to pressure. Constant pressure (i.e. the light application of an ultrasound probe) is not involved in the calculation as there is no baseline to allow comparison, but varying pressure (in this study, cardiovascular pulsations in the wrist) allows the amount of strain to be calculated by comparing extremes of distortion. It is this property that allows ambient strain elastography to have decreased operator reliance.
Despite this advantage, strain ratios remain an imperfect proxy to elasticity because they rely on certain mathematical assumptions. For example, they assume that the relationship between pressure and strain is linear for nervous tissue, a relationship not yet demonstrated. Furthermore, to calculate a strain ratio, one must assume the pressure is evenly distributed throughout the tissue, which may not always be true. A known artifact of strain elastography occurs when pressure builds up along curved boundaries, making the nearby tissues appear softer due to increased distortion. This can be due to excess pressure applied by the ultrasound probe, regardless of whether that pressure is constant or changing (Shiina et. al., 2015). In the images observed for this study, there were instances in which the color map seemed to be offset from the objects it appeared to represent, which may be an example of this artifact. This could indicate that the pressure applied by the ultrasound probe was too great.
In addition, it is possible the radial and ulnar arteries were not able to provide the required even pressure throughout the complex environment of the wrist. This consideration is supported by the relatively large standard deviations in strain measurements, which are comparable to (and in one case larger than) the means themselves (Table 2). In previous studies using different techniques, standard deviations 50% to 77% of the mean are not uncommon (Orman et. al., 2013; Kantarci et. al., 2014; Miyamoto et. al., 2014b; Yoshii et. al., 2014; Shiina et. al., 2015), although other studies have found greater reliability (Yoshii et. al., 2014; Liao et. al., 2015).
Other possible causes for the observations seen in this study include potential changes to the tendons. In the present study, tendons were used as a reference point to calculate a strain ratio that would be inversely proportional to the stiffness of the median nerve; however, this assumes that the stiffness of the tendons remains relatively constant from patient to patient. With this in mind, it is possible that the changes affecting the median nerve in CTS also affect the nearby tendons so that the ratio of the two does not change. Any changes might be expected to affect the contents of the carpal tunnel more than the rest of the wrist, which would lead to less difference between the groups in the longitudinal images (in which the tendons the nerve is compared to are within the carpal tunnel) than in the cross-sectional images (in which the tendon is outside the tunnel).
A final, interesting consideration is the possibility that BMI acts as a confounding factor. BMI is a known risk factor for carpal tunnel syndrome (Shiri et. al., 2015), was found to be significantly higher in the patient population in this study, and has a strong correlation with strain ratio even when the presence of disease was controlled for. If the effect of increased BMI were to soften the nerve, it could potentially serve as a significant confounding factor in elastographic studies of the median nerve that has not been examined in past studies (Orman et. al., 2013; Kantarci et. al., 2014; Liao et. al., 2015; Shiina et. al., 2015). Alternatively, if the effect of BMI were to harden tendons, this would increase the strain ratio in patients with increased BMI – and an increased risk of CTS – without directly relating to the presence of disease itself. With this in mind, reevaluation of the data was performed without controlling for BMI, but did not result in any new significant difference between the groups (p=0.70 for cross-sectional images, p=0.12 for longitudinal). If an effect of BMI is truly present, it is unclear why it would only be manifested in the cross-sectional images, though it may relate to the specific tendons that are used as the reference.
Limitations of this study include its relatively small sample size and the limitations of strain imaging as described above. Although we were able to demonstrate high reliability for measurements on previously captured video loops, this study was not designed to assess the reliability of obtaining the images. Therefore, although all images were obtained by the same ultrasonographer (MSC) to increase the reliability of obtaining similar elastograms, this value is not measurable with this study. Additionally, statistical analyses assumed independence of each data point, which may not always hold true for bilateral measurements. However, the possible lack of independence, which itself is debatable, would not significantly alter the statistical results as correlation was not detected for strain ratio between wrists. Finally, patient blood pressure was not controlled in the study; although this may affect the cardiovascular pulsations use to obtain the images, it is unclear how this would affect the imaging or results.
Further research in the area of ultrasound elastography for CTS will play an important role in developing this field for potential clinical use. The present study highlights several further areas that will be particularly important to examine, in particular the relationship between BMI, CTS, and median nerve strain, as well as the elastography technique most suited to the wrist. Future research should also explore the use of elastography in other neuromuscular diseases, as it could potentially serve as a responsive biomarker in conditions such as myopathy and motor neuron disease.
Ambient strain elastography is reliable but does not show changes in median nerve stiffness with carpal tunnel syndrome. BMI may be a confounding factor, and further studies should be conducted to clarify this relationship.
Acknowledgments
COI and Source of Funding: Authors Dr. Cartwright and Mr. Martin have no financial disclosures or other conflict of interest. Financial support for this study was provided to Dr. Cartwright by the NIH/NINDS (K23NS062892).
Footnotes
Presentations: This paper has not been presented.
References
- Bodofsky EB, Green berg WM, Wu KD. Median nerve compression at the wrist: is it ever unilateral? Electromyography and Clinical Neurophysiology. 2001;41(8):451–456. [PubMed] [Google Scholar]
- Buchberger W, Schön G, Strasser K, Jungwirth W. High-resolution ultrasonography of the carpal tunnel. J Ultrasound Med. 1991;10(10):531–537. doi: 10.7863/jum.1991.10.10.531. [DOI] [PubMed] [Google Scholar]
- Cartwright MS, Griffin LP, Dowlen H, et al. A randomized trial of diagnostic ultrasound to improve outcomes in focal neuropathies. Muscle and Nerve. 2015;52(5):746–753. doi: 10.1002/mus.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajarzadeh M, Dadgostar M, Sarraf P, Emami-Razavi SZ, Miri S, Malek M. Application of ultrasound elastography for determining carpal tunnel syndrome severity. Jpn J Radiol. 2015;33(5):273–278. doi: 10.1007/s11604-015-0416-3. [DOI] [PubMed] [Google Scholar]
- Jablecki CK, Andary MT, Floeter MK, et al. Practice parameter: Electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2002;58(11):1589–1592. doi: 10.1212/wnl.58.11.1589. [DOI] [PubMed] [Google Scholar]
- Kantarci F, Ustabasioglu FE, Delil S, et al. Median nerve stiffness measurement by shear wave elastography: a potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur Radiol. 2014;24(2):434–440. doi: 10.1007/s00330-013-3023-7. [DOI] [PubMed] [Google Scholar]
- Klauser AS, Halpern EJ, De Zordo T, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250(1):171–177. doi: 10.1148/radiol.2501080397. [DOI] [PubMed] [Google Scholar]
- Liao Y-Y, Lee W-N, Lee M-R, et al. Carpal tunnel syndrome: US strain imaging for diagnosis. Radiology. 2015;275(1):205–214. doi: 10.1148/radiol.14140017. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Halpern EJ, Kastlunger M, et al. Carpal tunnel syndrome: diagnosis by means of median nerve elasticity–improved diagnostic accuracy of US with sonoelastography. Radiology. 2014a;270(2):481–486. doi: 10.1148/radiol.13122901. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Siedentopf C, Kastlunger M, et al. Intracarpal tunnel contents: evaluation of the effects of corticosteroid injection with sonoelastography. Radiology. 2014b;270(3):809–815. doi: 10.1148/radiol.13131083. [DOI] [PubMed] [Google Scholar]
- Molitor PJ. A diagnostic test for carpal tunnel syndrome using ultrasound. Journal of Hand Surgery British and European Volume. 1988;13(1):40–41. doi: 10.1016/0266-7681_88_90048-4. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Fukuda K, Kobayashi S, et al. Real-time tissue elastography as a tool for the noninvasive assessment of liver stiffness in patients with chronic hepatitis C. Journal of Gastroenterology. 2011;46:350–358. doi: 10.1007/s00535-010-0301-x. [DOI] [PubMed] [Google Scholar]
- Newington L, Harris EC, Walker-Bone K. Carpal tunnel syndrome and work. Best Pract Res Clin Rheumatol. 2015;29(3):440–453. doi: 10.1016/j.berh.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newington L, Harris EC, Walker-Bone K. Carpal tunnel syndrome and work. Best Pract Res Clin Rheumatol. 2015;29(3):440–453. doi: 10.1016/j.berh.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgur T, Yakut ZI, Teber MA, et al. Ultrasound elastographic evaluation of the median nerve in pregnant women with carpal tunnel syndrome. European Review for Medical and Pharmalogical Sciences. 2015;19(1):23–30. [PubMed] [Google Scholar]
- Orman G, Ozben S, Huseyinoglu N, Duymus M, Orman KG. Ultrasound elastographic evaluation in the diagnosis of carpal tunnel syndrome: initial findings. Ultrasound Med Biol. 2013;39(7):1184–1189. doi: 10.1016/j.ultrasmedbio.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41(5):1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Shiri R, Pourmemari MH, Falah-Hassani K, Viikari-Juntura E. The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev. 2015 Sep; doi: 10.1111/obr.12324. [DOI] [PubMed] [Google Scholar]
- Wiesler ER, Chloros GD, Cartwright MS, Smith BP, Rushing J, Walker FO. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;31(5):726–732. doi: 10.1016/j.jhsa.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Yoshii Y, Ishii T, Etou F, Sakai S, Tanaka T, Ochiai N. Reliability of automatic vibratory equipment for ultrasonic strain measurement of the median nerve. Ultrasound Med Biol. 2014;40(10):2352–2357. doi: 10.1016/j.ultrasmedbio.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Yoshii Y, Ishii T, Tanaka T, Tung W-L, Sakai S. Detecting median nerve strain changes with cyclic compression apparatus: a comparison of carpal tunnel syndrome patients and healthy controls. Ultrasound Med Biol. 2015;41(3):669–674. doi: 10.1016/j.ultrasmedbio.2014.09.020. [DOI] [PubMed] [Google Scholar]


