Abstract
CaCl2 treatment followed by heat shock is the most common method for artificial transformation. Here, the cells were transformed using CaCl2 treatment either with heat shock (standard protocol) or without heat shock (lab protocol) to comprehend the difference in transformation efficiency. The BL21 strain of Escherichia coli (E. coli) was being susceptible using CaCl2 treatment. Some Cells were kept at -80 oC while the others were kept at 4 ˚C. Afterwards the susceptible cells were transformed using either standard or lab protocol. The transformation efficiency between cells experienced heat shock and those were not influenced by heat shock was almost the same. Moreover, regardless of transformation protocol, the cells kept at 4 ˚C were transformed more efficiently in compared to those were kept at -80 oC.
Key Words: E. coli, Artificial transformation, Heat shock, Transformation efficiency
INTRODUCTION
Bacteria can naturally obtain new generic information through 3 various mechanisms; conjugation, transduction and transformation [1]. Transferring of DNA directly from one organism to another one is called conjugation but in transduction DNA transferring is occurred with the aid of bacteriophage. During transformation naked DNA is bounden to the cell surface and passed through wall- membrane complex [2]. Transformation can happen either naturally or artificially in bacteria. Natural transformation is a rare mechanism used by some bacterial cells to take up DNA from the environment. In artificial transformation, bacterial cells should be susceptible under certain laboratory conditions prior to transformation. There are two main methods for artificial transformation in bacteria; CaCl2 treatment followed by brief heat shock and electroporation [3, 4]. Either of methods has been modified during the past century to achieve more transformation efficiency.
The exact mechanism of how CaCl2 treatment could facilitate transferring DNA from extracellular environment into cytoplasm is still unrevealed. It is assumed that DNA molecules can be absorbed on the cell surface with the help of divalent cation Ca2+ and heat shock step make entering DNA into cytosol possible [2].
In this study, E. coli bacteria were transformed using two methods; (1) CaCl2 treatment followed by heat shock step and (2) CaCl2 treatment without using heat shock step. The transformation efficiency was calculated for both methods. It seems that heat shock step may not have the crucial role for transformation protocol.
MATERIALS AND METHODS
The BL21 strain of E. coli bacteria was used for transformation. The pEGFP-N1 vector originally purchased from Clontech Laboratories, Inc. (USA). The vector is kanamycin resistant and contains enhanced green fluorescent protein (EGFP) gene.
E. coli bacteria were grown in Luria Bertani (LB) medium. 15 µL of overnight cultured E. coli was inoculated to 25 ml LB broth and was incubated on a shaker at 37 ˚C and 250 rpm until the optical density of suspension at 600 nm reached in the range of 0.4-0.7. Then the suspensions were kept on ice for 30 min [5]. Following this step, the bacteria were pelleted using Dragon Lab centrifuge (D3024R, Dragon Laboratory Instruments Limited, China) at 1717 for 20 min at 4 ˚C. The bacterial pellet was re-suspended in 12.5 ml CaCl2 (100mM) and placed on ice for 30 min. Next, the cell suspensions were centrifuged as above and pellets were dissolved in 25 ml CaCl2 (100mM). At this stage, suspensions kept on ice overnight. Again the bacteria were pelleted at 1717 for 20 min at 4 ˚C and the pellets were re-suspended in 2250 µl CaCl2 (100mM) and 750 µl glycerol 60% and stored at -80 ˚C and/or 4 ˚C for further experiments.
Transformations were performed using either standard protocol or lab protocol as follows; 100 µl of thawed competent bacteria and 0.65 ng of pEGFP-N1 DNA (isolated from E. coli strain DH5-alpha with AccuPrep® plasmid mini extraction kit from Bioneer and qualified with gel agarose electrophoresis) were added to pre-chilled tube and pipetted gently. In standard protocol, the suspensions were kept on ice for 30 min and after that the mixture were heated at 42 ˚C for 45 seconds. Following heat shock step the tubes were kept on ice for another 5 min. Then 1 ml LB broth was added and serially diluted suspensions were prepared and incubated in a shaker at 37 ˚C for 2 hours. But in our lab protocol, the ice and heat shock steps were completely omitted and 1 ml LB broth was immediately added to the mixture of DNA and bacterial cells (at 25 ˚C) and the suspension was serially diluted and incubated in a shaker at 250 rpm for 2 hours at 37 ˚C. At the end of both protocols, the cells were centrifuged at 1717 for 5 min. and pellets were re-suspended in 100 µl LB and streaked on kanamycin resistant plate.
The experiments were performed in quadruple. The data are reported as mean ± standard deviation. The unpaired t- test was performed in order to understand the significance of difference. The two-tailed P values were assigned less than 0.05. The GraphPad Prism 5 software was used for analysis.
RESULTS AND DISCUSSION
The transformation was performed using cells kept at either 4 ˚C (fresh) or -80 ˚C with both standard and lab protocol. The cells were successfully transformed with standard and lab protocol. The number of bacteria which were able to uptake DNA molecules is defined as Transformation Efficiency (TE) [2]. The TE was calculated from Equation 1.
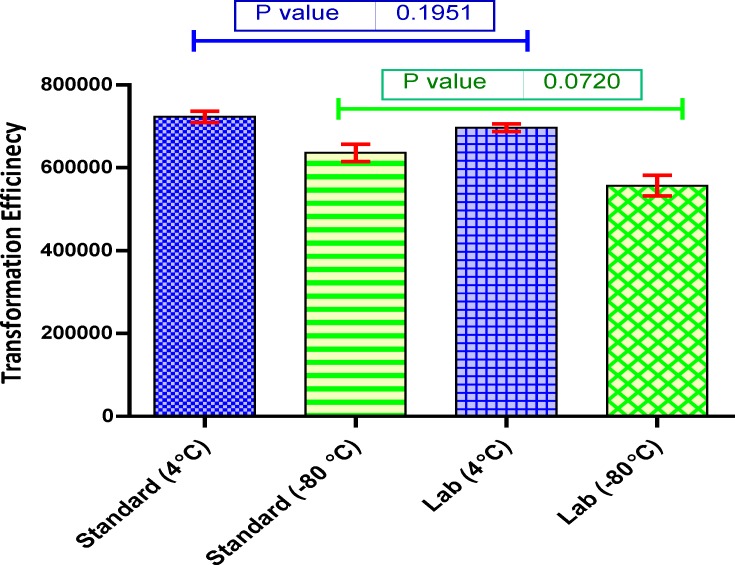
In general, the TE of fresh cells (kept at 4 ˚C) was more than those were kept for longer period at -80 oC. Notably, the difference between lab and standard protocol was quite low and bacteria could be transformed without applying heat shock. The transformation efficiencies are displayed in Figure 1. The successful transformation plates are presented in Figure 2.
Figure 1.
Transformation efficiencies of various conditions
Figure 2.
Successful diluted bacterial transformation plates using standard and lab protocol (The samples were diluted 100 times
It was suggested that heat shock step could facilitate DNA entry but still there is not enough clues. Panja et al (2006) reported that heat-pulse step cause reduction in membrane potential [2]. The cellular inside potential is became less negative as a result of membrane potential decrease therefore, the negative DNA could enter the cytosol easier [2].
Although the exact role of Ca2+ ions is not known yet, it is believed that Ca2+ could develop the interaction between DNA molecules and LPS (lipopolysaccharide) of outer membrane. Also, Ca2+ ions could alter the physio-chemical properties of lipids and induce phase transition of phosphatidylglycerol and LPS [6-8]. Furthermore, Ca2+ divalent cations enhance structural changes in phosphatidylcholine-cardiolipin bilayers which lead to increased permeability [6, 9-11].
Our results suggest that Ca2+ ions play more crucial role in artificial transformation. Also, it seems that the ability of Ca2+ in increasing the membrane permeability is more than heat shock. At the same time, it should be noted that in the lab protocol cells were undergone a brief heat shock (0 →25 → 37 ˚C) which is appeared to be enough for transferring DNA. In order to understand the exact mechanism of Ca2+ ions and heat shock step in transformation, more investigation need to be performed.
Acknowledgment
This research project was financially supported by a grant from the Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran. The authors declare that there is no conflict of interests.
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.Chen I, Dubnau D. DNA uptake during bacterial transformation. Nat Rev Microbiol. 2004;2:241–249. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- 2.Panja S, Saha S, Jana B, Basu T. Role of membrane potential on artificial transformation of E coli with plasmid DNA. J Biotechnol. 2006;127:14–20. doi: 10.1016/j.jbiotec.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Divya Prakash G, Anish RV, Jagadeesh G, Chakravortty D. Bacterial transformation using micro-shock waves. Anal Biochem. 2011;419:292–301. doi: 10.1016/j.ab.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Chung CT, Niemela SL, Miller RH. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 6.van Die IM, Bergmans HE, Hoekstra WP. Transformation in Escherichia coli: studies on the role of the heat shock in induction of competence. J Gen Microbiol. 1983;129:663–670. doi: 10.1099/00221287-129-3-663. [DOI] [PubMed] [Google Scholar]
- 7.Verkleij AJ, de Kruyff B, Ververgaert PH, Tocanne JF, van Deenen LL. The influence of pH, Ca2+ and protein on the thermotropic behaviour of the negatively charged phospholipid, phosphatidylglycerol. Biochim Biophys Acta. 1974;339:432–437. doi: 10.1016/0005-2736(74)90171-0. [DOI] [PubMed] [Google Scholar]
- 8.van Alphen L, Verkleij A, Burnell E, Lugtenberg B. 31P nuclear magnetic resonance and freeze-fracture electron microscopy studies on Escherichia coli II Lipopolysaccharide and lipopolysaccharide-phospholipid complexes. Biochim Biophys Acta. 1980;597:502–517. doi: 10.1016/0005-2736(80)90223-0. [DOI] [PubMed] [Google Scholar]
- 9.Gerritsen WJ, de Kruijff B, Verkleij AJ, de Gier J, van Deenen LL. Ca2+-induced isotropic motion and phosphatidylcholine flip-flop in phosphatidylcholine-cardiolipin bilayers. Biochim Biophys Acta. 1980;598:554–560. doi: 10.1016/0005-2736(80)90035-8. [DOI] [PubMed] [Google Scholar]
- 10.Mandersloot JG, Gerritsen WJ, Leunissen-Bijvelt J, van Echteld CJ, Noordam PC, de Gier J. Ca2+-induced changes in the barrier properties of cardiolipin/ phosphate-dylcholine bilayers. Biochim Biophys Acta. 1981;640:106–113. doi: 10.1016/0005-2736(81)90536-8. [DOI] [PubMed] [Google Scholar]
- 11.Burnell E, van Alphen L, Verkleij A, de Kruijff B. 31P nuclear magnetic resonance and freeze-fracture electron microscopy studies on Escherichia coli I Cytoplasmic membrane and total phospholipids. Biochim Biophys Acta. 1980;597:492–501. doi: 10.1016/0005-2736(80)90222-9. [DOI] [PubMed] [Google Scholar]