Abstract
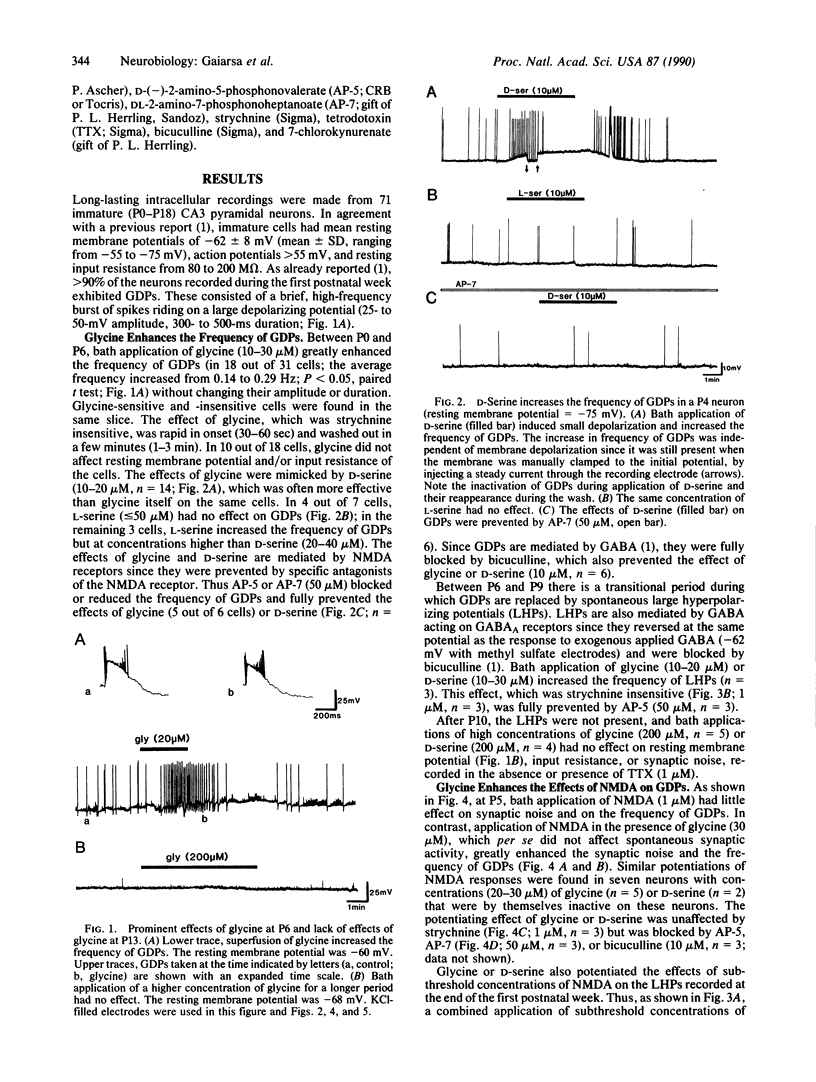
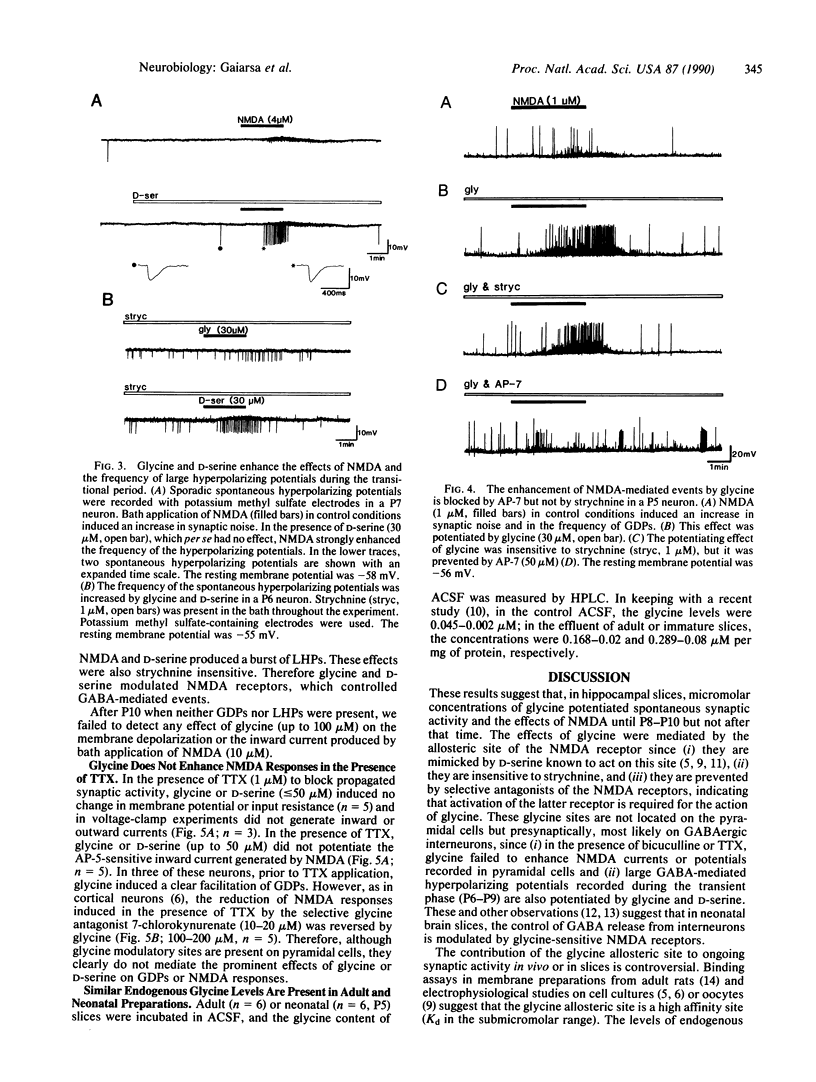
We report in this study that, in the presence of magnesium, bath application of micromolar concentrations of glycine have prominent effects on synaptic events and N-methyl-D-aspartate (NMDA) responses in neonatal but not in adult hippocampal slices. Intracellular recordings were made from 71 rat CA3 hippocampal neurons in neonatal slices. In keeping with our earlier study, during the first postnatal week, CA3 neurons exhibited giant depolarizing potentials (GDPs). These GDPs are mediated by gamma-aminobutyric acid (GABA) acting on type A GABA (GABAA) receptors and modulated presynaptically by NMDA receptors. In the majority of cells (18 out of 31), glycine (10-30 microM) increased the frequency of GDPs (from 0.14 to 0.29 Hz). This effect was mimicked by D-serine (10-20 microM) and blocked by the NMDA receptor antagonists D-(-)-2-amino-5-phosphonovalerate (50 microM) and DL-2-amino-7-phosphonoheptanoate (50 microM) and by the GABAA antagonist bicuculline (10 microM) but not by strychnine (1 microM). Subthreshold concentrations of glycine (or D-serine) and NMDA, when given together, enhanced synaptic noise and the frequency of GDPs. In the presence of tetrodotoxin (1 microM), glycine and D-serine (up to 50 microM) did not modify the NMDA-induced inward currents in CA3 pyramidal cells. However the reduction of NMDA-mediated currents by 7-chlorokynurenate (10-20 microM) was reversed by glycine and D-serine (100-200 microM). In contrast, glycine (up to 100 microM) had no effect on membrane potential, input resistance, or NMDA responses after postnatal day 10. It is concluded that GABA-mediated events are facilitated by glycine acting on presynaptically located NMDA receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Dent J. A. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981 Jan 1;195(1):51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Mo N. Inhibitory postsynaptic potentials in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1989 Mar;410:267–281. doi: 10.1113/jphysiol.1989.sp017532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont J. L., Gardette R., Crepel F. Postnatal development of the chemosensitivity of rat cerebellar Purkinje cells to excitatory amino acids. An in vitro study. Brain Res. 1987 Jul;431(1):59–68. doi: 10.1016/0165-3806(87)90195-7. [DOI] [PubMed] [Google Scholar]
- Fletcher E. J., Millar J. D., Zeman S., Lodge D. Non-competitive antagonism of N-methyl-d-aspartate by displacement of an endogenous glycine-like substance. Eur J Neurosci. 1989;1(3):196–203. doi: 10.1111/j.1460-9568.1989.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L., Mayer M. L. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988 Oct;8(10):3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M., Miller R. J. Excitatory amino acid-evoked release of [3H]GABA from hippocampal neurons in primary culture. Brain Res. 1989 Mar 13;482(1):23–33. doi: 10.1016/0006-8993(89)90538-6. [DOI] [PubMed] [Google Scholar]
- Hegstad E., Langmoen I. A., Hablitz J. J. Zinc and glycine do not modify low-magnesium-induced epileptiform activity in the immature neocortex in vitro. Epilepsy Res. 1989 Mar-Apr;3(2):174–177. doi: 10.1016/0920-1211(89)90046-6. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Foster A. C., Leeson P. D., Priestley T., Tridgett R., Iversen L. L., Woodruff G. N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989 Apr;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto H., Simon J. R., Aprison M. H. Determination of the equilibrium dissociation constants and number of glycine binding sites in several areas of the rat central nervous system, using a sodium-independent system. J Neurochem. 1981 Oct;37(4):1015–1024. doi: 10.1111/j.1471-4159.1981.tb04489.x. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- McGale E. H., Pye I. F., Stonier C., Hutchinson E. C., Aber G. M. Studies of the inter-relationship between cerebrospinal fluid and plasma amino acid concentrations in normal individuals. J Neurochem. 1977 Aug;29(2):291–297. doi: 10.1111/j.1471-4159.1977.tb09621.x. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Olverman H. J., Nguyen L., Watkins J. C., Cotman C. W. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9836–9840. doi: 10.1073/pnas.85.24.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom R. W., Deschenes N. L. Glycine modulation of NMDA-evoked release of [3H]acetylcholine and [3H]dopamine from rat striatal slices. Neurosci Lett. 1989 Jan 30;96(3):323–328. doi: 10.1016/0304-3940(89)90399-6. [DOI] [PubMed] [Google Scholar]
- Represa A., Tremblay E., Ben-Ari Y. Transient increase of NMDA-binding sites in human hippocampus during development. Neurosci Lett. 1989 Apr 24;99(1-2):61–66. doi: 10.1016/0304-3940(89)90265-6. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- This week in science. Science. 1988 Jul 22;241(4864):395–395. doi: 10.1126/science.241.4864.395. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Walker V. E., Flynn D. M. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature. 1989 Mar 30;338(6214):422–424. doi: 10.1038/338422a0. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Roisin M. P., Represa A., Charriaut-Marlangue C., Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988 Oct 4;461(2):393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Woodson W., Nitecka L., Ben-Ari Y. Organization of the GABAergic system in the rat hippocampal formation: a quantitative immunocytochemical study. J Comp Neurol. 1989 Feb 8;280(2):254–271. doi: 10.1002/cne.902800207. [DOI] [PubMed] [Google Scholar]



