Abstract
As an aromatic and colorful plant of substantive taste, saffron (Crocus sativus L.) owes such properties of matter to growing class of the secondary metabolites derived from the carotenoids, apocarotenoids. Regarding the critical role of microRNAs in secondary metabolic synthesis and the limited number of identified miRNAs in C. sativus, on the other hand, one may see the point how the characterization of miRNAs along with the corresponding target genes in C. sativus might expand our perspectives on the roles of miRNAs in carotenoid/apocarotenoid biosynthetic pathway. A computational analysis was used to identify miRNAs and their targets using EST (Expressed Sequence Tag) library from mature saffron stigmas. Then, a gene co- expression network was constructed to identify genes which are potentially involved in carotenoid/apocarotenoid biosynthetic pathways. EST analysis led to the identification of two putative miRNAs (miR414 and miR837-5p) along with the corresponding stem- looped precursors. To our knowledge, this is the first report on miR414 and miR837-5p in C. sativus. Co-expression network analysis indicated that miR414 and miR837-5p may play roles in C. sativus metabolic pathways and led to identification of candidate genes including six transcription factors and one protein kinase probably involved in carotenoid/apocarotenoid biosynthetic pathway. Presence of transcription factors, miRNAs and protein kinase in the network indicated multiple layers of regulation in saffron stigma. The candidate genes from this study may help unraveling regulatory networks underlying the carotenoid/apocarotenoid biosynthesis in saffron and designing metabolic engineering for enhanced secondary metabolites.
Key Words: Crocus sativus, EST sequences analysis, Co-expression network
INTRODUCTION
Saffron, the desiccated stigma of Crocus sativus L., contains volatile and non- volatile compounds. Saffron is an important plant because of its color, taste and aroma which are resulted from accumulation of the apocarotenoids such as crocin, picrocrocin and safranal, respectively [1]. In addition, these compounds have a broad range of pharmacological effects [2, 3]. Biosynthesis and regulation of apocarotenoids is a complicated process that occurs during the growth of saffron stigma. Apocarotenoid biosynthesis occurs mainly in the context of stigma color [4]. Transcriptome analysis of saffron stigmas is a prerequisite to provide a comprehensive view of the molecular basis of carotenoid/apocarotenoid biosynthesis and corresponding regulatory networks, the gynoecium biology, and the genomic organization [5, 6]. Post-transcriptional mechanisms, such as RNA silencing, have been found to play regulatory roles in secondary metabolic synthesis [7-9]. MicroRNAs (miRNAs), recognized as vital negative post-transcriptional regulators, are 19~24 nucleotides non-coding small RNAs which can direct transcriptional repression of target genes through mRNA cleavage or translational inhibition [10-14]. Guleria et al. 2012 predicted three miRNAs of C. sativus, csa-miR1, csa-miR2 and csa-miR3 by using in silico methods of EST analysis [15]. In addition, predicted targets for respective miRNAs were reported to play roles in regulation of plant growth, disease resistance, senescence, stress responses, mRNA export, protein synthesis and post-translational modifications [15]. Identification and characterization of miRNAs are important, especially for metabolic engineering in crop plants. However, little is known about regulatory roles of miRNAs in saffron stigma. Mature miRNAs are highly evolutionarily conserved and homologs of known miRNAs can be identified among plant species; this has the advantage of being able to use the comparative analysis as an effective means of determination of conserved miRNAs [16]. ESTs analysis can be considered as a valuable approach for the exploration of pre- miRNAs, particularly in plant species with unavailable genome sequences. This approach has been widely performed to determine miRNAs in a growing number of plant species [17-21]. In this work, an EST library was used to discover the miRNAs expressed in Crocus stigma. Afterwards, the regulatory functions of these miRNAs were predicted by searching for potential target genes. Finally, network modeling was used to investigate the roles of putative miRNAs in carotenoid/apocarotenoid biosynthesis in Crocus stigma.
MATERIALS AND METHODS
Data source: One Publicly available 5´-EST library of saffron stigma (Crocus sativus L., Iridaceae) containing 6202 ESTs was retrieved from NCBI (http://www.ncbi.nlm.nih.gov/). This includes the EST sequences generated by D'Agostino et al. (2007) [5]. Moreover, all currently available mature miRNAs (include 35828 sequences from 223 species) were downloaded from miRBase (http://www.mirbase.org/) [22].
EST sequence processing and assembly: The library was screened for vector contamination, sequence length and complexity using EGassembler webserver (http://egassembler.hgc.jp) [23]. The vector, sequences having similarity to plastids (chloroplast, mitochondrial) and repetitive sequences were masked and trimmed (less than 100 bp or having greater than 4% ambiguous bases) sequences were discarded [23, 24] and then, the remaining high quality ESTs were clustered and assembled into unigenes consisting of contigs and singletons using EGassembler webserver with overlap percent identity cutoff >80 %.
miRNAs prediction: The contigs were subjected against the plant mature miRNAs sequences using C-mii, a tool for plant miRNA and target identification in plants [25], with expect values=10, to increase the hit chance for more potential sequences. The minimal folding free energy index (MFEI) value of more than 0.6 is used to recognize miRNAs from other non-coding RNAs. Contig sequences having maximum 4 mis- matches were subjected to similarity searches against the non-coding RNA database 10 (Rfam 10) with removed miRNAs, and the UniProtKB/Swiss-Prot protein databases with C-mii BLAST option (E-value=10-10). All sequences with hit(s) were excluded from further analysis. miRNAs are different from other RNAs due to the distinguished property of their precursor sequences to form a secondary hairpin structure [26, 27]. Therefore, the putative miRNAs were further analyzed for being able to fold into appropriate stem–loop structures with UNAFold software embedded in C-mii. The parameters were adjusted as folding temperature (25 ºC), maximum bulges/interior loop size (30), and all others with defaults values.
miRNA-targeted genes prediction and gene ontology: The putative target genes of C. sativus predicted miRNAs were identified using C-mii software, with default parameters (no more than one mismatch at positions 1-9, no mismatches at positions 10 and 11 and no more than two consecutive mismatches). The mature miRNA sequences were queried against unigenes obtained from analysis of EST library of saffron stigma and unigenes of Arabidopsis thaliana (DFCI Gene Index (AGI), version 15) to search for putative target mRNAs. Gene ontology (GO) annotation of identified target genes was performed using the DAVID Bioinformatics Resources 6.7 [28, 29] and KEGG pathway database [30].
Gene co-expression network: The identified miRNA-targeted genes were networked using GeneMANIA based on automatically selected weighting method [31]. It provides a number of co-expressed genes relevant to target genes to make the regulatory networks more complete.
Identification of genes encoding transcription factor and protein kinases:
Homology-based search against the plant transcription factor database (PlnTFDB) [32] was performed in order to identify genes encoding transcription factor in the network. Also, homology-based search against the Kinase Sequence Database (KSD) [33] was performed in order to identify genes encoding protein kinases in the network.
RESULTS AND DISCUSSION
Pre-processing of 6202 EST sequences derived from saffron stigma resulted in 6020 high quality sequences. Assembling pre-processed ESTs resulted in 912 unigenes consisting of 609 contigs and 303 singletons, in which 5717 (94.93%) of ESTs fell into the contigs. The average contigs length was 508bp. The number of ESTs ranged from 2 to 555 in the contigs indicating that the genes have been expressed at different levels. 303 ESTs remained as singletons that probably represented the genes that are expressed at low level.
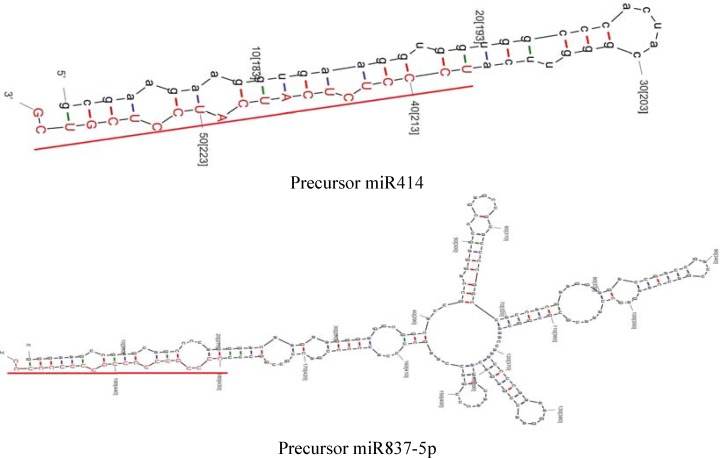
The alignment of total contigs obtained from EST analysis with known miRNAs from the miRNA Registry (miRBase) identified two contig sequences (contig 65 and contig 441) possessing the features so as to be considered as a putative miRNA and showed complementarities with two conserved plant mature miRNAs (ppt-miR414 and aly-miR837-5p, respectively). The number of ESTs in contig 65 and contig 441 was 8 and 2, respectively. The lengths of pre-miRNA414 and pre-miRNA837 were 58bp and 200bp, respectively. Both in silico predicted miRNAs were located at 3' ends of their respective pre-miRNAs. Minimum free energy (MFE), minimum free energy index (MFEI) values and some features of miRNAs were presented in Table 1. The secondary structures of the identified miRNAs were presented in Fig. 1. miRNAs are key components of large gene regulatory networks. Identification of miRNAs and miRNA target genes has a great significance to plant genetic improvement. In saffron, identification and characterization of miRNAs through genetic screening and direct cloning is difficult due to the non-availability of genomic sequence data and the tissue and time specific expressions of some miRNAs [34]. Computational approaches as powerful tools have simplified the identification and characterization of potential miRNAs. In doing so, two putative miRNAs including miR837-5p and miR414 along with the corresponding stem-looped precursors were computationally identified among the contigs, that have broadened our understanding of miRNAs which may play crucial regulatory roles in flavor and color biogenesis, the gynoecium biology, and the genomic organization. Unlike highly conserved mature miRNAs, pre-miRNAs are known to be species specific. Because it shows direct evidence of the of miRNA gene expression, the identification of pre-miRNAs in any organism is of fundamental importance [16].
Table 1.
Some features of predicted miRNAs from EST analysis of C. sativus
| Known mature miRNA | ppt-miR414 | aly-miR837-5p |
|---|---|---|
| Input sequence name | Contig65 | Contig441 |
| Number of mismatches between miRNA and miRNA* | 3 | 4 |
| Number of bulges | 2 | 0 |
| Bulge size | 1 | 0 |
| Number of flanked bases | 2 | 8 |
| Precursor start | 174 | 251 |
| Precursor stop | 231 | 450 |
| Primary miRNA MFE(kcal/mol) | -187.405 | -293.481 |
| Precursor miRNA MFE(kcal/mol) | -28.295 | -66.852 |
| Precursor miRNA MFEI(kcal/mol) | -0.832 | -0.777 |
| Number of homolog mismatches | 4 | 4 |
| Known mature miRNA sequence | 5':UCAUCCUCAUCAUCCUCGUCC:3' | 5':CAUUGUUUCUUGUUUUUUUCA:3' |
| Predicted miRNA sequence | 5':UCCCUCUCAUCAUCCUCGUCG:3' | 5':CUUUGUUUCUUGUUUUCCUCC:3' |
The maximum asymmetry of miRNA/miRNA
duplex was 4 for prediction of a novel miRNA
Figure 1.
Predicted secondary structure of in silico identified precursor miRNAs in C. sativus L. The mature miRNAs sequences are underlined and highlighted in red
According to Xie et al. (2010), the miR414 existed across the plant kingdom several million years ago prior to the divergence of monocots and dicots. It can therefore be found in almost all plant species [35]. According to research by Guleria and Yadav (2011) on miR414 expression in Stevia rebaudiana, miR414 primarily targets transcriptional regulators which participate in vital plant functions including growth, development, physiological and morphological changes, metabolism and defense responses. Hence, miR414 seems to play an essential role in the regulation of growth and development of plant [36]. A number of researchers have reported the important role of miR414 targets in post-transcriptional modifications, including SNF2 transcriptional regulator, high mobility group proteins, pentatricopeptide repeat- containing proteins, C2H2 zinc finger proteins, F-Box family proteins, DNA store keepers and RNA recognition motifs [37-40]. In addition, miR414 was reported to be a drought stress-associated miRNA in Physcomitrella patens, indicating that it may play important roles in drought stress tolerance in P. patens. It has been shown that mir414 targets mainly are involved in transportation of protein or sugar. Mir414 targets including group 3 late embryogenesis abundant (LEA) proteins and sucrose transporter showed overexpression in response to drought stress [41]. To our awareness, there has been little discussion about the processes regulated by miR837-5p in plants. Hua and coworkers conducted a survey and showed that miR837-5p in Arabidopsis, targets genes related to iron deficiency [42].
Target prediction is the first step to infer the roles of the putative miRNAs. So, miRNA targets were predicted. Each of putative miRNAs targeted 5 loci. Target prediction of newly identified miRNAs is presented in Table 2. Substantiated by UPE (unpaired energy), the most putative target genes for miR414 and miR837-5p were AT4G38480 and AT5G04670, respectively (Table 2). When mature miRNA sequences were used as queries to search for putative targets in unigenes obtained from analysis of saffron stigma EST library, only one EST (accession: EX143431.1) was considered to be miR414 target. The targets of miRNAs were also investigated in respect to their gene ontology. The putative miRNAs targets presented diverse biological process, molecular functions and cellular components (Table 2). Predicting the targets of miRNAs can provide informative clues about potential functions of miRNA and the miRNA regulatory network in saffron stigma. In our EST library one singletone (accession: EX143431.1) is found to be targeted by miR414. Subjecting this singletone to a tBLASTn search against nucleotide collection (nr/nt) database, showed it probably was beta carbonic anhydrase 5. Annotation of target protein suggested that this protein is involved in carbonate dehydratase activity. Search against KEGG pathway database was performed to elucidate the biochemical pathway in which this gene is involved.
Table 2.
The list of potential targets predicted for the putative miRNAs from C. sativus and their corresponding gene ontology
| miRNA | Target genes | Description | UPE* | Inhibition mechanism |
Gene Ontology
|
||
|---|---|---|---|---|---|---|---|
| process | function | component | |||||
| miR414 | gb|EX143431.1| EX143431 | Beta carbonic anhydrase 5 | 20.468 | Translation | Carbonate dehydratas e activity | Metal ion binding | Chloroplas, plastid |
| AT2G13700.1 | Transposable element gene | 9.032 | Cleavage | ||||
| AT4G06613.1 | Transposable element gene | 20.119 | Cleavage | ||||
| AT3G29783.1 | Transposable element gene | 6.812 | Cleavage | ||||
| AT4G38480.1 | Transducin/WD40 | 16.214 | Cleavage | Nucleotide | CUL4 RING | ||
| (F20M13_40) | repeat-like superfamily protein | binding | ubiquitin ligase complex | ||||
| miR837-5p | AT2G15670.1 | SEC14 cytosolic factor family protein / phosphoglyceride transfer family protein | 14.724 | Cleavage | Integral component of membrane | ||
| AT5G04670.1 (T1E3_30) | Enhancer of polycomb-like protein | 16.168 | Cleavage | Regulation of | Nucleus(Picco lo NuA4 | ||
| transcription | histone acetyltransfera se complex) | ||||||
| AT5G48790.1 | Domain of unknown function (DUF1995) | 15.571 | Cleavage | photosynt hesis, light reaction, | Chloroplast, plastid | ||
| regulation of protein dephospho | |||||||
| rylation | |||||||
| AT1G52565.1 | unknown protein | 15.838 | Cleavage | Endomembran e system | |||
| AT3G42770.1 | F-box/RNI-like/FBD- like domains- | Cleavage | |||||
Based on the results, it might be involved in nitrogen metabolism. No target gene was found for miR837-5p in unigenes obtained from analysis of saffron stigma EST library. However, 5 target genes for miR837-5p were predicted in Arabidopsis. Gene ontology analysis showed that some of the predicted target genes were involved in transcription regulation, photosynthesis, light reaction, and regulation of protein dephosphorylation.
A study by Eisen and colleagues suggested that co-expressed genes were functionally related. Sets of co-expressed genes that may be associated with target genes can be elucidated by gene co-expression network building. Co-expression analyses can lead to characterize genes of unknown function [43]. In doing so, the gene co- expression network was constructed, using potential target genes of predicted miR414 and miR837-5P. The list of predicted co-expressed genes is presented in Table 3.
Table 3.
The list of co-expressed genes in the co-expression network
| Symbol | Tair ID | Description | Pathway |
|---|---|---|---|
| AT1G52565 ATBCA5 | AT1G52565 AT4G33580 | unknown protein beta carbonic anhydrase 5 | Nitrogen |
| metabolism | |||
| AT2G32640 | AT2G32640 | Lycopene beta/epsilon cyclase protein | |
| T1E3_30 | AT5G04670 | Enhancer of polycomb-like transcription factor protein | |
| AT2G15670 | AT2G15670 | BEST Arabidopsis thaliana protein match is: SEC14 cytosolic factor family protein / phosphoglyceride transfer family protein | |
| F20M13.40 | AT4G38480 | Transducin/WD40 repeat-like superfamily protein | |
| AT3G42770 | AT3G42770 | F-box/RNI-like/FBD-like domains-containing protein | |
| AT5G48790 | AT5G48790 | Domain of unknown function (DUF1995) | |
| AT4G34220 | AT4G34220 | Leucine-rich repeat protein kinase family protein | |
| ATSWI3C | AT1G21700 | SWITCH/sucrose nonfermenting 3C | |
| PR5K | AT5G38280 | PR5-like receptor kinase | |
| T17F3.6 | AT1G69910 | Protein kinase superfamily protein | |
| T1E22_170 | AT5G02410 | alpha-1,2-glucosyltransferase | N-Glycan biosynthesis |
| ATDOF2.4 | AT2G37590 | DNA binding with one finger 2.4 | |
| AT3G18570 | AT3G18570 | Oleosin family protein | |
| AT5G17700 | AT5G17700 | MATE efflux family protein | |
| AT3G13240 | AT3G13240 | unknown protein | |
| AT1G11720 | AT1G11720 | starch synthase 3 | |
| AT5G28470 | AT5G28470 | Major facilitator superfamily protein | |
| AtMYB40 | AT5G14340 | myb domain protein 40 | |
| ERF043 | AT4G32800 | Integrase-type DNA-binding superfamily protein | |
| ATGLR1.3 | AT5G48410 | glutamate receptor 1.3 | |
| PSBI | ATCG0008 | photosystem II reaction center protein I | |
| T22C5_12 | AT1G27670 | unknown protein; BEST Arabidopsis thaliana protein match is: | |
| unknown protein (TAIR:AT1G75360.1) | |||
| AT2G28320 | AT2G28320 | Pleckstrin homology (PH) and lipid-binding START domains- | |
| T24D18.12 | AT1G16020 | Protein of unknown function (DUF1712) | |
| TRFL3 | AT1G17460 | TRF-like 3 | |
| AT1G77800 | AT1G77800 | PHD finger family protein |
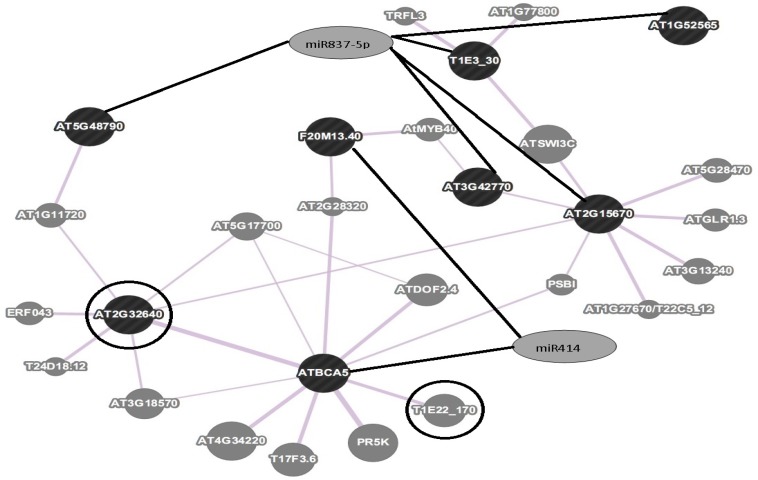
The network uncovered the relationships between the target genes and the co- expressed genes. Lycopene beta/epsilon cyclase protein (AT2G32640) was shown to be a part of the co-expression network that included 28 relationships (Fig. 2) and was directly or indirectly connected to almost all of the target genes. Earlier reports have also shown increased expression of LCYβ and LCYε in stigma leading to increased production of secondary metabolites [44]. Notably, lycopene beta/epsilon cyclase protein within the predicted network was connected to the carotenoid biosynthesis. Additionally, beta carbonic anhydrase 5, target of miR414, had a relationship with alpha-1,2-glucosyltransferase (Fig. 2). Glucosyltransferases are enzymes that catalyze the transfer of a sugar molecule to a speci fi c acceptor, thereby forming a glucosidic bond. Many secondary metabolites of higher plants, including important fl avour compounds, are glucosylated [45]. Carotenoid-derived metabolites (apocarotenoids) constitute a growing class of secondary metabolites that are found as glucosides in fruits and other plant parts [46]. In C. sativus, crocetin and 3-Hydroxy-β-cyclocitral are transformed to crocin and picrocrocin respectively by enzymatic activities of UDP- glucosyltransferases [6]. To date the gene encoding alpha-1,2-glucosyltransferase has not been reported in crocus. Therefore, kinetic analysis and substrate specificity of this enzyme from crocus may help elucidate the role it plays in apocarotenoid biosynthetic pathway. Identification and characterization of glycosyltransferase genes from saffron stigma could help explain the accumulation of a specific glycosylated metabolite [47]. It is noteworthy that miR414 and miR837-P targeted the genes that co-expressed with gene such as lycopene beta/epsilon cyclase protein (AT2G32640) which was reported to be involved in carotenoid biosynthesis. This finding suggested the significant role of predicted miRNAs in carotenoid biosynthetic process (Fig. 2).
Figure 2.
A graphical view of co-expression network of potential target genes. Network was constructed using GeneMANIA based on automatically selected weighting method. Network includes predicted miRNAs, miRNA-target genes and co-expressed genes. Relations between miRNAs and target genes are shown as black edges, whereas relations between target genes and co-expressed genes are depicted by gray edges. Lycopene beta/epsilon cyclase protein (AT2G32640) was directly or indirectly connected to almost all of the target genes of predicted miR414 and miR837-5P. The co-expression network also unraveled the relationship between beta carbonic anhydrase 5 (a target of miR414) and alpha-1,2- glucosyltransferase (T1E22_170). Genes involved in carotenoid/apocarotenoid biosynthesis (AT2G32640 and T1E22_170) are highlighted with a circle
Despite the significant progress concerning carotenoid/apocarotenoid pathway [1, 48], enzymes, intermediates [6] and the network regulating the carotenoid/apocarotenoid biosynthesis still remain elusive. Based on the co-expression network presented here, it can be suggested that these putative miRNAs will provide a knowledge base about regulatory network of carotenoid/apocarotenoid biosynthesis. Identification of transcription factor and protein kinase genes in this network may provide a new insight into the regulatory mechanism of carotenoid/apocarotenoid biosynthesis. Six unigenes including Arabidopsis thaliana switching protein 3C, Dof- type zinc finger domain-containing protein, AtMYB40, AP2 domain-containing transcription factor TINY, TRF-LIKE 3 and PHD finger family protein showed similarity to transcription factors belonging to various families (Table 4).
Table 4.
The list of co-expressed genes encoding transcription factors in the co-expression network
| Symbol | Tair ID | Description | Family |
|---|---|---|---|
| ATSWI3C | AT1G21700 | ATSWI3C (Arabidopsis thaliana switching protein 3C); DNA binding | MYB-related |
| ATDOF2.4 | AT2G37590 | Dof-type zinc finger domain-containing protein | C2C2-Dof |
| AtMYB40 | AT5G14340 | AtMYB40 (myb domain protein 40); DNA binding / transcription factor | MYB |
| ERF043 | AT4G32800 | AP2 domain-containing transcription factor TINY, putative | AP2-EREBP |
| TRFL3 | AT1G17460 | TRFL3 (TRF-LIKE 3); DNA binding / transcription factor | MYB-related |
| AT1G77800 | AT1G77800 | PHD finger family protein | PHD |
Transcription factors are known to play key roles in plant growth, development and response to various environmental stimuli. They are also reported to be involved in regulation of secondary metabolite biosynthesis [6]. Presence of the identified transcription factors in the co-expression network supports previous researches into investigation expression of regulatory carotenoid pathway genes. Recent evidence suggest that several members of Myb family have regulatory roles in different secondary metabolic pathways [49-51]. Gargouri et al. demonstrated that PHD7 was positively correlated with zeaxanthin epoxidase (ZEO), enzyme involved in carotenoid biosynthesis in Chlamydomonas [52]. A survey conducted by Baba et al. (2015) showed that zinc finger protein DOF5.4 and zinc finger protein DOF2.4 were expressed in saffron stigma. Since little is known about the transcriptional regulators controling the expression of structural genes in Crocus carotenoid/apocarotenoid biosynthetic pathway, the transcription factors identified from this survey are good candidates to experimentally validate their roles in carotenoid/apocarotenoid biosynthetic pathway and flower development [6]. Also, protein kinases are involved in proteinsphosphorylation. Phosphorylation and dephosphorylation as the major general mechanism controlling proteins [53] regulates diverse cellular functions in eukaryotes such as cell division, cell differentiation, signal transduction, etc [6]. One unigene showed similarity to leucine-rich repeat transmembrane protein kinase (AT4G34220) belonging to family98.
Taken together, these findings indicated the presence of miRNAs in regulatory pathway of carotenoid/apocarotenoid biosynthesis in saffron stigma. Target prediction and co-expression network led to the inference regarding the roles of the predicted miRNAs. The identified miRNAs and their target and co-expressed genes including transcription factors and protein kinase in C. sativus may help unraveling regulatory networks underlying the carotenoid/apocarotenoid biosynthesis and designing metabolic engineering for enhanced secondary metabolites. Due to the existence of false positive results in bioinformatics prediction, putative miRNA discovered through this approach need to be further verified with laboratory experiments. This survey provides an overview of the existence and expression of miRNAs in saffron sigma for future research.
Acknowledgments:
We would like to greatly thank Department of Agroecology of Agriculture and Natural Resources of Darab for supporting this research.
Conflict of Interest:
The authors declare that they have no competing interest.
References
- 1.Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Robio- Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S, Giuliano G. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci USA. 2014;111:12246–12251. doi: 10.1073/pnas.1404629111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Wang CZ, Wen XD, Shoyama Y, Yuan CS. Role of saffron and its constituents on cancer chemoprevention. Pharm Biol. 2013;51:920–924. doi: 10.3109/13880209.2013.771190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez-Gómez L, Rubio-Moraga A, Ahrazem O. Understanding carotenoid metabolism in saffron stigmas: unraveling aroma and colour formation. Funct Plant Sci Biotechnol. 2010;4:56–63. [Google Scholar]
- 5.D'Agostino N, Pizzichini D, Chiusano ML, Giuliano G. An EST database from saffron stigmas. BMC Plant Biol. 2007;7:53. doi: 10.1186/1471-2229-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba SA, Mohiuddin T, Basu S, Swarankar MK, Malik AH, Wani ZA, Singh AK, Ashraf N. Comprehensive transcriptome analysis of Crocus sativus for discovery and expression of genes involved in apocarotenoid biosynthesis. BMC Genomics. 2015;16:698. doi: 10.1186/s12864-015-1894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boke H, Ozhuner E, Turktas M, Parmaksiz I, Ozcan S, Unver T. Regulation of the alkaloid biosynthesis by miRNA in opium poppy. Plant Biotechnol J. 2015;13:409–420. doi: 10.1111/pbi.12346. [DOI] [PubMed] [Google Scholar]
- 8.Robert-Seilaniantz A, MacLean D, Jikumaru Y, Hill L, Yamaguchi S, Kamiya Y, Jones JDG. The microRNA miR393 redirects secondary metabolite biosynthesis away from camalexin and towards glucosinolates. Plant J. 2011;67:218–231. doi: 10.1111/j.1365-313X.2011.04591.x. [DOI] [PubMed] [Google Scholar]
- 9.Ng DWK, Zhang CQ, Miller M, Palmer G, Whiteley M, Tholl D, Chen ZJ. cis- and trans-regulation of miR163 and target genes confers natural variation of secondary metabolites in two Arabidopsis species and their allopolyploids. Plant Cell. 2011;23:1729–1740. doi: 10.1105/tpc.111.083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 12.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Baumberger N, Baulcombe DC. Arabidopsis ARGONAUTE1 is an RNA slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci USA. 2005;102:11928–11933. doi: 10.1073/pnas.0505461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 15.Guleria p, Goswami D, Yadav K. Computational identification of miRNAs and their targets from Crocus sativus L. Arch Biol Sci. 2012;64:65–70. [Google Scholar]
- 16.Yusuf NH, Ong WD, Redwan RM, Latip MA, Kumar SV. Discovery of precursor and mature microRNAs and their putative gene targets using high-throughput sequencing in pineapple (Ananas comosus var comosus) Gene. 2015;571:71–80. doi: 10.1016/j.gene.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Luan F, Zhu H, Shao Y, Chen A, Lu C, Luo Y, Zhu B. Computational identification of microRNAs and their targets in wheat (Triticum aestivum L) Sci China C Life Sci. 2009;52:1091–1100. doi: 10.1007/s11427-009-0144-y. [DOI] [PubMed] [Google Scholar]
- 18.Zanca AS, Vicentini R, Ortiz-Morea FA, Del Bem LE, da Silva MJ, Vincentz M, Nogueira FT. Identification and expression analysis of microRNAs and targets in biofuel crop sugarcane. BMC Plant Biol. 2010;10:260. doi: 10.1186/1471-2229-10-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akter A, Islam MM, Mondal SI, Mahmud Z, Jewel NA, Ferdous S, Amin MR, Rahman MM. Computational identification of miRNA and targets from expressed sequence tags of coffee (Coffea arabica) Saudi J Biol Sci. 2013;21:3–12. doi: 10.1016/j.sjbs.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vishwakarma NP, Jadeja VJ. Identification of miRNA encoded by Jatropha curcas from EST and GSS. Plant Signal Behav. 2013;8:e23152. doi: 10.4161/psb.23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Din M, Barozai MYK. Profiling microRNAs and their targets in an important fleshy fruit: tomato (Solanum lycopersicum) Gene. 2014;535:198–203. doi: 10.1016/j.gene.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masoudi-Nejad A, Tonomura K, Kawashima S, Moriya Y, Suzuki M, Itoh M, Kanehisa M, Endo T, Goto S. EGassembler: online bioinformatics service for large- scale processing, clustering and assembling ESTs and genomic DNA fragments. Nucleic Acids Res. 2006;34:W459–462. doi: 10.1093/nar/gkl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson JE, Leebens-Mack JH, Wall PK, Zahn LM, Mueller LA, Landherr LL, Hu Y, Ilut DC, Arrington JM, Choirean S, Becker A, Field D, Tanksley SD, Ma H, dePamphilis CW. EST database for early flower development in California poppy (Eschscholzia californica Cham, Papaveraceae) tags over 6000 genes from a basal eudicot. Plant Mol Biol. 2006;62:351–369. doi: 10.1007/s11103-006-9025-y. [DOI] [PubMed] [Google Scholar]
- 25.Numnark S, Mhuantong W, Ingsriswang S, Wichadakul D. C-mii: a tool for plant miRNA and target identification. BMC Genomics. 2012;13:S16. doi: 10.1186/1471-2164-13-S7-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhardwaj J, Mohammad H, Yadav SK. Computational identification of microRNAs and their targets from the expressed sequence tags of horsegram (Macrotyloma uniforum (Lam) Verdc) J Struct Funct Genomics. 2010;11:233–240. doi: 10.1007/s10969-010-9098-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang BH, Pan XP, Wang QL, Cobb GP, Anderson TA. Identification and characterization of new plant microRNAs using EST analysis. Cell Res. 2005;15:336–360. doi: 10.1038/sj.cr.7290302. [DOI] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-Rodríguez P, Riaño-Pachón DM, Corrêa LGG, Rensing SA, Kersten B, Mueller-Roeber B. PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res. 2010;38:D822–D827. doi: 10.1093/nar/gkp805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buzko O, Shokat KM. Kinase sequence database: sequence alignments and family assignment. Bioinformatics. 2002;18:1274–1275. doi: 10.1093/bioinformatics/18.9.1274. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Pan X, Wang Q, Cobba GP, Anderson TA. Computational identification of microRNAs and their targets. Comp Biol Chem. 2006;30:395–407. doi: 10.1016/j.compbiolchem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Xie F, Frazier TP, Zhang B. Identification and characterization of microRNAs and their targets in the bioenergy plant switchgrass (Panicum virgatum) Planta. 2010;232:417–434. doi: 10.1007/s00425-010-1182-1. [DOI] [PubMed] [Google Scholar]
- 36.Guleria P, Yadav SK. Identification of miR414 and expression analysis of conserved miRNAs from Stevia rebaudiana. Genomics Proteomics Bioinformatics. 2011;9:211–217. doi: 10.1016/S1672-0229(11)60024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suo J, Liang X, Pu L, Zhang Y, Xue Y. Identification of GhMYB109 encoding a R2R3MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L) Biochim Biophys Acta. 2003;1630:25–34. doi: 10.1016/j.bbaexp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Wu Q, Pwee KH, Manjunatha Kini R. Interaction of wheat high- mobility-group proteins with four-way-junction DNA and characterization of the structure and expression of HMGA gene. Arch Biochem Biophys. 2003;409:357–366. doi: 10.1016/s0003-9861(02)00630-6. [DOI] [PubMed] [Google Scholar]
- 39.Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Identification of multiple distinct SNF2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;43:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Q, Xiang A, Yang Q. Bioinformatic identification of microRNAs and their target genes from Solanum tuberosum expressed sequence tags. Chin Sci Bull. 2007;52:2380–2389. [Google Scholar]
- 41.Wan P, Wu J, Zhou Y, Xiao J, Feng J, Zhao W, Xiang S, Jiang G, Chen JK. Computational analysis of drought stress-associated miRNAs and miRNA co- regulation network in Physcomitrella patens. Genomics Proteomics Bioinformatics. 2011;9:37–44. doi: 10.1016/S1672-0229(11)60006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua L, Ming-Qun G, Xiao-Yu S, Hui-Jie Z. Identification of microRNA responsed to iron deficiency in Arabidopsis. Chin J Biochem Mol Biol. 2014;30:291–297. [In Chinese] [Google Scholar]
- 43.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castillo R, Fernandez J, Gomez-Gomez L. Implications of carotenoid biosynthetic genes in apocarotenoid formation during the stigma development of Crocus sativus and its closer relatives. Plant Physiol. 2005;139:674–689. doi: 10.1104/pp.105.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winterhalter P, Skouroumounis GK. Glycoconjugated aroma compounds: occurrence, role and biotechnological transformation. Adv Biochem Eng Biotechnol. 1997;55:73–105. doi: 10.1007/BFb0102063. [DOI] [PubMed] [Google Scholar]
- 46.Winterhalter P, Rouseff RS, editors. Carotenoid-derived aroma compounds. Washington, DC: American Chemical Society; 2001. Carotenoid-derived aroma compounds: an introduction; pp. 1–17. [Google Scholar]
- 47.Barvkar VT, Pardeshi VC, Kale SM, Kadoo NY, Gupta VS. Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns. BMC Genomics. 2012;13:175. doi: 10.1186/1471-2164-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubio-Moraga A, Rambla JL, Fernández-de-Carmen A, Trapero-Mozos A, Ahrazem O, Orzáez D, Granell A, Gómez-Gómez L. New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Mol Biol. 2014;86:555–569. doi: 10.1007/s11103-014-0250-5. [DOI] [PubMed] [Google Scholar]
- 49.Onkokesung N, Gaquerel E, Kotkar H, Kaur H, Baldwin IT, Galis I. MYB8 controls inducible phenolamide levels by activating three novel hydroxycinnamoyl- coenzyme A: polyamine transferases in Nicotiana attenuata. Plant Physiol. 2012;158:389–407. doi: 10.1104/pp.111.187229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koyama K, Numata M, Nakajima I, Goto Yamamoto N, Matsumura H, Tanaka N. Functional characterization of a new grapevine MYB transcription factor and regulation of proanthocyanidin biosynthesis in grapes. J Exp Bot. 2014;65:4433–4449. doi: 10.1093/jxb/eru213. [DOI] [PubMed] [Google Scholar]
- 51.Yuan Y, Wu C, Liu Y, Yang J, Huang L. The Scutellaria baicalensis R2R3-MYB transcription factors modulates flavonoid biosynthesis by regulating GA metabolism in transgenic tobacco plants. PLoS One. 2013;8:e77275. doi: 10.1371/journal.pone.0077275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gargouri M, Park JJ, Holguin FO, Kim MJ, Wang H, Deshpande RR, Shachar-Hill Y, Hicks LM, David RG. Identification of regulatory network hubs that control lipid metabolism in Chlamydomonas reinhardtii. J Exp Bot. 2015;66:4551–4566. doi: 10.1093/jxb/erv217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartels S, Gonzalez Besteiro MA, Lang D, Ulm R. Emerging functions for plant MAP kinase phosphatases. Trends Plant Sci . 2010;15:322–329. doi: 10.1016/j.tplants.2010.04.003. [DOI] [PubMed] [Google Scholar]