Abstract
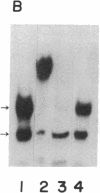
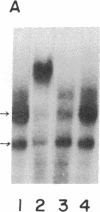
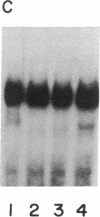
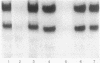
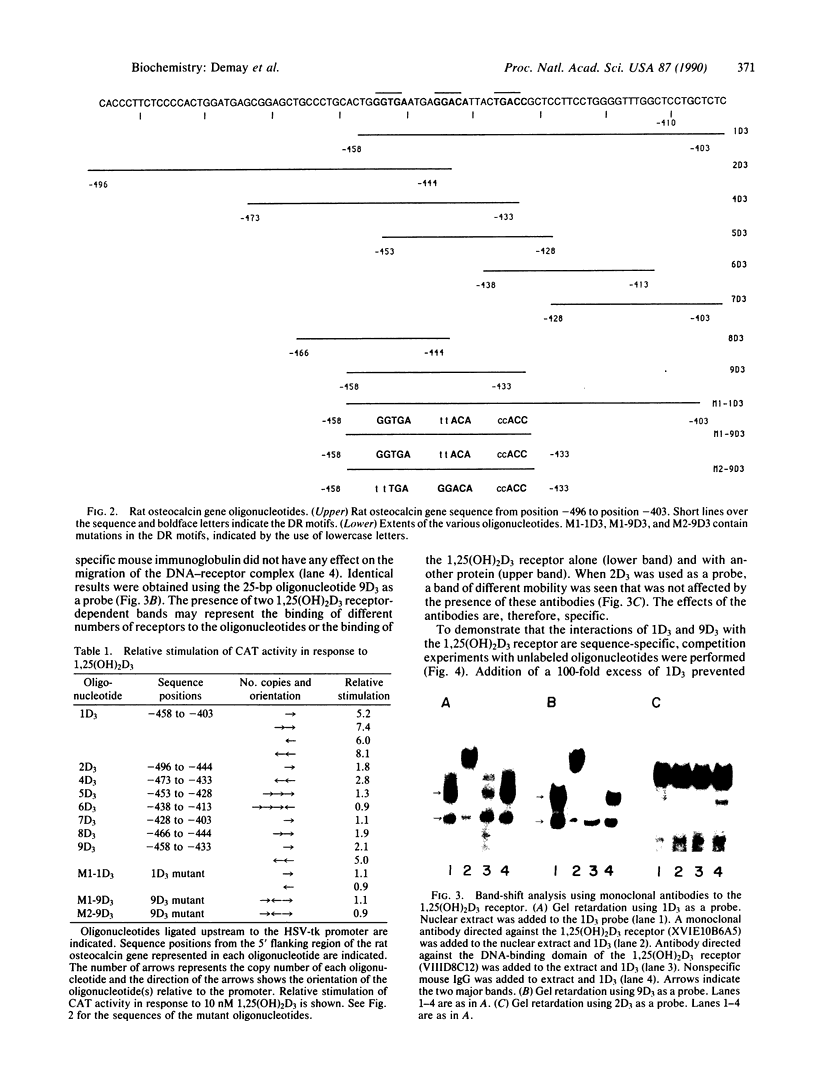
The 5' flanking region of the rat osteocalcin gene has been shown to confer responsiveness to 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] after transfection of fusion genes into ROS 17/2.8 cells. Deletion analysis has demonstrated that there are at least two domains in this 5' flanking region that contribute to 1,25(OH)2D3 responsiveness; however, only the downstream region is able to confer 1,25(OH)2D3 responsiveness to either the native osteocalcin promoter or to a heterologous viral promoter (herpes simplex virus thymidine kinase). The proximal region responsible for 1,25(OH)2D3 induction of the rat osteocalcin gene lies 458 base pairs upstream from the transcription start site of this gene. A 25-base-pair oligonucleotide corresponding to the sequences in this region is able to confer 1,25(OH)2D3 responsiveness to the thymidine kinase promoter in an orientation-independent fashion. This sequence contains three copies of a short sequence that are homologous to "half-sites" of steroid response elements. Gel-retardation assays using porcine intestinal nuclear extract as a rich source of 1,25(OH)2D3 receptor demonstrated retardation in the migration of probes containing the sequence noted above. A monoclonal antibody directed against the 1,25(OH)2D3 receptor caused further retardation in the migration of these protein-DNA complexes. Therefore, the sequences represented in this oligonucleotide encompass the sequences necessary for binding of the 1,25(OH)2D3 receptor to DNA as well as those sequences necessary for 1,25(OH)2D3 to induce osteocalcin gene transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brent G. A., Harney J. W., Chen Y., Warne R. L., Moore D. D., Larsen P. R. Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol Endocrinol. 1989 Dec;3(12):1996–2004. doi: 10.1210/mend-3-12-1996. [DOI] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., DeLuca H. F. Identification of the porcine intestinal 1,25-dihydroxyvitamin D3 receptor on sodium dodecyl sulfate/polyacrylamide gels by renaturation and immunoblotting. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7825–7829. doi: 10.1073/pnas.82.23.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame M. C., Pierce E. A., Prahl J. M., Hayes C. E., DeLuca H. F. Monoclonal antibodies to the porcine intestinal receptor for 1,25-dihydroxyvitamin D3: interaction with distinct receptor domains. Biochemistry. 1986 Aug 12;25(16):4523–4534. doi: 10.1021/bi00364a011. [DOI] [PubMed] [Google Scholar]
- Demay M. B., Roth D. A., Kronenberg H. M. Regions of the rat osteocalcin gene which mediate the effect of 1,25-dihydroxyvitamin D3 on gene transcription. J Biol Chem. 1989 Feb 5;264(4):2279–2282. [PubMed] [Google Scholar]
- Kerner S. A., Scott R. A., Pike J. W. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Lian J., Stewart C., Puchacz E., Mackowiak S., Shalhoub V., Collart D., Zambetti G., Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Price P. A., Baukol S. A. 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J Biol Chem. 1980 Dec 25;255(24):11660–11663. [PubMed] [Google Scholar]
- Prost E., Moore D. D. CAT vectors for analysis of eukaryotic promoters and enhancers. Gene. 1986;45(1):107–111. doi: 10.1016/0378-1119(86)90138-1. [DOI] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. G., Rutledge S. J., Buenaga R. F., Rodan G. A. Characterization of the rat osteocalcin gene: stimulation of promoter activity by 1,25-dihydroxyvitamin D3. Biochemistry. 1988 Nov 15;27(23):8521–8526. doi: 10.1021/bi00423a003. [DOI] [PubMed] [Google Scholar]