Abstract
Background
In a context of controversy about influenza antiviral treatments, this study assessed primary health care physicians’ prescription of neuraminidase inhibitors (NIs) in France during pandemic and seasonal influenza between 2009 and 2013.
Methods
This observational study, using data recorded in three national databases, estimated the rate of NIs’ prescription among influenza like-illness (ILI) patients seen in GPs’ and paediatricians’ consultations, and determined factors associated with this prescription according to a multivariate analysis. NIs’ delivery by pharmacists was also evaluated.
Results
Rates of NIs’ prescription were estimated to 61.1% among ILI patients with a severe influenza risk factor seen in GPs’ consultation during the A(H1N1)pdm2009 pandemic versus an average rate of 25.9% during the three following seasonal influenza epidemics. Factors associated with NIs’ prescription were a chronic disease in patients under 65 years (OR, 14.85; 95%CI, 13.00–16.97) and in those aged ≥ 65 and older (OR, 7.54; 5.86–9.70), an age 65 years in patients without chronic disease (OR, 1.35; 1.04–1.74), a pregnancy (OR, 10.63; 7.67–15.76), obesity (OR, 4.67; 3.50–6.22), and a consultation during the pandemic A(H1N1)pdm2009 (OR, 3.19; 2.93–3.48). The number of antiviral treatments delivered by pharmacists during the A(H1N1)pdm2009 pandemic was 835 per 100 000 inhabitants, and an average of 275 per 100 000 inhabitants during the three following seasonal influenza epidemics.
Conclusions
Although physicians seem to follow the recommended indications for NIs in primary health care practice, this study confirms the low rate of NIs prescription to ILI patients with a severe influenza risk factor, especially during seasonal epidemics.
Introduction
Influenza is an acute infectious respiratory disease, which can lead to serious and life-threatening complications, especially in specific populations such as the elderly and patients with a chronic disease. In France, seasonal epidemics are associated with 700,000 to 4,800,000 consultations for influenza like-illness (ILI) (1–8 % of the general population) [1, 2], and with 0 to 24 deaths per 100,000 inhabitants [3]. Although vaccination remains the most effective prophylactic intervention for influenza infection in the population at risk of complications [4, 5], its effectiveness is estimated only around 60% in adults between 18 and 65 years old, and less certain in the other age groups including the elderly [6–8].
Antiviral treatments thus constitute a complementary prophylactic and therapeutic approach. NIs are recommended by French public health authorities [9], as in most other countries [10], to treat, as soon as possible, ILI patients i) at risk of influenza complications, defined by patients with at least one of the following characteristics: age ≥ 65 years old, chronic underlying disease, obesity defined as BMI ≥ 40, or pregnancy; ii) who is hospitalized; iii) or has severe, complicated, or progressive illness. Currently only neuraminidase inhibitors (NIs) (oseltamivir and zanamivir) may be prescribed, amandatanes are no longer recommended because of viral resistance [11]. They reduce the time for alleviation of symptoms in patients with a virologically confirmed influenza infection by one day [12, 13, 14, 15]. They decrease the occurrence of respiratory complications related to influenza in healthy patients and those at risk, the occurrence of pneumonia, and generally the prescription of antibiotics [12, 13, 14–16]. Finally, they reduce nasal shedding which could limit the spread of the disease [17].
Despite the results of these studies and the recommendations, the effectiveness of NIs to reduce infectivity and transmission, when used as treatment for symptomatic infected patients, remains questioned. The latest reviews and meta-analysis challenge the literature [13]. They highlight the fact that the populations studied did not specifically include patients with a severe influenza risk factor, as the elderly, and that the level of evidence regarding the effect of NIs on mortality and hospitalization was low. There might be a publication bias, suggesting that studies showing no impact of antivirals have not been published [12, 15, 18]. To better understand how physicians apply recommendations in this debated context, the present study was designed to describe NIs’ prescriptions by GPs and paediatricians in France during the last pandemic A(H1N1)pdm2009 and the three seasonal epidemics that followed.
Methods
In the follow-up to the BIVIR clinical trial [19, 20], an observational study of NIs’ prescriptions has been conducted using three French national databases: i) French GPs’ Sentinel network database to study NIs’ prescription in ILI patients seen in general practitioners (GPs)’ consultations, ii) GROG network database to study NIs’ prescription in ILI patients seen in GPs’ and paediatricians’ consultations ≤ 48h after onset of symptoms, iii) and IMS Health database to study NIs’ delivery in pharmacies.
French GPs’ Sentinel network database
French GPs’ Sentinels network [21], focused on infectious diseases, consists of 1,298 general practitioners (GPs), 2.1% of all GPs with a liberal or mixed activity in France. Sentinels GPs’ characteristics are comparable to those of all French GPs regarding regional distribution, proportion in rural practice, type of practice and distribution of main clinical skills [22]. Sentinel physicians transmit every week by Internet the number of patients seen in consultation for ILI. ILI was defined as a sudden onset of fever over 39°C with myalgia and respiratory symptoms (cough, sore throat). For each case, data collected by GPs were age, sex, influenza vaccination for the current season, prescription of antiviral treatment, chronic underlying disease without any specification, obesity, pregnancy and if a hospitalisation was requested. The date of onset of symptoms was not reported.
GROG network database
GROG network [23], focused on influenza and respiratory virus, consists of 526 representative physicians (391 GPs and 114 paediatricians) distributed over the French territory. Physicians transmit every week by Internet the number of patients seen in consultation for ILI, with the same definition as Sentinel physicians. They are asked to perform, every week, a clinical questionnaire only for the first ILI case seen in consultation ≤ 48h after onset of symptom, reporting the same data than Sentinel physicians.
Inclusion criteria for Sentinel and Grog databases
All cases reported by Sentinel physicians from July 1, 2009 to June 30, 2013 were included in this study, corresponding to four influenza epidemic seasons, 2009/2010, 2010/2011, 2011/2012, 2012/2013. From January 1, 2009 to June 30, 2013, influenza epidemic periods were determined by applying a periodic regression model on weekly ILI incidence rates below a cut-off value [1]. All cases reported by Grog physicians during these four epidemics periods were included in this study
Pharmacists’ database
Private IMS-Pharmastat network consists of 14,000 representative pharmacies distributed over the French territory (60% of pharmacies), excluding hospital pharmacies. All medication sales are collected weekly. Data are aggregated into therapeutic classes from the Anatomical Classification of the European Pharmaceutical Marketing Research Association (EphMRA) [24] and an estimation of medication sales in France, out of hospital, is calculated each week. From January 1, 2009 to June 30, 2013, sales of influenza antivirals agents (class J05B4=oseltamivir and zanamivir), measured in boxes (10 pills per box), were included in the study.
Statistical analysis
For the two physicians’ databases, a descriptive analysis was performed on all variables collected for included cases. A new variable named “Presence of at least one severe influenza risk factor” was created, defined by patients with at least one of the following characteristics: age ≥ 65 years old, chronic underlying disease, obesity defined as BMI≥ 40, or pregnancy. To prevent a misinterpretation of our results, the rate of NIs’ prescription was analysed for the whole study population, but particularly for cases with the presence of at least one severe influenza risk factor.
Factors associated with NIs’ prescription were studied by performing univariate and then multivariate logistic regression. The following explanatory variables were analysed: age, sex, influenza vaccination, chronic underlying disease, obesity, pregnancy, pandemic vs. influenza season, hospitalisation requested at the end of the consultation, and seen in consultation by a GP or a paediatrician. A composite variable was created from “age” and “chronic underlying disease” due to a strong interaction (data not shown) between these two variables. From the univariate analyses results, a multivariate model was built with all variables with p-values <0.20 and then a backward selection approach was used. Analysis were performed in three populations: i) all ILI cases, ii) ILI cases seen in consultation ≤ 48h after onset of symptoms, iii) women between 15 and 50 years old.
Medication sales as influenza antivirals agents were analysed weekly during the study period. A reference period of July (year n) to June (year n +1) was chosen to estimate the rate of boxes delivered during the influenza epidemic period.
All analyses were performed with the R software (version 2.13.2; R Development Core Team 2011).
Results
Over the four influenza epidemics analysed herein, Sentinel GPs reported 35,188 ILI cases distributed in 11,067 ILI cases during the A(H1N1)pdm2009 pandemic, 7,809 during the seasonal influenza epidemic in 2010–2011, 4,984 in 2011–2012 and 11,328 in 2012–2013. Mean age of patients was 26 years and 48.9% (n=16,923) of them were men. Patients having at least one severe influenza risk factor represent 8.9% (n=2,866) of the population. Detailed characteristics of cases are given in Table 1. Over the same influenza epidemic periods, GROG physicians reported 1,854 ILI cases seen in consultation ≤ 48h after onset of symptoms. Among them, 48.2% (n=894) were included by a GP, and 51.8% (960) by a paediatrician. Mean age was 12 years (22.7 years for GPs’ patients and 2.4 years for paediatricians’ patients) and 51.7% (n=950) were men (Table 1).
Table 1.
Description of ILI cases included between 2009 and 2013, 35188 cases seen in GPs’ consultation (French GPs’ Sentinels database) and 1854 seen in GPs’ and paediatricians’ consultation ≤ 48h after onset of symptoms (GROG network database)
| French GPs’ Sentinels database | GROG network database | ||
|---|---|---|---|
| Included by GPs N=35188 |
Included by GPs N=894 |
Included by Paediatricians N=960 |
|
| Age, Median [Quartile] | 21 [9 ; 40] | 15 [6 ; 37] | 2 [1 ; 3] |
| < 15 yo, n (%) | 14003 (39.8) | 438 (49.0) | 960 (100) |
| Male, n (%) | 16923 (48.9) | 451 (50.7) | 499 (52.6) |
| Seasonal influenza vaccinated, n (%) | 1413 (4.1) | 49 (5.5) | 16 (1.7) |
| Presence of at least one severe influenza risk factor*, n (%) | 2866 (8.9) | 101 (13.7) | 35 (4.3) |
| ≥65 yo, n (%) | 1413 (4.0) | 34 (3.8) | 0 |
| Chronic underlying disease, n (%) | 1487 (4.2) | 75 (8.4) | 35 (3.6) |
| Obesity, n (%) | 309 (0.9) | 7 (0.8) | 0 |
| Pregnancy, n (%) | 121 (0.3) | 2 (0.2) | 0 |
Presence of a severe influenza risk factor was defined by patients with at least one of the following characteristics: age ≥ 65 years old, chronic underlying disease, obesity defined as BMI ≥ 40, and pregnancy
NIs’ prescription
For the whole study period, rate of NIs’ prescription among ILI patients seen in Sentinel GPs’ consultation during influenza epidemics was 8.8% (n=2845) (Table 2). The rate of NIs’ prescription among ILI patients with at least one severe influenza risk factor was 33.4% (n=919) (Table 2); 61.1% during the A(H1N1)pdm2009 pandemic (n=410), 34.5% during the seasonal influenza in 2010–2011 (n=176), 23.6% in 2011–2012 (n=146) and 19.6% in 2012–2013 (n=187). The rate of NIs’ prescription among ILI patients without any severe influenza risk factor was 6.4% (n=1863) (Table 2); between 12.3% during the A(H1N1)pdm2009 pandemic (n=991) and 3.2% in 2012–2013 (n=314). During influenza epidemics, 66.7% of NIs’ prescriptions were to patients without any severe influenza risk factor. Oseltamivir represented 99.9% of NIs prescription (n=2793), zanamivir 0.1% (n=4).
Table 2.
NIs’ prescription among ILI cases included between 2009 and 2013, 35188 cases seen in GPs’ consultation (French GPs’ Sentinels database) and 1854 seen in GPs’ and paediatricians’ consultation ≤ 48h after onset of symptoms (GROG network database)
| 2009/2010 | 2010/2011 | 2011/2012 | 2012/2013 | Total | ||
|---|---|---|---|---|---|---|
|
Sentinelles GPs’ database
* N=35188 |
NIs’ prescription, n (%) | 1440 (16.1) | 493 (6.5) | 402 (8.2) | 510 (4.6) | 2845 (8.8) |
| among ILI patients with at least one influenza risk factor***, n (%) | 410 (61.1) | 176 (34.5) | 146 (23.6) | 187 (19.6) | 919 (33.4) | |
| among ILI patients without any influenza risk factor***, n (%) | 991 (12.3) | 309 (4.4) | 249 (5.9) | 314 (3.2) | 1863 (6.4) | |
|
Grog GPs database
** N=894 |
NIs’ prescription, n (%) | 61 (16.8) | 15 (8.3) | 14 (11.5) | 24 (10.7) | 114 (12.8) |
| among ILI patients with at least one influenza risk factor***, n (%) | 17 (43.6) | 3 (21.4) | 6 (26.1) | 6 (25) | 32 (32) | |
| among ILI patients without any influenza risk factor***, n (%) | 27 (15.3) | 11 (6.6) | 8 (8.1) | 18 (9.3) | 64 (10.1) | |
|
Grog paediatricians’ database
** N=960 |
NIs’ prescription, n (%) | 57 (15.7) | 18 (9.2) | 16 (9.8) | 15 (6.4) | 106 (11.1) |
| among ILI patients with at least one influenza risk factor***, n (%) | 9 (50) | 5 (55.6) | 0 (0) | 0 (0) | 14 (40) | |
| among ILI patients without any influenza risk factor***, n (%) | 35 (17.2) | 13 (7.0) | 16 (10.1) | 14 (6.2) | 78 (10.1) |
All ILI cases seen in consultation
ILI cases seen in consultation ≤ 48h after onset of symptoms
Presence of a severe influenza risk factor was defined by patients with at least one of the following characteristics: age ≥ 65 years old, chronic underlying disease, obesity defined as BMI ≥ 40, and pregnancy
Rate of NIs’ prescription among ILI patients seen in GROG GPs’ consultation ≤ 48h after onset of symptom during influenza epidemics was 12.8% (n=114), and 32% (n=32) among ILI patients with at least one severe influenza risk factor (Table 2). These data are respectively of 11.1% (n=106) and 40% (n=14) among ILI patients seen in GROG paediatricians’ consultation ≤ 48h after onset of symptom.
During the study period, 6670 ILI cases were declared by Sentinel GPs outside of the epidemic periods. Among these cases, 487 (7.5%) had a NIs’ prescription. Patients receiving NI outside of the epidemic periods were older (mean age 34.8 vs 29.5, p<0.001) and had more frequently obesity (22.6% vs 12.5%, p=0.003) than those during the epidemic periods. There was no difference for the other characteristics (sex, seasonal influenza vaccination, presence of at least one severe influenza risk factor, chronic underlying disease and pregnancy) (data not shown).
Factors associated with NIs’ prescription
In multivariate analysis, factors associated with NIs’ prescription during influenza epidemics were a chronic disease in patients under 65 years (OR, 14.85; 95% CI, 13.00–16.97) and in those aged 65 and older (OR, 7.54; 5.86–9.70), an age ≥ 65 years in patients without chronic disease (OR, 1.35; 1.04–1.74), obesity (OR, 4.67; 3.50–6.22), and a consultation during the pandemic A(H1N1)pdm2009 (OR, 3.19; 2.93–3.48) versus the other three seasons (Table 3). Pregnancy was also associated with NIs’ prescription in women population aged between 15 and 50 years old (OR, 10.63; 7.17–15.76) (Table 4). Among ILI patients seen in consultation ≤ 48h after onset of symptoms, same factors were associated with NIs’ prescription (data not shown). The medical speciality of the physician (GP or paediatrician) was not associated with NIs’ prescription.
Table 3.
Factors associated with NIs’ prescription during influenza epidemics since 2009 among 32,458 ILI patients seen in GPs’ consultation (univariate and multivariate analysis)
| Univariate analysis | Multivariate analysis N=31,154 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | Treated with NIs n (%) | OR | OR (IC 95%) | p | OR | OR (IC 95 %) | P |
| Male | 15,657 | 1,419 (9.1) | 1.08 | [1.01 ; 1.17] | 0.047 | - | ||
| Female | 16,289 | 1,374 (8.4) | ||||||
| Age ≥ 65 yo with chronic underlying disease | 327 | 105 (32.1) | 6.46 | [5.10 ; 8.18] | <0.001 | 7.54 | [5.86 ; 9.70] | <0.001 |
| Age ≥ 65 yo without chronic underlying disease | 986 | 70 (7.1) | 1.04 | [0.81 ; 1.34] | 0.737 | 1.35 | [1.04 ; 1.74] | 0.023 |
| Age <65 yo with chronic underlying disease | 1,134 | 607 (53.5) | 15.72 | [13.87 ; 17.82] | <0.001 | 14.85 | [13.00 ; 16.97] | <0.001 |
| Age <65 yo without chronic underlying disease | 29,640 | 2,023 (6.8) | ||||||
| Seasonal influenza vaccinated | 1,363 | 262 (19.2) | 2.64 | [2.29 ; 3.04] | <0.001 | - | ||
| Not seasonal influenza vaccinated | 30,620 | 2,533 (8.3) | ||||||
| Obesity | 303 | 126 (41.6) | 7.73 | [6.13 ; 9.75] | <0.001 | 4.67 | [3.50 ; 6.22] | <0.001 |
| No obesity | 31,793 | 2,681 (8.4) | ||||||
| Hospitalisation requested | 131 | 17 (13.0) | 1.55 | [0.93 ; 2.58] | 0.094 | - | ||
| No hospitalisation requested | 31,352 | 2,757 (8.8) | ||||||
| Pandemic A(N1N1)2009 | 8,918 | 1,440 (16.1) | 3.03 | [2.81 ; 3.28] | <0.001 | 3.19 | [2.93 ; 3.48] | <0.001 |
| Seasonal influenza epidemic | 23,540 | 1,405 (6.0) | ||||||
Table 4.
Factors associated with the NIs’ prescription during influenza epidemics since 2009 among 8008 women aged between 15 and 50 yo seen in GPs’ consultation for ILI (univariate and multivariate analysis)
| Univariate analysis | Multivariate analysis N= 7 200 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | Treated with NIs n (%) | OR | OR (IC 95%) | p | OR | OR (IC 95 %) | P |
| Pregnant | 116 | 53 (45.7) | 8.53 | [5.87 ; 12.40] | <0.001 | 10.63 | [7.17 ; 15.76] | <0.001 |
| Not pregnant | 7084 | 636 (9.0) | ||||||
| Seasonal influenza vaccinated | 169 | 42 (24.9) | 3.27 | [2.28 ; 4.70] | <0.001 | - | ||
| Not seasonal influenza vaccinated | 6977 | 641 (9.2) | ||||||
| Chronic underlying disease | 244 | 133 (54.5) | 13.79 | [10.57 ; 18.00] | <0.001 | 12.86 | [9.70 ; 17.05] | <0.001 |
| No chronic underlying disease | 6956 | 556 (8.0) | ||||||
| Obesity | 91 | 39 (42.9) | 7.45 | [4.88 ; 11.38] | <0.001 | 6.56 | [4.02 ; 10.68] | <0.001 |
| No obesity | 7109 | 650 (9.1) | ||||||
| Hospitalisation requested | 23 | 4 (17.4) | 1.95 | [0.66 ; 5.75] | 0.225 | - | ||
| No hospitalisation requested | 7023 | 684 (9.7) | ||||||
| Pandemic A(N1N1)2009 | 1877 | 339 (18.1) | 3.09 | [2.64 ; 3.63] | <0.001 | 3.05 | [2.57 ; 3.62] | <0.001 |
| Seasonal influenza epidemic | 5395 | 357(6.6) | ||||||
NIs’ delivery by pharmacists
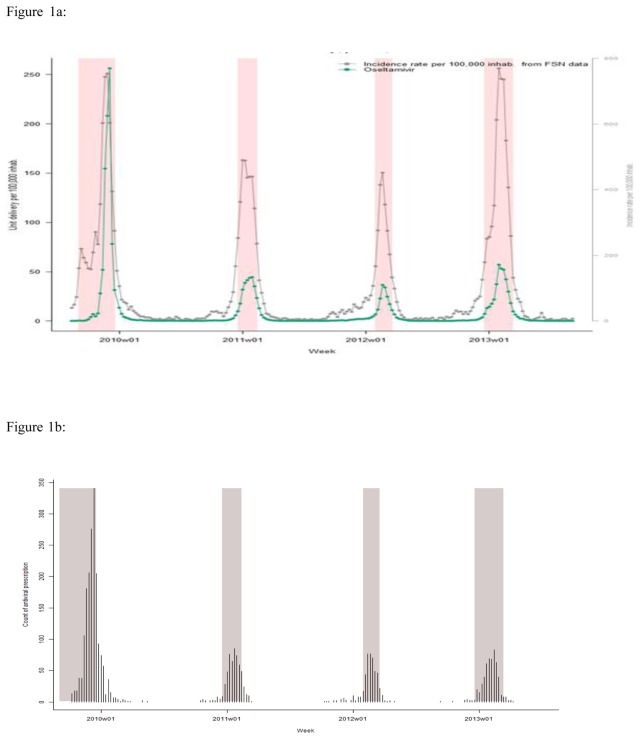
Between January 2009 and June 2013, the weekly number of NIs boxes delivered in France by pharmacists followed strictly the dynamics of influenza epidemics (Figure 1a), and the GPs NIs’ prescription (figure 1b). This volume delivered fluctuated according to epidemics with a maximum during the pandemic A(H1N1)pdm2009 (n=524,773 or 835.5/100 000 inhabitants), and a lower level during seasonal epidemics in 2010–2011 (n=181,005 or 286.6/100 000), 2011–2012 (n=104 624 or 164.7/100 000), and 2012–2013 (n=238,777 or 373.9/100 000). Zanamivir represented only 157 boxes in 2009–2010, 72 in 2010–2011, 10 in 2011–2012 and 35 in 2012–2013, other boxes delivered were oseltamivir.
Figure 1.
Figure 1a: Number of boxes of oseltamivir delivered per 100 000 inhabitants per week in France between 2009 and 2013, weekly incidence of ILI cases seen in general practice.
* Shaded periods represent influenza epidemic periods determined by applying a periodic regression model on weekly ILI incidence rates below a cut-off value [1]
Figure 1b: Number of cases included in the study who received a NIs’ prescription in and outside of the influenza epidemic periods in the Sentinel GPs database.
* Shaded periods represent influenza epidemic periods determined by applying a periodic regression model on weekly ILI incidence rates below a cut-off value [1]
Over a reference period of July (year n) to June (year n +1), the rate of boxes delivered outside the epidemic periods was 4.8% in 2009–2010, 14.3% in 2010–2011, 23.1% in 2011–2012 and 8.9% in 2012–2013 (Figure 3), corresponding respectively to 26649 NIs’ boxes in 2009–2010, 30266 in 2010–2011, 31393 in 2011–2012 and 23186 in 2012–2013. Considering a margin of 3 weeks before and 3 weeks after the official dates of influenza epidemics, the rate of NIs’ prescription outside this “large epidemic period” was 2.4% in 2009–2010, 4.7% in 2010–2011, 12.4% in 2011–2012 and 3.8% in 2012–2013, corresponding respectively to 13282, 9869, 16825 and 10040 boxes.
Discussion
In a context of controversy about prescription of influenza antiviral treatments [25, 26], this study established in France an inventory of NIs’ prescription and delivery in primary health care practice during seasonal epidemics and pandemic between 2009 and 2013.
As previously reported [27–29], the population which consult a GP or a paediatrician for an influenza like-illness, included in our study, was relatively young and healthy. A minority of patients included in our study received influenza vaccination. One of the reasons is that influenza vaccination, as NIs’ prescription, is recommended in France only for patients at risk of severe influenza, who represent a minority of ILI cases seen in GPs consultation during influenza epidemics. Our results are consistent with those (5–14.5%) reported in European countries [27, 28].
Recommendations regarding NIs’ prescription in the treatment of ILI patients at risk of influenza complication are consensual, both at the national and international level [9, 10]. However, this study showed a low and decreasing NIs’ prescription rate in targeted patients. This reluctance of physicians to prescribe NIs to all ILI patients with a severe influenza risk factor and follow strictly NIs’ recommendation in primary health care practice has been studied recently in United States [30, 31]. In this study, only 195 of 1021 (19%) of ILI participants who met high-risk criteria received an antiviral prescription. These low NIs’ prescription rates can be explained by different factors. Concerns remain about the NIs’ effectiveness, [12, 13, 15, 18], serious side effects are evocated [32], and pharmaceutical companies are suspected of a lack of transparency [33, 34]. Moreover, some doubts about public influenza expertise have been mentioned with a perception of conflicts of interest between experts and pharmaceutical industry [35]. At last, French primary care practitioners are very concerned in advices coming from independent educational medical reviews, which during the whole study period expressed doubts about oseltamivir effectiveness and interest [36]. The gap between the rate of NIs’ prescription among high risk ILI cases seen in consultation by GPs in France during the 2009 pandemic and the following seasonal epidemics could reflect the physicians’ perception that seasonal epidemic are less severe, that an unknown influenza virus which emerge during a pandemic could have a higher morbidity and mortality, and that a NIs’ treatment has to be prescribed more frequently during influenza pandemic.
However, in the present study when NIs were prescribed, there was a good concordance between physicians’ practices and the recommendations. A clear association between NIs’ prescription and the presence of at least one severe influenza risk factor which reflects the recommendation of the public health authorities [9, 10]. The delivery of NIs by pharmacists strictly followed the dynamics of influenza epidemics. Respiratory specimens are not collected routinely in GPs’ or pediatricians’ offices for influenza testing in France. Furthermore, influenza laboratory confirmations were not available for ILI cases included in the present study. Although shown in a recent article [30], the association between NIs’ prescription and influenza confirmation could not be tested.
Another important point is that 6.4% of ILI patients without any severe influenza risk factor but seeking physician consultation received NIs. It is difficult to know whether these prescriptions adhere to the recommendations or not; these latter specify that NIs have to be prescribed for ILI patients with severe symptoms or according to the discretion of the physician [9, 10]. Regardless, these prescriptions for patients without severe influenza risk factors represented a high proportion (66.7%) of all NIs’ prescriptions during influenza epidemic periods since 2009, and should be explored. Another intriguing result found in this study is the existence of NIs’ prescription outside the epidemic periods. The recommendations stipulate that NIs are to be prescribed only during the circulation period of influenza virus. French official epidemic periods, used in our study, are defined by a statistical method [1], which does not forcibly reflect the circulation of influenza virus. It is possible that the perception of the influenza epidemic periods was quite different for the physicians. However, even if we take a margin of 3 weeks before and 3 weeks after the official dates, NIs’ prescriptions outside this “large epidemic period” were still found. These results are difficult to explain, and the reasons for such prescription should be explored in subsequent studies.
We must recognize several limitations to this study. The fact that we use pre-existent physicians’ database did not permit the collection of more precise biomedical, psychosocial and socio-demographic data about ILI patients, which could be associated to NIs’ prescription. In the same way, we did not have data about physicians’ characteristics. As expected [29], the rate of hospitalizations requested by GPs among ILI cases seen in primary health care consultations was low. The fact that it is an infrequent event could explain the lack of power to show an association between hospitalization and NIs’ prescription, although it is a specific factor in the French NIs’ recommendations. Pharmacist’s database was limited to the number of boxes delivery without any clinical or social information about patients who received NIs. Conversely this study presented the advantage of the large number of subjects included in the physicians’ databases. Inclusion of the whole ILI patients seen in Sentinel GPs’ consultations during the epidemic period avoided a risk of a selection bias. The use of two different and complementary physicians’ databases, showing close results, reinforces our conclusions. Another advantage of the study’s design is the use of physicians’ and pharmacists’ databases, which permit to present data about prescription and delivery.
Although physicians seem to follow recommended indications for NIs’ antiviral use in primary health care practice, this study confirms their hesitation to prescribe NIs to all ILI patients with a severe influenza risk factor. It would be useful to try to understand the reasons of the gap between experts’ recommendations and physicians’ practices. A prospective study would better assess biomedical, psychosocial and socio-demographic factors associated with NIs’ prescription. Considering this reluctance from GPs to prescribe NI systematically to ILI patients with a severe influenza risk factor, it could be interesting to modulate our public health messages. Improvement of adherence to NIs’ prescription might be focused among those for whom influenza vaccination is less effective. For example, when the early estimate suggests that the influenza vaccine has low effectiveness against circulating influenza, as against the (H3N2) viruses circulating this year [37].
Acknowledgments
Funding. This study was supported by the French National Institute of Health and Medical Research (Inserm) and the University Pierre and Marie Curie (UPMC). The funding was not specific for the study described in this article. The funder had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit this article for publication. All researchers’ decisions have been entirely independent from funders.
Footnotes
Contributors. Designed the experiments/the study: TB, FG, CT, ID, CL, XD, BL, TH, AM, CL ; Analysed the data: TB, FG, CT, CL ; Collected data/did experiments for the study: TB, FG, ID, BL, AM ; Wrote the first draft of the paper: TB, FG, CL ; Contributed to the writing of the paper: TB, FG, CT, ID, CL, XD, BL, TH, AM, CL.
Disclosure statement. All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf. AM and ID report grants from Laboratoire Roche, grants from Laboratoire GSK, grants from Laboratoire Argène, grants from Laboratoire Sanofi Pasteur MSD, grants from Laboratoire Abbott Products SAS, outside the submitted work; others authors have nothing to disclose.
References
- 1.Costagliola D, Flahault A, Galinec D, Garnerin P, Menares J, Valleron AJ. A routine tool for detection and assessment of epidemics of influenza-like syndromes in France. Am J Public Health. 1991;81:97–9. doi: 10.2105/ajph.81.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turbelin C, Souty C, Pelat C, et al. Age distribution of influenza like illness cases during post-pandemic A(H3N2): comparison with the twelve previous seasons, in France. PLoS One. 2013;8(6):e65919. doi: 10.1371/journal.pone.0065919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemaitre M, Carrat F, Rey G, Miller M, Simonsen L, Viboud C. Mortality Burden of the 2009 A/H1N1 Influenza Pandemic in France: Comparison to Seasonal Influenza and the A/H3N2 Pandemic. PLoS ONE. 2012;7(9):e45051. doi: 10.1371/journal.pone.0045051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leophonte P. Flu vaccine and anti-neuraminidases. Synergy or concurrence? Rev Mal Respir. 2001;18:487–89. [PubMed] [Google Scholar]
- 5.Haut Conseil de la Santé Publique (France) 2013 vaccination schedule and recommendations from the “Haut Conseil de la Santé Publique” in France. Bull Epidémiol Hebd. 2013;14–15:129–59. [Google Scholar]
- 6.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;(2):CD004876. doi: 10.1002/14651858.CD004876.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013;31(50):6030–3. doi: 10.1016/j.vaccine.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 9.Haut Conseil de la Santé Publique (France) On the use of influenza antivirals in patients outside of the hospital for curative treatment and post-exposure treatment during circulation of seasonal influenza virus. Paris: The Institute; 2012. [Google Scholar]
- 10.Centers for Disease Control and Prevention (USA) Seasonal Influenza (Flu) - Antiviral Medications: Summary for Clinicians. Atlanta: The Institute; 2014. [Google Scholar]
- 11.Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children--diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(8):1003–32. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebell MH, Call M, Shinholser J. Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract. 2013;30:125–33. doi: 10.1093/fampra/cms059. [DOI] [PubMed] [Google Scholar]
- 13.Michiels B, Van Puyenbroeck K, Verhoeven V, Vermeire E, Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS One. 2013;8(4):e60348. doi: 10.1371/journal.pone.0060348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burch J, Corbett M, Stock C, et al. Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:537–45. doi: 10.1016/S1473-3099(09)70199-9. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson T, Jones M, Doshi P, Del Mar C, Dooley L, Foxlee R. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. 2010;2:CD001265. doi: 10.1002/14651858.CD001265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev. 2012;1:CD002744. doi: 10.1002/14651858.CD002744.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355(9218):1845–50. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 18.Doshi P, Jefferson T. Clinical study reports of randomised controlled trials: an exploratory review of previously confidential industry reports. BMJ Open. 2013;3(2) doi: 10.1136/bmjopen-2012-002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med. 2010;7(11):e1000362. doi: 10.1371/journal.pmed.1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchon T, Mentré F, Charlois-Ou C, et al. Factors associated with clinical and virological response in patients treated with oseltamivir or zanamivir for influenza A during the 2008–2009 winter. Clin Microbiol Infect. 2013;19(2):196–203. doi: 10.1111/j.1469-0691.2011.03751.x. [DOI] [PubMed] [Google Scholar]
- 21.Flahault A, Blanchon T, Dorléans Y, Toubiana L, Vibert JF, Valleron AJ. Virtual surveillance of communicable diseases: a 20-year experience in France. Stat Methods Med Res. 2006;15(5):413–21. doi: 10.1177/0962280206071639. [DOI] [PubMed] [Google Scholar]
- 22.Legrand J. French GPs Sentinelles network: study of the representativity and participation of GPs sentinel physicians. Paris: Inserm; 2001. [Google Scholar]
- 23.Hannoun C, Dab W, Cohen JM. A new influenza surveillance system in France: the Ile-de-France “GROG”: Principles and methodology. Eur J Epidemiol. 1989;5(3):285–93. doi: 10.1007/BF00144828. [DOI] [PubMed] [Google Scholar]
- 24.Anatomical Classification of Pharmaceutical Products. European Pharmaceutical Market Research Association (EphMRA); [Accessed May 26, 2014]. Available from http://www.ephmra.org/Classification. [Google Scholar]
- 25.Cohen D. Questions remain over safety and effectiveness of oseltamivir. BMJ. 2012;344:e467. doi: 10.1136/bmj.e467. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz HM, Jackevicius CA, Ross JS. Tamiflu: 14 flu seasons and still questions. BMJ. 2013;346:f547. doi: 10.1136/bmj.f547. [DOI] [PubMed] [Google Scholar]
- 27.Kissling E, Valenciano M, Buchholz U, et al. Influenza vaccine effectiveness estimates in Europe in a season with three influenza type/subtypes circulating: the I-MOVE multicentre case-control study, influenza season 2012/13. Euro Surveill. 2014;19(6) doi: 10.2807/1560-7917.es2014.19.6.20701. [DOI] [PubMed] [Google Scholar]
- 28.Kissling E, Valenciano M, Cohen JM, et al. I-MOVE multi-centre case control study 2010–11: overall and stratified estimates of influenza vaccine effectiveness in Europe. PLoS One. 2011;6(11):e27622. doi: 10.1371/journal.pone.0027622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelat C, Lasserre A, Xavier A, Turbelin C, Blanchon T, Hanslik T. Hospitalization of influenza-like illness patients recommended by general practitioners in France between 1997 and 2010. Influenza Other Respir Viruses. 2013;7(1):74–84. doi: 10.1111/j.1750-2659.2012.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havers F, Thaker S, Clippard JR, et al. Use of Influenza Antiviral Agents by Ambulatory Care Clinicians During the 2012–2013 Influenza Season. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ison MG. Failing Our Patients by Suboptimally Treating Influenza Infections. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu425. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman KB, Demakas A, Erdman CB, Dimbil M, Doraiswamy PM. Neuropsychiatric adverse effects of oseltamivir in the FDA Adverse Event Reporting System, 1999–2012. BMJ. 2013;347:f4656. doi: 10.1136/bmj.f4656. [DOI] [PubMed] [Google Scholar]
- 33.Gotzsche PC. European governments should sue Roche and prescribers should boycott its drugs. BMJ. 2012;345:e7689. doi: 10.1136/bmj.e7689. [DOI] [PubMed] [Google Scholar]
- 34.Cohen D. Roche offers researchers access to all Tamiflu trials. BMJ. 2013;346:f2157-f. doi: 10.1136/bmj.f2157. [DOI] [PubMed] [Google Scholar]
- 35.Milon A, Autain F. A(H1N1)2009 pandemic infuenza : the first pandemic of the XXIth century. Paris: Sénat; 2010. [Google Scholar]
- 36.Oseltamivir : retention of information, marketing authorisation unjustifiable. La Revue Prescrire. 2013;33(354):261. [Google Scholar]
- 37.Flannery B, Clippard J, Zimmerman RK, et al. Early estimates of seasonal influenza vaccine effectiveness - United States, january 2015. MMWR Morb Mortal Wkly Rep. 2015;64(1):10–5. [PMC free article] [PubMed] [Google Scholar]