Abstract
Pulmonary arterial hypertension (PAH) is caused by extensive pulmonary vascular remodeling that increases right ventricular (RV) afterload and leads to RV failure. PAH predominantly affects women; paradoxically, female PAH patients have better outcomes than men. The roles of estrogen in PAH remain controversial, which is referred to as “the estrogen paradox”. Here, we sought to determine the role of estrogen in pulsatile pulmonary arterial hemodynamic changes and its impact on RV functional adaption to PAH. Female mice were ovariectomized and replenished with estrogen or placebo. PAH was induced with SU5416 and chronic hypoxia (SuHx). In vivo hemodynamic measurements showed that (1) estrogen prevented loss of pulmonary vascular compliance with limited effects on the increase of pulmonary vascular resistance in PAH; (2) estrogen attenuated increases in wave reflections in PAH and limited its adverse effects on PA systolic and pulse pressures; and (3) estrogen maintained the total hydraulic power and preserved transpulmonary vascular efficiency in PAH. This study demonstrates that estrogen preserves pulmonary vascular compliance independent of pulmonary vascular resistance, which provides a mechanical mechanism for ability of estrogen to delay disease progression without preventing onset. The estrogenic protection of pulsatile pulmonary hemodynamics underscores the therapeutic potential of estrogen in PAH.
Keywords: Pulmonary Vascular Compliance, Pulmonary Vascular Resistance, Wave Reflection, Estrogen Paradox, Transpulmonary Vascular Efficiency
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare but rapidly progressing, fatal disease with 3-year survival rate of 55% 10 characterized by progressive increase in resistance and loss of compliance that increases right ventricular (RV) afterload. The typical cause of death in PAH is RV failure; thus the ability of the RV to adapt to increased afterload determines outcomes of PAH 27. In contrast to systemic hypertension, PAH preferentially affects women by 4:1 3. Paradoxically, female PAH patients have better RV function and thus better survival rates than their male counterparts 10,12. Intuitively, these clinical findings suggest that the female sex hormone estrogen is an important contributor to PAH development. However, animal studies show that estrogen attenuates the severity of PAH and ovariectomy exacerbates the disease 17,42. The discrepant role of estrogen in PAH is referred to as “the estrogen paradox”2,13. As a step toward resolving this paradox, we sought to determine the role of estrogen in pulmonary vascular hemodynamic changes and their impact on RV functional adaption.
The protective effects of estrogen on the cardiovascular system are well-studied in the systemic circulation 25. In the pulmonary circulation, estrogen has been shown to attenuate hypoxia- and monocrotaline-induced PAH via vasorelaxation and anti-myogenesis 17,41. Our recent studies on mice exposed to a combination of VEGF receptor inhibitor SU5416 and chronic hypoxia (SuHx) have shown that estrogen preserves compliance of proximal pulmonary arteries (PA), which is linked to reduced RV afterload and improved RV function in PAH 21,22. However, it remains unknown whether the change in PA compliance resulting from estrogen treatment is dependent on the change in resistance, since pulmonary circulatory resistance and compliance follow an inverse relationship regardless of disease severity or treatment 18,19. Furthermore, in our previous studies, conduit PAs were isolated and mechanically tested under the same pressure and frequency ranges ex vivo rather than under their respective conditions in vivo. Given the nonlinear viscoelastic behavior of conduit PAs 15,39, in vivo differences in pressure and hemodynamics can affect the measured arterial compliance.
Here we test the hypothesis that estrogen attenuates PA stiffening independent of PA narrowing in PAH. We also hypothesize that estrogen attenuates pulmonary wave reflection and enhances ventricular-vascular energy transmission efficiency by preserving artery compliance in PAH. The objectives of this study are to determine the effect of estrogen on (a) PA functional remodeling (compliance and resistance); (b) wave reflection; and (c) transpulmonary vascular efficiency 7, a sensitive measure of the ease of blood flow through pulmonary vasculature. We used a mouse model of angioproliferative PAH, created via SuHx as done previously 21,22. To quantify PA hemodynamics, we measured PA pressure and flow in vivo, from which we derived pulmonary vascular impedance, a measure that provides the most complete characterization of pulmonary vascular functional status and steady and pulsatile afterload11. Our results demonstrate that estrogen preserves PA compliance despite increased resistance, attenuates wave reflection and enhances transpulmonary vascular efficiency in PAH. These findings provide a functional mechanism by which estrogen protects RV function and shed light on sex disparities in PAH outcomes.
MATERIALS and METHODS
Animal handling
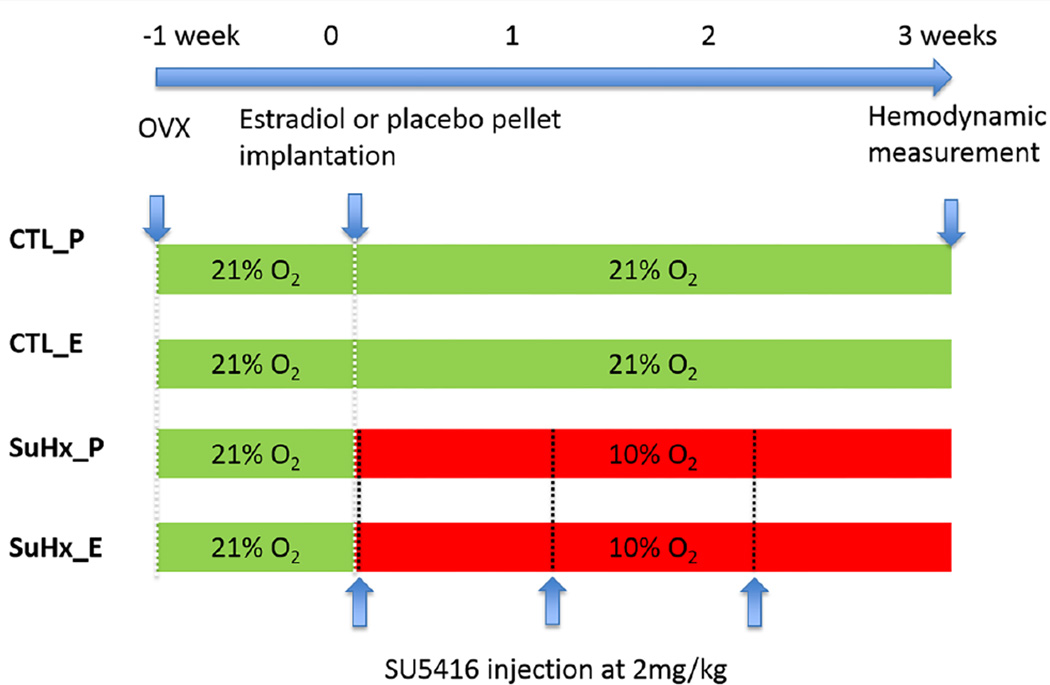
All procedures (Figure 1) were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. Ovariectomized (OVX) female C57BL/6 mice, 9–10 weeks-old, were purchased from a commercial vendor (Jackson Laboratory) to eliminate natural fluctuations in estrogen levels. After 1 week to ensure depletion of endogenous estrogen store, OVX mice were implanted subcutaneously either with estradiol-17β pellets (0.1 mg per 21-day-release, Innovation Research of America) to achieve a plasma estrogen level at or slightly higher than the physiological levels or with placebo pellets as control for estrogen supplement 21. Immediately after pellet implantation, half of the mice (n = 10 mice treated with estrogen and n = 10 treated with placebo) were injected intraperitoneally (i.p.) with SU5416 at 20 mg kg−1 weekly and exposed to normobaric hypoxia (10% O2) for 21 days (SuHx). The animals without SuHx were kept at room air for 21 days. All animals were housed at room temperature with a 12-hour dark/light cycle and with free access to food and water.
Figure 1.
Experimental groups and timeline of experiments. OVX, ovariectomy, CTL_P, placebo-treated control group; CTL_E, estrogen-treated control group; SuHx_P, placebo-treated SuHx group; and SuHx_E, estrogen-treated SuHx group.
In vivo PA hemodynamic measurement
PA pressure and flow were measured simultaneously in vivo following the procedures described by Tabima 35. Briefly, after a mouse was deeply anesthetized with an i.p. injection of urethane at 2 g kg−1 to maintain heart rate, the mouse was intubated and ventilated at a tidal volume of ~225 μl and respiratory rate of ~200 breaths per min while supine on a heated pad to maintain body temperature at 38°C. To expose the RV, a ventral midline incision was made. A 1.2F pressure catheter (Millar Instruments, Houston, TX) was then inserted into the RV apex and advanced to the main PA just distal to the pulmonary valve.
The blood velocity (V) and diameter (D) at the same location in the PA were acquired simultaneously using echocardiography (Visualsonics, Toronto, and Canada) with a 40 MHz probe. Doppler angles were set parallel to the blood flow. The Doppler sample window was chosen sufficiently downstream from the pressure catheter to avoid the jetting around the catheter; PA diameters were obtained during diastole. A specialized probe holder was used to replicate the angle and positioning of the probe for each mouse as much as possible. PA pressure and flow were recorded and analyzed using a customized device (Cardiovascular Engineering, Norwood, MA). Surgery and data collection were completed within ~60 min.
Pulmonary vascular impedance analysis
The pressure (P) and flow (Q = V*πD2/4) waveforms were analyzed in the time domain to derive pulmonary vascular impedance 26. Mean PA pressure (mPAP), pulse pressure (PP = systolic P − diastolic P), stroke volume (SV), cardiac output (CO), and global pulmonary arterial compliance (SV/PP) were obtained. Total pulmonary vascular resistance Z0 (or tPVR = mPAP/CO) and characteristic impedance (ZC) were calculated as described previously 35, except that ZC = dP/dQ was taken prior to when Q reaches 45% of its maximum value to ensure the linearity of P and Q waveforms. The pulse wave reflection (Ʈ) was defined as Γ = (Z0 − ZC)/(Z0 + ZC), which characterizes impedance mismatch between proximal and distal beds 38.
Wave separation analysis
The pulse wave velocity (PWV) at the main PA was determined from ZC, main PA luminal area (A) and blood density (ρ):
Here we assume that ρ is constant at 1060 kg m−3 as the blood density changes only 1.4% between a hematocrit of 40 to 70% 37. The forward and backward pressure and flow waves were determined using wave separation analysis 32,40.
The index of global wave reflection or reflection magnitude was calculated as the ratio of the amplitude of Pf and Pb (Pb/Pf), which captures the wave reflection of the whole vascular bed 38. The effect of wave reflection on pulmonary hemodynamics was quantified using two indices 9: ΔSP defined as the difference between the systolic pressure of measured and forward pressure waves, and ΔPP defined as the difference between the PP of measured and forward pressure waves. To quantify the effect of PAH and estrogen on the timing of wave reflection, we defined the normalized time between the beginning of systolic to the peak of the reflected pressure wave (tpeak = Δt/T), where T is the period of cardiac cycle. This index allows us to quantify the timing of the return of the predominant reflected wave to the measuring site in the proximal PA.
Energy analysis
The total hydraulic power (Wt) was calculated as the time-average of the product of the instantaneous pulmonary PA pressure (P(t)) and flow (Q(t)) over the cardiac cycle, ignoring the small kinetics terms 9. The steady power (Ws) was calculated as the product of mPAP and CO, and the oscillatory power (Wo) was the difference between Wt and Ws.
Right ventricular-vascular coupling was assessed by two indices 9, i.e., the oscillatory power fraction ratio, defined as Wo/Wt and transpulmonary vascular efficiency defined as CO/Wt (energy cost of maintaining or increasing CO).
Morphology and histological analysis
Immediately after hemodynamic measurement, RV free wall and left ventricle (LV) plus interventricular septum (S) were dissected and weighed to derive RV hypertrophy indices, i.e., RV weight normalized by the body weight (BW), or by the tibia bone, or by the weight of LV+S.
For histology, whole lungs were pressure-perfused with 10% formalin at 15 mmHg. The formalin-fixed lungs and left extralobar PA were then embedded in paraffin. Tissue sections (5 μm) were stained with verhoff Van Geisen (VVG) to identify elastin, and picro-sirius red (SR) to measure collagen. 5 blocks were sequentially selected from the left lung lobe; at least 100 arteries were analyzed. Arteries were grouped by the outer diameters ranging from 20 to 50 μm, 50 to 100 μm, 100 to 200 μm and 200 to 500 μm. Images were captured on an inverted microscope (TE-2000; Nikon, NY) at 10X using a Spot camera and analyzed using an imaging analysis software Metavue (Optical Analysis Systems, NH).
Wall thickness and medial wall thickness was measured as the distance between the intima and the adventitia or the internal and external elastic membranes, respectively, with a line measurement tool and averaged over 5–6 positions. To quantify the area fraction of collagen or elastin, the area positive for staining was identified using thresholding and normalized to the total area of PA wall at the region of interest. The content of the protein of interest was calculated as the product of area faction and the total wall thickness of the PAs. Presented values are the mean of 5 fields per mouse lung section and 4 mice per group.
Statistical analysis
The study was powered to detect an effect size of at least 0.75 for the main comparison of Control/Placebo vs. SuHx/Estrogen groups in the 2 × 2 analysis of variance model with 81% power at the two-sided 0.05 significance level. The planned sample size for detecting this difference was 10 per experimental condition. All results are presented as mean ± standard error. The significance of the changes in PA hemodynamics with estrogen treatment and SuHx exposure was assessed with two-way analysis of variance followed with Tukey multiple comparisons at a significance level of 0.05. Statistical analysis was performed using R software (Foundation for statistical computing, version 2.5.1).
RESULTS
Estrogen restored pulsatile RV load but not steady RV load in early PAH
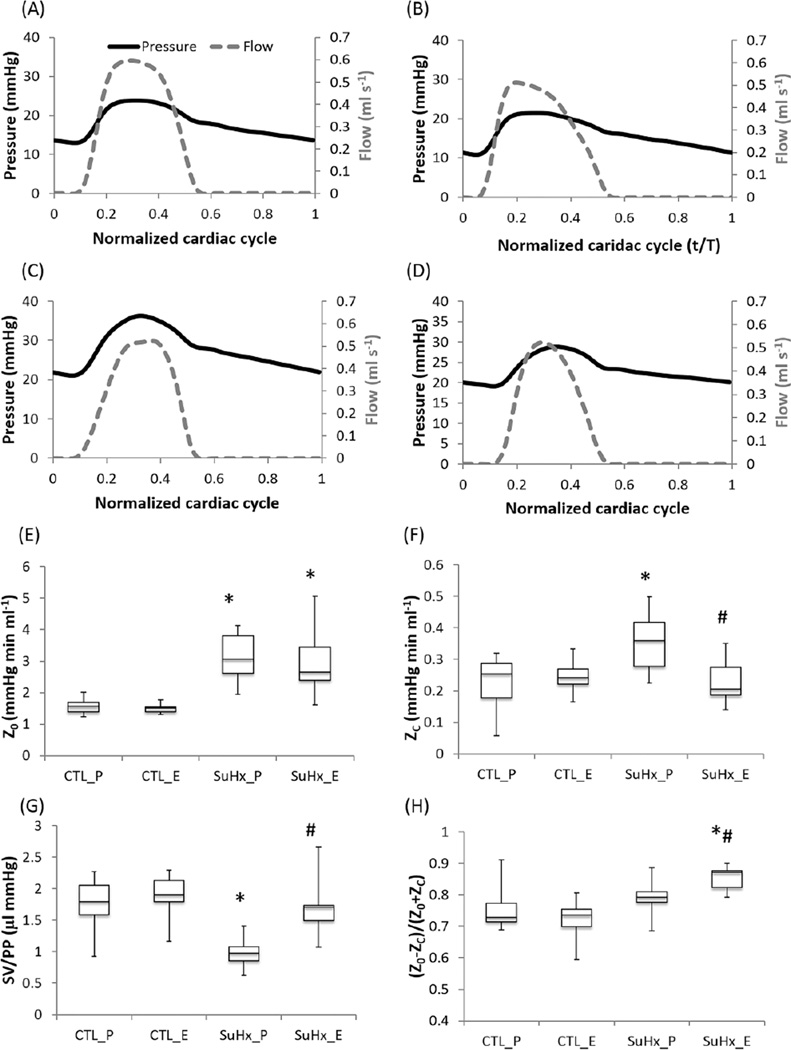
Representative pulmonary pressure and flow waveforms are plotted against a normalized cardiac cycle for all the groups (Figure 2A–D). Pulmonary pressures were significantly higher in the SuHx groups compared to the control groups (Table 1), indicating PAH. No significant difference in mPAP was detected between the estrogen- and placebo-treated SuHx groups. Whereas SV was significantly lower in the placebo-treated SuHx group compared to the control group, CO and CI were preserved in both SuHx groups.
Figure 2.
Effect of estrogen on the resistance and compliance of pulmonary vasculature in PAH. (a) The placebo-treated control group (CTL_P), (b) the estrogen-treated control group (CTL_E), (c) the placebo-treated SuHx group (SuHx_P), (d) the estrogen-treated SuHx group (SuHx_E), (e) Resistance (Z0), (f) Characteristic impedance (ZC), (g) Global compliance, and (h) Pulse wave reflection index. * P<;0.05 vs. Control, # P<0.05 vs. Placebo.”
Table 1.
Pulmonary arterial hemodynamics in control and PAH groups with/without estrogen treatment
| Parameters | CTL_P (n = 10) | CTL_E (n = 10) | SuHx_P (n = 8) | SuHx_E (n = 9) |
|---|---|---|---|---|
| HR (bpm) | 532±9 | 486±14# | 573±5 | 519±14*# |
| mPAP (mmHg) | 16.7±0.8 | 16.7±1.2 | 27.1±0.6* | 24.4±1.1* |
| sPAP (mmHg) | 21.5±1.4 | 22.3±1.5 | 36.4±1.0* | 30.5±1.2*# |
| PP (mmHg) | 11.8±0.5 | 11.5±0.6 | 16.7±1.1* | 10.6±0.9# |
| SV (µl) | 20.2±1.2 | 21.2±1.1 | 15.9±1.3* | 17.5±0.1 |
| CO (ml min−1) | 10.8±0.6 | 10.3±0.5 | 9.1±0.7 | 8.9±0.8 |
| CI (ml min−1 g−1) | 0.48±0.03 | 0.47±0.02 | 0.44±0.04 | 0.41±0.03 |
Data presented as mean±SE. CTL, control; P, placebo; E, estrogen; mPAP and sPAP, mean and systolic pulmonary arterial pressure; PP, pulse pressure; SV, stroke volume; CO, cardiac output; CI, cardiac index.
P<0.05 vs. Control,
P<0.05 vs. Placebo.
The resistance (Z0) significantly increased in both SuHx groups compared to the controls; no difference was found between the estrogen and placebo-treated SuHx groups (Figure 2E). In contrast, the characteristic impedance (ZC) increased significantly in the placebo-treated SuHx group (Figure 2F), indicating proximal PA stiffening with PAH without either endogenous or exogenous estrogen. Pulse pressure (PP) and the global compliance index SV/PP also increased in the placebo-treated SuHx group (Table 1 and Figure 2G), which supports the interpretation of proximal PA stiffening in PAH without either endogenous or exogenous estrogen. However, exogenous estrogen significantly limited the increase in ZC, PP and SV/PP compared to the placebo-treated SuHx group, such that they were not different from the control levels. Since mPAP was similar between these groups, the difference in ZC was unlikely to be due to the pressure dependence of arterial stiffness 4. The pulse wave reflection was not altered in the placebo-treated SuHx group (Figure 2H), but significantly increased in the estrogen-treated SuHx group.
Estrogen attenuates elevated wave reflection in PAH
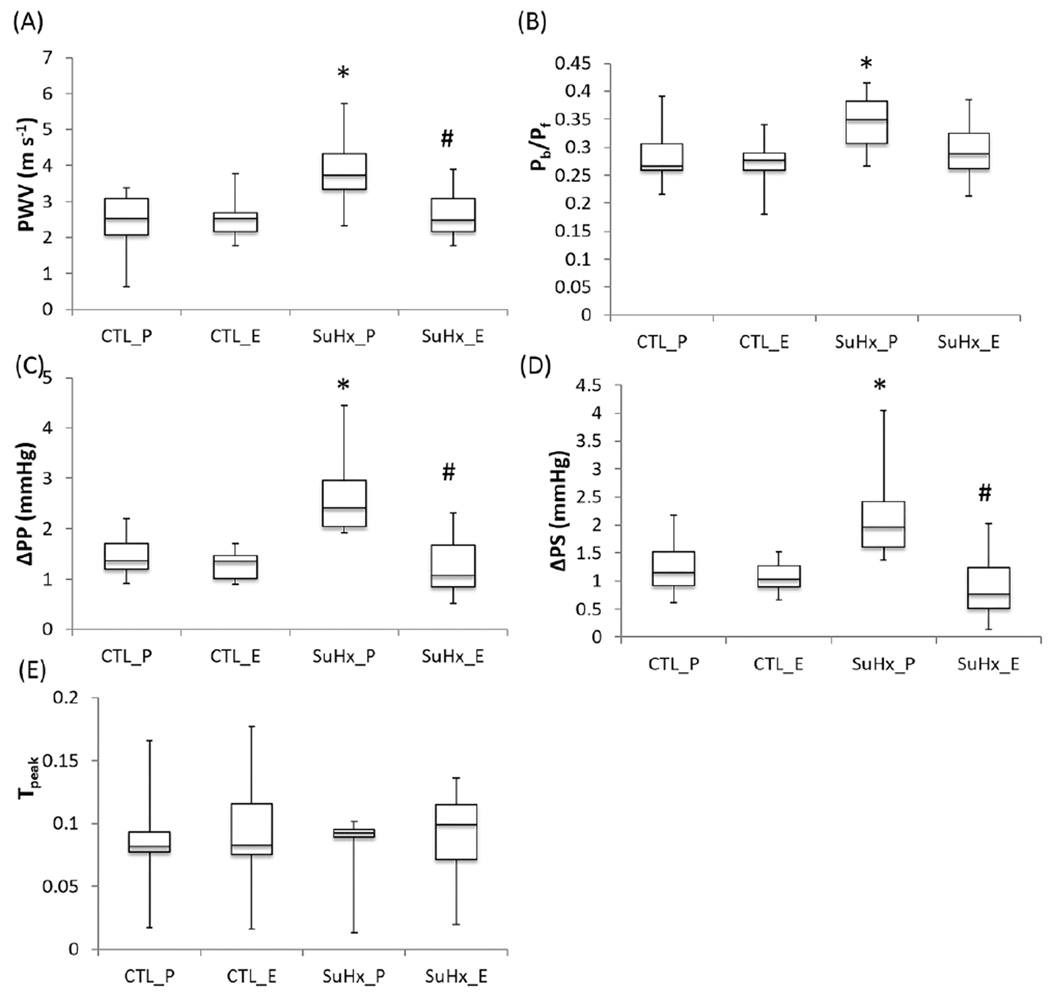
The pulse wave velocity (PWV) increased in the placebo-treated SuHx group (Figure 3A). Estrogen restored it to the control level, confirming that estrogen attenuates PAH-induced proximal arterial stiffening. ΔSP and ΔPP, indices of the effects of wave reflection on the systolic pressure and pulse pressure, respectively, were significantly higher in the placebo-treated SuHx group (Figure 3C and D), indicating that the reflected wave significantly increased the systolic pressure and the pulse pressure in PAH. No changes in ΔSP and ΔPP were found in the estrogen-treated SuHx group, indicating that estrogen limited the effects of wave reflection on these pressures. The global wave reflection index (Pb/Pf) increased in the placebo-treated SuHx group (Figure 3B) and estrogen attenuated the increase in Pb/Pf. Although estrogen treatment tended to delay the arrival of reflected wave (tpeak), no significant difference in the timing of wave reflection was found between the control and PAH groups or between the estrogen and placebo-treated groups (Figure 3D).
Figure 3.
Effects of estrogen on wave reflection in PAH. (A) Pulse wave velocity (PWV), (B) Global wave reflection index, (C) difference in systolic pressure between measured and forward pressure waves, (D) difference in pulse pressure between measured and forward pressure waves, and (E) normalized time between the beginning of systolic pressure wave to the peak of the reflected pressure wave (tpeak = Δt/T). *, P<0.05 vs. Control, #, P<0.05 vs. Placebo.
Estrogen protects transpulmonary vascular efficiency
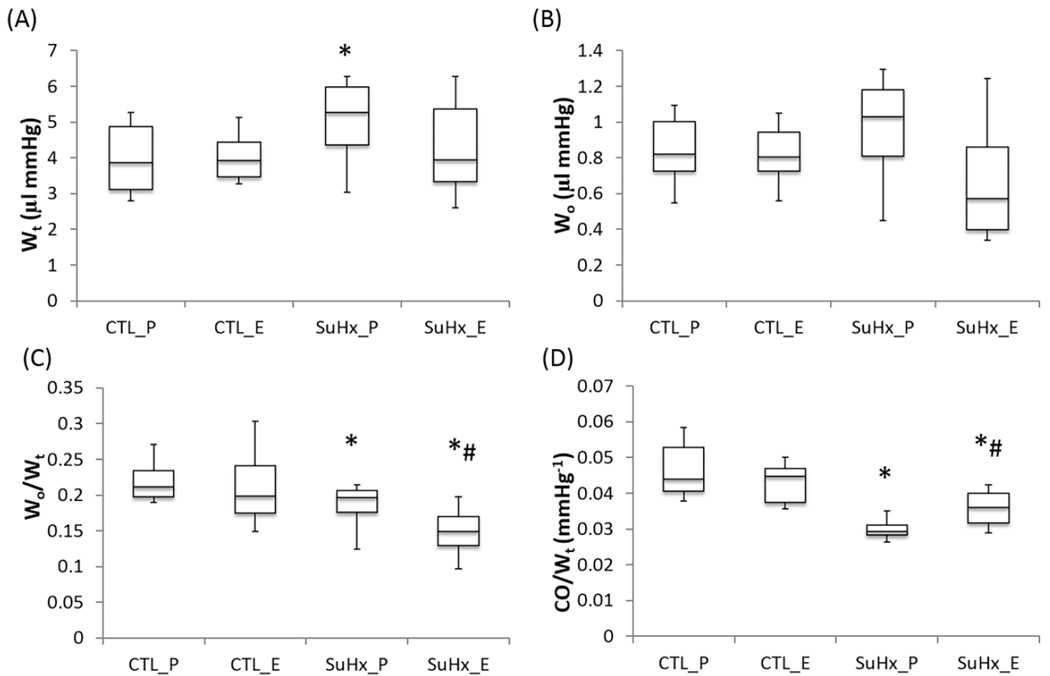
The total hydraulic power (Wt) significantly increased in the placebo-treated SuHx group, and estrogen attenuated the increase and restored it to the control level (Figure 4A). Although the oscillatory power (Wo) was not significantly changed, the oscillatory power fraction was significantly lower in both SuHx groups and estrogen treatment further decreased the oscillatory power fraction (Figure 4B and C). The transpulmonary vascular efficiency was significantly decreased in both SuHx groups compared to the control group (Figure 4D). Estrogen treatment attenuated this decrease in the PAH group.
Figure 4.
The effects of estrogen on hydraulic powers and energy transmission in PAH. (A) Total Hydraulic power (Wt), (B), Oscillatory hydraulic power (Wo), (C) Oscillatory power fraction (Wo/Wt), and (D) Transpulmonary vascular efficiency (CO/Wt). *, P<0.05 vs. Control; #, P<0.05 vs. Placebo.
Estrogen modified resistance-compliance relationship of the pulmonary circulation
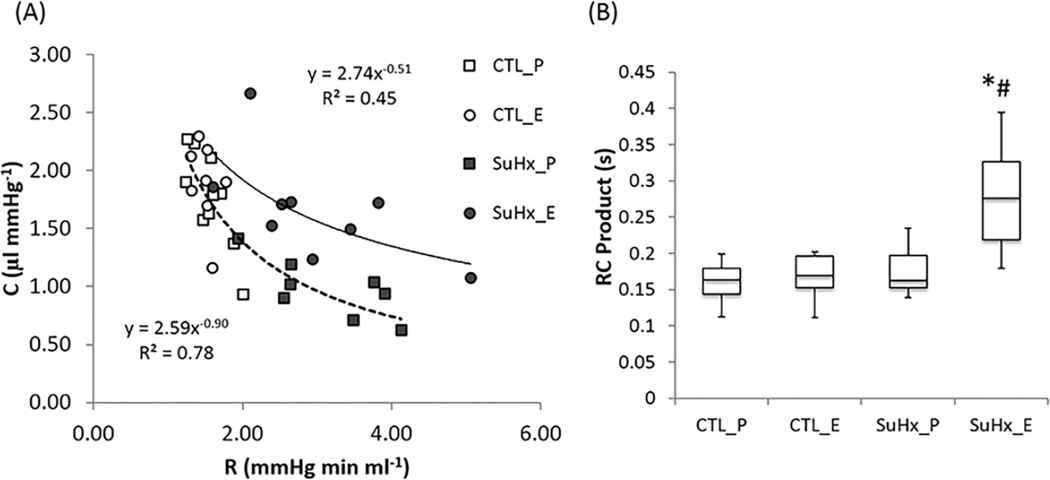
The resistance (R= Z0) and compliance (C= SV/PP) of the placebo-treated SuHx group and control groups followed an inverse relationship (Figure 5A), suggesting that PAH did not alter this relationship which previously has been found to be conserved in the pulmonary circulation 19. Notably, estrogen-treatment in the SuHx group did alter this relationship by changing the exponent from about −1 to about −0.5. The upward shifting of the R-C curve indicates that at any given resistance, the pulmonary arterial network is more compliant with estrogen treatment. The RC-time, the product of R and C, was significantly larger in the estrogen-treated SuHx group compared to the control and the placebo-treated SuHx group (Figure 5B).
Figure 5.
Estrogen modifies the relationship between resistance (R) and compliance (C) in pulmonary vasculature. (A) R-C curves and (B) RC time constant. *, P<0.05 vs. Control; #, P<0.05 vs. Placebo.
Morphology and structural changes
The RV was significantly hypertrophied in both PAH groups and to a similar degree (Table 2), consistent with the changes in mPAP. The uterine weight in the estrogen-treated mice (128±6 g) was significantly higher than that in the placebo group (12±1g) and confirmed the success of OVX and pellet implantation.
Table 2.
Morphologic parameters in control and PAH groups with/without estrogen treatment
| Parameters | CTL_P (n = 10) | CTL_E (n = 10) | SuHx_P (n = 8) | SuHx_E (n = 9) |
|---|---|---|---|---|
| BW (g) | 22.5±0.3 | 22.1±0.5 | 20.7±0.3* | 22.0±0.4# |
| RV/BW (mg g−1) | 0.88±0.03 | 0.85±0.03 | 1.59±0.10* | 1.51±0.03* |
| RV/Tibia (mg mm−1) | 1.08±0.05 | 1.08±0.06 | 1.84±0.13* | 1.84±0.05* |
| RV/LVS (mg mg−1) | 0.28±0.01 | 0.29±0.01 | 0.42±0.02* | 0.35±0.01*# |
| RV wall thickness (mm) | 0.27±0.02 | 0.28±0.02 | 0.36±0.02* | 0.40±0.03* |
| RV ID (mm) | 1.45±0.03 | 1.38±0.03 | 1.31±0.07 | 1.38±0.09 |
| Main PA ID (mm) | 1.34±0.02 | 1.31±0.03 | 1.36±0.02 | 1.40±0.02* |
BW, body weight; RV, RV weight; LVS, left ventricle and septum weight; PA, pulmonary artery; ID, inner diameter.
P<0.05 vs. Control,
P<0.05 vs. Placebo.
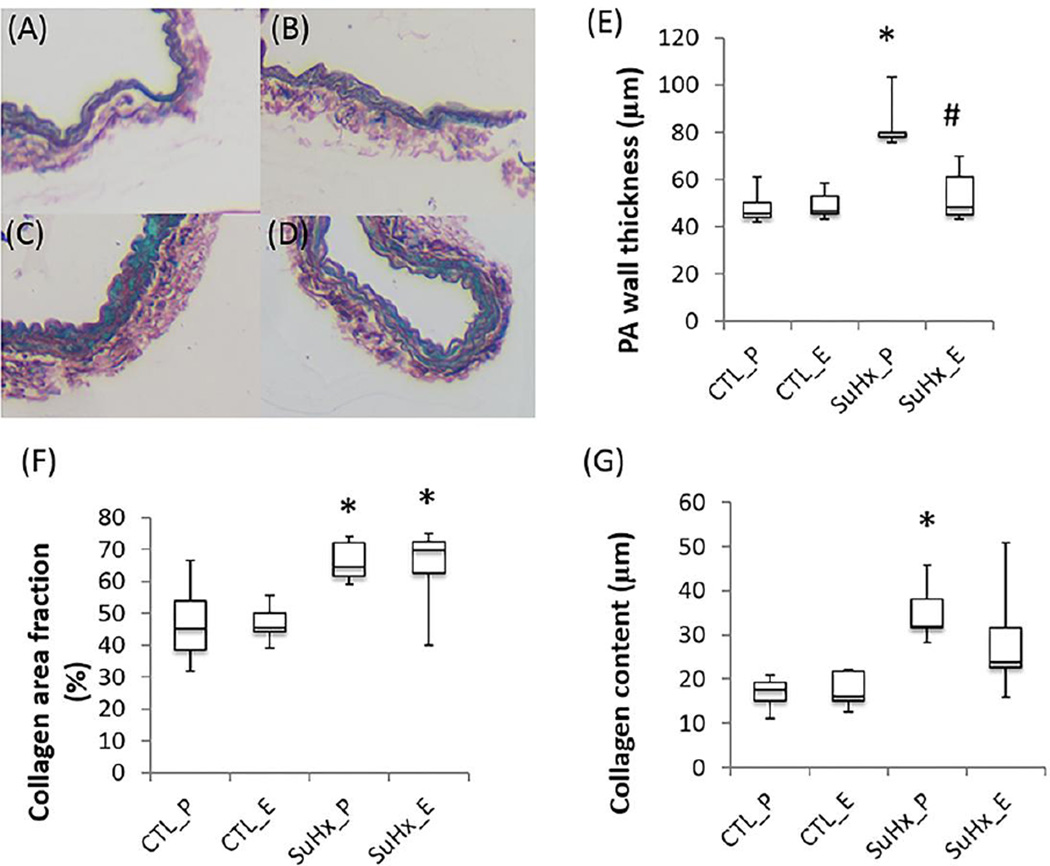
SuHx increased collagen and wall thickness in the left PA measured histologically and estrogen attenuated this increase (Figure 6). Elastin was not significantly altered in the left PA either by PAH or estrogen treatment (data not shown). We did not find significant changes in the collagen or elastin area fraction or in the medial wall thickness in distal pulmonary arteries between the estrogen and placebo-treated SuHx groups (data not shown).
Figure 6.
Representative conduit PA histology and semi-quantitative analysis in the placebo- and estrogen-treated control and SuHx groups. (A–D) Sirius red staining for collagen (red), (E) PA wall thickness, (F) collagen area fraction and (G) content. *, P<0.05 vs. Control; #, P<0.05 vs. Placebo.
DISCUSSION
In this study, we investigated the effects of estrogen on pulsatile pulmonary hemodynamics in PAH. The novel findings in this study include (a) that estrogen modifies the inverse relationship between PA compliance and resistance: estrogen attenuates decrease in PA compliance independent of PA resistance; and (b) that estrogen attenuates PA wave reflection and enhances transpulmonary vascular efficiency. The protective effects of estrogen on PA hemodynamics reduce RV afterload and energy demand, which contribute to better RV adaption and may be responsible for better outcomes in female PAH patients.
Estrogen attenuates pulmonary arterial compliance independent of pulmonary vascular resistance in PAH
In the pulmonary circulation, resistance (R) and compliance (C) are coupled such that the product of R and C (or the RC time) is constant, which has been attributed to the large number of small distal arteries that are both highly resistant and highly compliant29. In most types of PH, the RC time constant does not change with disease severity or treatment 18,19; however, changes in the distribution of R or C can affect this relationship. For example, proximal obstruction CTEPH patients have shortened RC-time due to an proximal decrease in compliance with unchanged PVR 23. Here we found that estrogen altered this relationship such that at any given resistance, the pulmonary arterial network is more compliant (Figure 4). This is consistent with our finding that estrogen did not significantly affect distal PA remodeling in hypertensive animals. Instead, estrogen treatment significantly increased the diameter of main PA (Table 2), which may be mediated through enhanced eNOS expression and activation 6,24. Furthermore, our study showed that estrogen significantly attenuated collagen deposition and wall thickness in conduit PAs, as found previously 22.
The physiological basis for localized effects of estrogen on PAs may be the phenotypical heterogeneity of smooth muscle and endothelial cells throughout the vascular tree due to different regional environment, developmental origin, or vascular functional requirement 1,33. The possible differential responses of SMCs and ECs in the proximal and distal PAs to PAH and to estrogen treatment warrant future mechanistic study. The ability of estrogen to preserve arterial compliance implies that estrogen likely serves a novel therapy for pulmonary arterial stiffening in PAH, which is a powerful predictor of disease outcome.
It is noteworthy that the finding that estrogen has limited effects on total pulmonary vascular resistance (tPVR) does not agree with our previous result that estrogen protects against a PAH-induced increase in tPVR 21. In the current study, tPVR was calculated directly from mPAP and CO measured in the PA (tPVR = mPAP/CO); in the prior study, CO was computed from SV times HR, which will overestimate CO if significant tricuspid regurgitation exists. Since tricuspid regurgitation is likely estrogen-dependent 20 and often observed in PAH patients 30, we anticipate that the SV-method overestimated CO and thus underestimated tPVR in the estrogen-treated SuHx animals, which accounts for the discrepancy between these studies.
The impact of estrogen on wave reflection
Wave reflection, in part, contributes to increased burden on the heart by increasing pressure and reducing blood flow during systole 8. Arterial stiffness and wave reflection are greater in menopausal women than age-matched men independent of body size and heart rate 31. Treating postmenopausal women with estrogen significantly decreases aortic wave reflection 34 and estrogen receptors are linked to increased wave reflection and adverse cardiac events 28. Here we found that PAH increased PA PWV in the placebo-treated group, consistent with the clinical findings that male IPAH patients have higher PA-PWV than male healthy controls 16. Estrogen restored the wave velocity in the SuHx group to the control level, consistent with the clinical finding that female IPAH patients have lower PA-PWV than male IPAH patients 16.
As the hemodynamic consequences of wave reflections are highly dependent on their magnitude and timing relative to the cardiac cycle, we used global reflection index and tpeak to quantify the magnitude and timing of wave reflection. PAH increased the magnitude of wave reflection but did not alter the timing of wave reflection despite of increased PWV. The hemodynamic consequences of increase wave reflection included elevated systolic and pulse pressures that increase RV afterload and distal blood flow pulsatility, two major contributors to PAH progression. Estrogen attenuated the increase in the magnitude and tended to delay the arrival of reflected waves, which likely explain the lower PP and systolic pressure in the estrogen-treated SuHx group. It is interesting to note that in the estrogen-treated SuHx group (Table 1), the global wave reflection index, which captures the wave reflection of the whole pulmonary vasculature, did not reconcile with the higher pulse wave reflection index, which reflects the mismatch in distal and proximal impedance. These two indexes are usually consistent when Z0, a measure of total pulmonary resistance, and ZC, a measure of proximal compliance, are coupled in pulmonary circulation. Here, estrogen preserved ZC independent of increased Z0 in the PAH group that led to the increase in the pulse wave reflection index without affecting global wave reflection index.
The impact of estrogen on RV adaptation to PAH
Different types of RV loading result in distinct patterns of RV adaption 5. RV wall stress during RV ejection is the gold standard measure of RV afterload, which depends on RV wall thickness, chamber dimensions and vascular load. We did not find significant differences in normalized RV weight, wall thickness or RV inner diameter between estrogen treated and untreated groups (Table 2), indicating that the vascular load is the determining factor for RV adaptation. We found that estrogen significantly reduced the pulsatile load (ZC) with limited effect on the steady load (Z0). We did not find significant differences in RV hypertrophy between estrogen-treated and untreated PAH groups, suggesting that steady load Z0 is the dominant contributor to increased RV mass.
Energetically, we found that estrogen attenuated absolute RV total and oscillatory energy. Oscillatory energy fraction was significantly lower in the estrogen-treated PAH group compared to both control and placebo-treated PAH groups, suggesting estrogen improves energy efficiency. Whereas cardiac function (CO and CI) was little influenced (with compensation of the heart rate) by PAH, despite the marked increase in ZC and wave reflection in the untreated PAH group, the energetic cost to the heart for maintaining adequate flow was increased. This suggests a mechanism whereby pulmonary arterial stiffening and wave reflection may yield little functional decrement at baseline but limit reserve capacity under conditions of increased demand, as observed in our previous study 22. This finding is also consistent with data from the systemic circulation 14. The ability of estrogen to protect PA compliance and to attenuate wave reflection that attenuates pulsatile loading and increase energy efficiency explain the enhanced RV function at baseline and cardiac reserve under stress in the estrogen treated PAH group 21.
Study limitations
There are several limitations in this study. First, the pressure and flow data were measured in open-chest animals under anesthesia. To minimize the effect of surgery on the measurement, we used small diameter (1.2F) pressure catheter and ensured minimal blood loss during the surgery. Since data from all experimental groups were acquired under the same conditions, we expect that the conclusions derived in this study are applicable to physiological conditions. Second, PCWP was not measured due to technical difficulties in rodents. PCWP is known to affect the RC relationship 36 and estrogen may affect PCWP via its vasorelaxation property. In addition, we approximated pulmonary vascular resistance using total pulmonary vascular resistance (Z0), which excluded the effects of PCWP and relied on an indirect estimate of total pulmonary vascular compliance. Therefore, whether and how estrogen modulates PWCP needs to be confirmed in large animal and clinical studies. Third, the resolution of echocardiography (80 m) is considered marginal to discern the difference in the main PA inner diameter among experimental groups. However the trend of PA inner diameter is consistent with ex vivo PA outer diameter measured optically in our previous study using the same animal model22. Finally, the PAH generated was mild and not longstanding. To shed light on the estrogen paradox, as well as to test the therapeutic potential of estrogen, comparable studies on intact female animals with an intrinsic estrous cycle with severe, longstanding PAH are warranted.
CONCLUSIONS
Abnormalities in pulmonary arterial pulsatile hemodynamics (i.e., increase in pulmonary vascular resistance and decrease in compliance) contribute to PAH progression. Our study demonstrated that estrogen attenuates decreased compliance largely independent of resistance, which reduces pulsatile RV afterload and attenuates wave reflection in PAH. The protective effects of estrogen on pulmonary hemodynamics provide a functional mechanism for improved RV functional adaptation in female PAH patients. The differential modulation of remodeling of the pulmonary proximal and distal vasculature also sheds light on the estrogen paradox in PAH in that estrogen does not affect PAH incidence but prevents the disease progression by modifying PA compliance only. Finally, the ability of estrogen to attenuate loss of pulmonary arterial compliance constitutes an opportunity for novel therapeutic intervention in PAH.
Acknowledgments
We thank Dr. Guoqing Song for performing in vivo hemodynamic measurement in mice. This work was supported by National Institutes of Health R01HL-086939 (to N.C. Chesler) and American Heart Association 13POST16910091 (to A. Liu).
Abbreviations
- CI
cardiac index
- CO
cardiac output
- LV
left ventricle
- mPAP
mean pulmonary arterial pressure
- OVX
ovariectomy
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PCWP
pulmonary capillary wedge pressure
- PP
pulse pressure
- PWV
pulse wave velocity
- Pf
forward pressure
- Pb
backward pressure
- Pb/Pf
index of global wave reflection
- PVR
pulmonary vascular resistance
- RV
right ventricle
- SuHx
Sugen-hypoxia exposure
- SV
stroke volume
- SV/PP
global arterial compliance
- tPVR
total pulmonary vascular resistance
- Wt
total hydraulic power
- Wo
oscillatory hydraulic power
- Wo/Wt
oscillatory power fraction
- CO/Wt
transpulmonary vascular efficiency
- Z0
total vascular resistance
- ZC
characteristic impedance
- Γ= (Z0-ZC)/(Z0+ZC)
pulse wave reflection
REFERENCES
- 1.Aird WC. Phenotypic Heterogeneity of the Endothelium: I. Structure, Function, and Mechanisms. Circ. Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 2.Austin E, Johansen A, Alzoubi A, Lahm T, West J, Tofovic S, MacLean M, Oka M. Gender, sex hormones and pulmonary hypertension. Pulm. Circ. 2013;3:294. doi: 10.4103/2045-8932.114756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M. Pulmonary Arterial HypertensionBaseline Characteristics From the REVEAL Registry. CHEST J. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 4.Bellofiore A, Roldán-Alzate A, Besse M, Kellihan HB, Consigny DW, Francois CJ, Chesler NC. Impact of Acute Pulmonary Embolization on Arterial Stiffening and Right Ventricular Function in Dogs. Ann. Biomed. Eng. 2013;41:195–204. doi: 10.1007/s10439-012-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgdorff MAJ, Bartelds B, Dickinson MG, Steendijk P, de Vroomen M, Berger RMF. Distinct loading conditions reveal various patterns of right ventricular adaptation. Am. J. Physiol. -Heart Circ. Physiol. 2013;305:H354–H364. doi: 10.1152/ajpheart.00180.2013. [DOI] [PubMed] [Google Scholar]
- 6.Chambliss KL, Shaul PW. Estrogen Modulation of Endothelial Nitric Oxide Synthase. Endocr. Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 7.Cheifetz I, Craig DM, Kern FH, Black DR, Hillman ND, Greeley WJ, Ungerleider RM, Smith PK, Meliones JN. Nitric oxide improves transpulmonary vascular mechanics but does not change intrinsic right ventricular contractility in an acute respiratory distress syndrome model with permissive hypercapnia. Critical Care Medicine. 1996;24:1554–1561. doi: 10.1097/00003246-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Furuno Y, Nagamoto Y, Fujita M, Kaku T, Sakurai S, Kuroiwa A. Reflection as a cause of mid-systolic deceleration of pulmonary flow wave in dogs with acute pulmonary hypertension: comparison of pulmonary artery constriction with pulmonary embolisation. Cardiovasc. Res. 1991;25:118–124. doi: 10.1093/cvr/25.2.118. [DOI] [PubMed] [Google Scholar]
- 9.Grignola JC, Ginés F, Bia D, Armentano R. Improved right ventricular-vascular coupling during active pulmonary hypertension. Int. J. Cardiol. 2007;115:171–182. doi: 10.1016/j.ijcard.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Sitbon O, Yaïci A, Montani D, O’Callaghan DS, Jaïs X, Parent F, Savale L, Natali D, Günther S, Chaouat A, Chabot F, Cordier J-F, Habib G, Gressin V, Jing Z-C, Souza R, Simonneau G. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur. Respir. J. 2010;36:549–555. doi: 10.1183/09031936.00057010. [DOI] [PubMed] [Google Scholar]
- 11.Hunter KS, Lee P-F, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, Chan KC, Shandas R. Pulmonary Vascular Input Impedance is a Combined Measure of Pulmonary Vascular Resistance and Stiffness and Predicts Clinical Outcomes Better than PVR Alone in Pediatric Patients with Pulmonary Hypertension. Am. Heart J. 2008;155:166–174. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard H-J, Boonstra A, Vonk Noordegraaf A. THe right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145:1230–1236. doi: 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jesus Perez VA. Making Sense of the Estrogen Paradox in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2011;184:629–630. doi: 10.1164/rccm.201107-1184ED. [DOI] [PubMed] [Google Scholar]
- 14.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ. Res. 1992;71:490–502. doi: 10.1161/01.res.71.3.490. [DOI] [PubMed] [Google Scholar]
- 15.Kobs RW, Muvarak NE, Eickhoff JC, Chesler NC. Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1209–H1217. doi: 10.1152/ajpheart.01129.2003. [DOI] [PubMed] [Google Scholar]
- 16.Kopeć G, Moertl D, Jankowski P, Tyrka A, Sobień B, Podolec P. Pulmonary Artery Pulse Wave Velocity in Idiopathic Pulmonary Arterial Hypertension. Can. J. Cardiol. 2013;29:683–690. doi: 10.1016/j.cjca.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Demark MV, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17β-Estradiol Attenuates Hypoxic Pulmonary Hypertension via Estrogen Receptor–mediated Effects. Am. J. Respir. Crit. Care Med. 2012;185:965–980. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lankhaar J-W, Westerhof N, Faes TJC, Gan CT-J, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur. Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 19.Lankhaar J-W, Westerhof N, Faes TJC, Marques KMJ, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am. J. Physiol. - Heart Circ. Physiol. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 20.Limacher MC, Ware JA, O’Meara ME, Fernandez GC, Young JB. Tricuspid regurgitation during pregnancy: Two-dimensional and pulsed doppler echocardiographic observations. Am. J. Cardiol. 1985;55:1059–1062. doi: 10.1016/0002-9149(85)90746-5. [DOI] [PubMed] [Google Scholar]
- 21.Liu A, Schreier D, Tian L, Eickhoff JC, Wang Z, Hacker TA, Chesler NC. Direct and indirect protection of right ventricular function by estrogen in an experimental model of pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H273–H283. doi: 10.1152/ajpheart.00758.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu A, Tian L, Golob M, Eickhoff JC, Boston M, Chesler NC. 17β-Estradiol Attenuates Conduit Pulmonary Artery Mechanical Property Changes With Pulmonary Arterial Hypertension. Hypertension. 2015;66:1082–1088. doi: 10.1161/HYPERTENSIONAHA.115.05843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKenzie Ross RV, Toshner MR, Soon E, Naeije R, Pepke-Zaba J. Decreased time constant of the pulmonary circulation in chronic thromboembolic pulmonary hypertension. Am. J. Physiol. - Heart Circ. Physiol. 2013;305:H259–H264. doi: 10.1152/ajpheart.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ. Res. 1997;81:355–362. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- 25.Mendelsohn ME, Karas RH. The Protective Effects of Estrogen on the Cardiovascular System. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF, Pfeffer MA, Westerhof N, Pfeffer JM. Measurement of aortic input impedance in rats. Am. J. Physiol. - Heart Circ. Physiol. 1994;267:H1907–H1915. doi: 10.1152/ajpheart.1994.267.5.H1907. [DOI] [PubMed] [Google Scholar]
- 27.Noordegraaf AV, Galiè N. The role of the right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. 2011;20:243–253. doi: 10.1183/09059180.00006511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter I, Kelley-Hedgepeth A, Huggins GS, Housman DE, Mendelsohn ME, Vita JA, Vasan RS, Levy D, Benjamin EJ, Mitchell GF. Association between arterial stiffness and variations in oestrogen-related genes. J. Hum. Hypertens. 2009;23:636–644. doi: 10.1038/jhh.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Presson RG, Audi SH, Hanger CC, Zenk GM, Sidner RA, Linehan JH, Wagner WW, Dawson CA. Anatomic distribution of pulmonary vascular compliance. J. Appl. Physiol. 1998;84:303–310. doi: 10.1152/jappl.1998.84.1.303. [DOI] [PubMed] [Google Scholar]
- 30.Rogers JH, Bolling SF. The Tricuspid Valve: Current Perspective and Evolving Management of Tricuspid Regurgitation. Circulation. 2009;119:2718–2725. doi: 10.1161/CIRCULATIONAHA.108.842773. [DOI] [PubMed] [Google Scholar]
- 31.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MSV, Sacco RL, Tullio MRD. Arterial Stiffness and Wave Reflection Sex Differences and Relationship With Left Ventricular Diastolic Function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segers P, Rietzschel ER, Buyzere MLD, Vermeersch SJ, Bacquer DD, Bortel LMV, Backer GD, Gillebert TC, Verdonck PR. Noninvasive (Input) Impedance, Pulse Wave Velocity, and Wave Reflection in Healthy Middle-Aged Men and Women. Hypertension. 2007;49:1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 33.Shanahan CM, Weissberg PL. Smooth Muscle Cell Heterogeneity: Patterns of Gene Expression in Vascular Smooth Muscle Cells In Vitro and In Vivo. Arterioscler. Thromb. Vasc. Biol. 1998;18:333–338. doi: 10.1161/01.atv.18.3.333. [DOI] [PubMed] [Google Scholar]
- 34.Stefanadis C, Tsiamis E, Dernellis J, Toutouzas P. Effect of estrogen on aortic function in postmenopausal women. Am. J. Physiol. - Heart Circ. Physiol. 1999;276:H658–H662. doi: 10.1152/ajpheart.1999.276.2.H658. [DOI] [PubMed] [Google Scholar]
- 35.Tabima DM, Roldan-Alzate A, Wang Z, Hacker TA, Molthen RC, Chesler NC. Persistent vascular collagen accumulation alters hemodynamic recovery from chronic hypoxia. J. Biomech. 2012;45:799–804. doi: 10.1016/j.jbiomech.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary Capillary Wedge Pressure Augments Right Ventricular Pulsatile LoadingClinical Perspective. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, LP Lee, JS Lee. A linear relation between the compressibility and density of blood. J Acoust Soc Am. 2001;109:390–396. doi: 10.1121/1.1333419. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm. Circ. 2011;1:212–223. doi: 10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Lakes RS, Golob M, Eickhoff JC, Chesler NC. Changes in Large Pulmonary Arterial Viscoelasticity in Chronic Pulmonary Hypertension. PLoS ONE. 2013;8:e78569. doi: 10.1371/journal.pone.0078569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerhof N, Sipkema P, Bos GCVD, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc. Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 41.Xu DQ, Luo Y, Liu Y, Wang J, Zhang B, Xu M, Wang YX, Dong HY, Dong MQ, Zhao PT, et al. Beta-estradiol attenuates hypoxic pulmonary hypertension by stabilizing the expression of p27kip1 in rats. Respir. Res. 2010;11:182. doi: 10.1186/1465-9921-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan P, Wu W-H, Gao L, Zheng Z-Q, Liu D, Mei H-Y, Zhang Z-L, Jing Z-C. Oestradiol ameliorates monocrotaline pulmonary hypertension via NO, prostacyclin and endothelin-1 pathways. Eur. Respir. J. 2013;41:1116–1125. doi: 10.1183/09031936.00044112. [DOI] [PubMed] [Google Scholar]