Abstract
Phenotypic plasticity is expected to play a major adaptive role in the response of species to ocean acidification (OA), by providing broader tolerances to changes in pCO2 conditions. However, tolerances and sensitivities to future OA may differ among populations within a species because of their particular environmental context and genetic backgrounds. Here, using the climatic variability hypothesis (CVH), we explored this conceptual framework in populations of the sea urchin Loxechinus albus across natural fluctuating pCO2/pH environments. Although elevated pCO2 affected the morphology, physiology, development and survival of sea urchin larvae, the magnitude of these effects differed among populations. These differences were consistent with the predictions of the CVH showing greater tolerance to OA in populations experiencing greater local variation in seawater pCO2/pH. Considering geographical differences in plasticity, tolerances and sensitivities to increased pCO2 will provide more accurate predictions for species responses to future OA.
Keywords: phenotypic plasticity, tolerance, climate variability, upwelling, Loxechinus albus
1. Introduction
In an era of rapid environmental changes such as ocean acidification (OA), there is a pressing need to understand how organisms will respond to greater and less predictable variations in environmental conditions [1]. Intuitively, phenotypic plasticity seems a suitable strategy to cope with these changes by means of behavioural, physiological, life-history and morphological adjustments [2]. These plastic responses have long been recognized as important mechanisms by which organisms maximize fitness in heterogeneous environments [3], facilitating the persistence of natural populations by providing broader tolerances to environmental conditions [4]. Despite this central role of phenotypic plasticity, standard models aimed to predict the effect of climatic change on species persistence and distribution (i.e. the climate envelope models) do not incorporate differences in plastic responses among populations [5]. Geographical differences in plasticity may reflect contrasting selective pressures resulting from habitats with different environmental heterogeneity [6]. In this context, the climatic variability hypothesis (CVH) offers a powerful conceptual framework with which to view the impact of future climate change (e.g. OA) on species persistence, by linking physiology, climate and biogeographic distributions [5]. The CVH states that in more variable environments, organisms should have broader ranges of environmental tolerance and/or greater physiological flexibilities that enable them to cope with fluctuating environmental conditions [7].
Environmental variability is an intrinsic characteristic of coastal ecosystems along the southern Pacific coast of South America, where spatio-temporal changes in CO2, pH, temperature, nutrients and other factors are the result of dynamic processes such as upwellings, riverine discharges and biological activity [8]. In this region, populations of marine organisms are exposed to natural variability in pH/pCO2 with fluctuating CO2-supersaturated surface waters almost year-round in the northern section (18° S–30° S), seasonal CO2-supersaturated waters southward (30° S–39° S) and CO2-undersaturated surface waters in the southernmost section (more than 42° S) [9]. Like in other coastal regions, this natural variability can be far greater in magnitude than the predicted change due to OA [10], and may have prompted the evolution of a broad range of mechanisms by which coastal organisms can maintain the homeostasis for biological processes (e.g. calcification) [11]. Homeostatic capacity and tolerances to CO2/pH changes are known to differ across geographical regions [12], highlighting the importance of this component in the understanding of species susceptibilities to future OA. In order to test the CVH, we explored morpho-physiological and developmental responses to elevated pCO2, in natural populations of a keystone species of the Pacific coast of Chile, the sea urchin Loxechinus albus. We predict that populations experiencing greater local variation in CO2/pH (i.e. continuous and seasonal upwelling subregions) will be less susceptible to OA than populations from less variable CO2/pH environments.
2. Material and methods
Adult sea urchins were collected from nine localities along the upwelling system and fjords of the Pacific coast of Chile, spanning approximately 4500 km (electronic supplementary material, figure S1). Animals were maintained in flowing seawater aquaria (13–14°C) and fed ad libitum with kelp until experiments. Gametes were obtained by standard methods [13] within 2–3 days after collection. From each locality, 20 independent crosses of a single male with pooled eggs of three females were developed to avoid male–female incompatibility. Eggs were fertilized using filtered (0.1 µm) seawater at ambient conditions (13°C and 390 µatm pCO2) and distributed in the experimental pCO2 treatments (current global: 390 µatm and projected OA: 1200 µatm [10,14]) with controlled temperature (13–14°C) at a concentration of approximately 0.7 embryos ml−1. Each cross was cultured separately in two replicate buckets for each treatment, in which the seawater carbonate chemistry was maintained using a semi-automatic flow-through CO2-mixing system [15], modified following [16] (electronic supplementary material, table S1). Embryos were sampled daily to record developmental progression (DP) and survival until early pluteus (4 arms, 80–84 h) when physiological (metabolic rate (MR) by oxygen uptake-VO2) and morphometric (total larval length and postoral arm length) analyses were performed (see the electronic supplementary material). Traits were analysed using linear mixed models in the ‘lme4’ package of R v.3.3 (R Core Team, 2016), with CO2 treatment and subregion as fixed factors and locality as random factor. Significance tests were performed with the ‘lmerTest’ package. Post hoc comparisons for mixed effects models were done with the ‘multcomp’ and ‘lsmeans’ packages. DP curves were fitted and analysed with GraphPad Prism software (GraphPad, San Diego, CA, USA).
3. Results
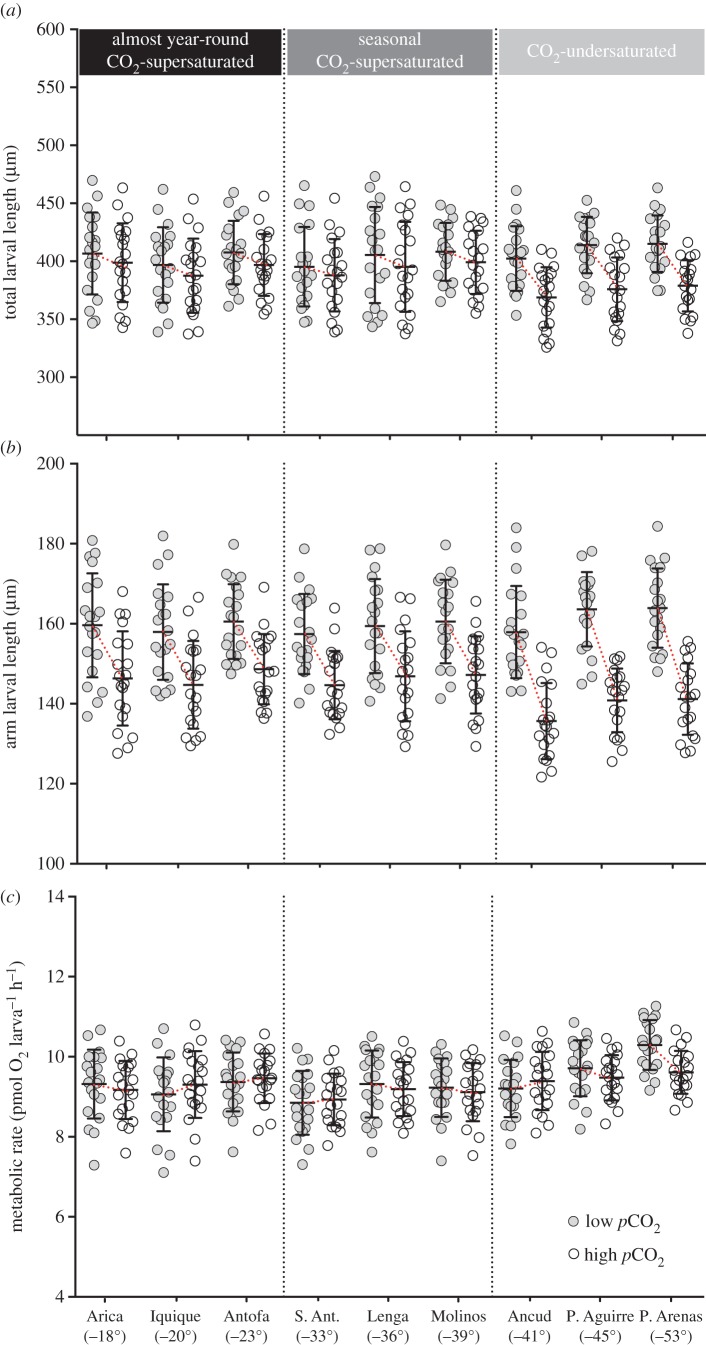
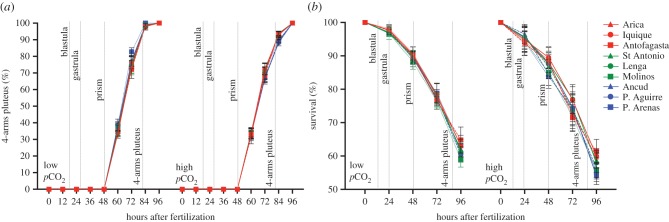
There were significant effects of high pCO2 on total larval length (TL: F1,348 = 32.4, p < 0.05), postoral arm length (POL: F1,348 = 221.7, p < 0.05), size-corrected MR (F1,348 = 35.8, p < 0.05), DP (F1,1792 = 11.35, p < 0.05) and survival (F1,348 = 494.9, p < 0.05) of L. albus. These effects varied among subregions (significant interaction) for all of the morpho-physiological (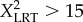 , p < 0.05) and developmental traits (electronic supplementary material, table S2). Although POL, survival and DP were significantly affected (F2,348 = 9.03, F2,348 = 33.1 and F20,1776 = 66.2; p < 0.05) by elevated pCO2 in populations from the three subregions (figures 1 and 2), the major negative effects were detected in those within the CO2-undersaturated subregion (post hoc tratio < −12, p < 0.05). These three populations were the only ones with negative effects of high pCO2 on TL and MR (post hoc tratio = −6.5 and −4.4, respectively, p < 0.05; figure 1a). Under low pCO2 conditions, most of the phenotypic traits did not differ among populations and subregions (TL: F2,177 = 1.08, p = 0.34; POL: F2,177 = 1.12, p = 0.32; MR: F2,177 = 1.92, p = 0.15; survival: F2,177 = 2.23, p = 0.11; figures 1 and 2). However, DP differed geographically (F8,864 = 6.634, p < 0.05; figure 2), showing a faster rate in the southernmost population (figures 1 and 2a).
, p < 0.05) and developmental traits (electronic supplementary material, table S2). Although POL, survival and DP were significantly affected (F2,348 = 9.03, F2,348 = 33.1 and F20,1776 = 66.2; p < 0.05) by elevated pCO2 in populations from the three subregions (figures 1 and 2), the major negative effects were detected in those within the CO2-undersaturated subregion (post hoc tratio < −12, p < 0.05). These three populations were the only ones with negative effects of high pCO2 on TL and MR (post hoc tratio = −6.5 and −4.4, respectively, p < 0.05; figure 1a). Under low pCO2 conditions, most of the phenotypic traits did not differ among populations and subregions (TL: F2,177 = 1.08, p = 0.34; POL: F2,177 = 1.12, p = 0.32; MR: F2,177 = 1.92, p = 0.15; survival: F2,177 = 2.23, p = 0.11; figures 1 and 2). However, DP differed geographically (F8,864 = 6.634, p < 0.05; figure 2), showing a faster rate in the southernmost population (figures 1 and 2a).
Figure 1.
Geographical responses in (a) total larval length, (b) postoral arm length and (c) size-corrected metabolic rate under pCO2 treatments. Mean ± s.d. S. Ant., St Antonio; P., Punta. (Online version in colour.)
Figure 2.
Effect of ocean acidification on (a) developmental progression and (b) survival of sea urchin larvae. Mean ± s.d.
4. Discussion
This study highlights the role of naturally fluctuating pCO2/pH environments in determining geographical differences in phenotypic responses to projected OA. Although elevated pCO2 affected larval morphology, physiology, development and survival, the magnitude of these effects differed among the three main subregions. The lack of clinal trends in phenotypic responses to simulated OA suggests that geographical differences in average pCO2 are not driving differences in tolerances among sea urchin populations. Instead, phenotypic differences in L. albus were consistent with the predictions of the CVH, showing greater tolerance to OA in populations experiencing greater local variation in seawater CO2/pH [7,17]. Total larval length, postoral arm length (a proxy of larval calcification [18]), DP and survival were less affected (approx. 3% change) in populations within the CO2-supersaturated subregions (i.e. continuous and seasonal; 18–39° S) than in populations from the CO2-undersaturated subregion (approx. 9% change; more than 40° S). This geographical pattern of phenotypic responses may result from spatial differences in OA-induced energetic costs for maintenance, growth and survival under elevated pCO2 conditions [19]. In fact, larval MR was only affected by acidified seawater in populations within the CO2-undersaturated subregion, supporting the idea that changes in energy allocation can be the main drivers of the negative effects of OA in L. albus [18,19]. Under present-day pCO2 conditions, larvae of L. albus showed similar morpho-physiological characteristics among populations. Nonetheless, the faster growth rate observed in the southernmost population is likely due to the rearing temperature, which was slightly higher (approx. 2°C) than the local conditions during its spawning/growing season (11°C) [13].
From a theoretical perspective, geographical differences in tolerances and sensitivities to high pCO2 may reflect contrasting selective pressures along the spatial distribution of L. albus [6]. Within the CO2-supersaturated subregions, local selection may have promoted highly plastic phenotypes with broad tolerance to pH variation [20], and greater fitness (e.g. survival) under high pCO2 conditions [21] in comparison with phenotypes from the CO2-undersaturated subregion. Similar geographical differences in performance and fitness have been documented in other marine species along upwelling systems (e.g. [12,22]), suggesting that regional differences in carbonate chemistry may have acted as selective pressures maintaining phenotypic and genetic variation necessary for adaptive responses to changing pH [21,22]. Although gene flow can impede the adaptive divergence in populations of L. albus [20], local adaptation may still occur if phenotype-specific mortality occurs after larval dispersal [23].
In a projected OA scenario, the stronger negative effects on larval growth and developmental dynamics in populations from the CO2-undersaturated region can lead to prolonged pelagic larval duration, increasing the susceptibility to predation and reducing the number of settlers due to the high mortality in the plankton [18]. For these populations, greater shifts in skeletal morphology caused by OA may influence larval feeding and their ability to disperse [24], affecting the energy transfer across trophic levels and potentially influencing population dynamics and predator–prey interactions in marine food webs [14]. In conclusion, our study reinforces the importance of considering geographical differences in plasticity, tolerances and sensitivities to increased pCO2 for predicting species responses to future OA.
Supplementary Material
Ethics
This study was conducted under approval of the UACH animal ethics committee no. 056/12.
Data accessibility
Data are available as the electronic supplementary material.
Authors' contributions
J.D.G.-E. and L.D.B. conceived the study, carried out analyses and drafted the manuscript. J.D.G.-E., P.A.V. and J.M.N. developed experiments. J.L. and R.T. analysed water chemistry. All authors approved the final version of the manuscript and agree to be held accountable for the content.
Competing interests
We have no competing interests.
Funding
J.D.G.-E. was supported by FONDECYT-Postdoctoral grant no. 3130381 and Duke/Oak Foundation Mini-Grant in Marine Conservation.
References
- 1.Botero C, Weissing F, Wright J, Rubenstein D. 2015. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189. ( 10.1073/pnas.1408589111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piersma T, van Gils J. 2011. The flexible phenotype. A body-centred integration of ecology, physiology and behaviour. New York, NY: Oxford University Press. [Google Scholar]
- 3.Pigliucci M. 2001. Phenotypic plasticity. In Evolutionary ecology: concepts and case studies (eds Fox CW, Roff DA, Fairbairn DJ), pp. 58–69. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Gabriel W, Luttbeg B, Sih A, Tollrian R, Gabriel W, Luttbeg B, Sih A, Tollrian R. 2005. Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am. Nat. 166, 339–353. ( 10.1086/432558) [DOI] [PubMed] [Google Scholar]
- 5.Molina-Montenegro M, Naya D. 2012. Latitudinal patterns in phenotypic plasticity and fitness-related traits: assessing the climatic variability hypothesis (CVH) with an invasive plant species. PLoS ONE 7, 23–28. ( 10.1371/journal.pone.0047620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaitán-Espitia JD, Marshall D, Dupont S, Bacigalupe LD, Bodrossy L, Hobday AJ. 2017. Geographical gradients in selection can reveal genetic constraints for evolutionary responses to ocean acidification. Biol. Lett. 13, 20160784 ( 10.1098/rsbl.2016.0784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janzen D. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 8.Vargas C, Contreras P, Perez C, Sobarzo M, Saldias G, Salisbury J. 2016. Influences of riverine and upwelling waters on the coastal carbonate system off Central Chile and their ocean acidification implications. J. Geophys. Res. 121, 1468–1483. ( 10.1002/2015JG003213) [DOI] [Google Scholar]
- 9.Torres R, et al. 2011. Air-sea CO2 fluxes along the coast of Chile: from CO2 outgassing in central northern upwelling waters to CO2 uptake in southern Patagonian fjords. J. Geophys. Res. 116, C09006 ( 10.1029/2010JC006344) [DOI] [Google Scholar]
- 10.Hofmann G, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendriks I, Duarte C, Olsen Y, Steckbauer A, Ramajo L, Moore T, Trotter J, McCulloch M. 2015. Biological mechanisms supporting adaptation to ocean acidification in coastal ecosystems. Estuar. Coast. Shelf Sci. 152, A1–A8. ( 10.1016/j.ecss.2014.07.019) [DOI] [Google Scholar]
- 12.Padilla-Gamiño J, Gaitán-Espitia J, Kelly M, Hofmann G. 2016. Physiological plasticity and local adaptation to elevated pCO2 in calcareous algae: an ontogenetic and geographic approach. Evol. Appl. 9, 1043–1053. ( 10.1111/eva.12411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence J. 2013. Sea urchins: biology and ecology, 3rd edn Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 14.Byrne M. 2011. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr. Mar. Biol. Annu. Rev. 49, 1–42. ( 10.1201/b11009-6) [DOI] [Google Scholar]
- 15.Torres R, Manriquez PH, Duarte C, Navarro JM, Lagos NA, Vargas CA, Lardies MA. 2013. Evaluation of a semi-automatic system for long-term seawater carbonate chemistry manipulation. Rev. Chil. Hist. Nat. 86, 443–451. ( 10.4067/S0716-078X2013000400006) [DOI] [Google Scholar]
- 16.Padilla-Gamiño J, Kelly M, Evans T, Hofmann G. 2013. Temperature and CO2 additively regulate physiology, morphology and genomic responses of larval sea urchins, Strongylocentrotus purpuratus. Proc. R. Soc. B 280, 20130155 ( 10.1098/rspb.2013.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupont S, Ortega-Martínez O, Thorndyke M. 2010. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19, 449–462. ( 10.1007/s10646-010-0463-6) [DOI] [PubMed] [Google Scholar]
- 18.Byrne M, Lamare M, Winter D, Dworjanyn S, Uthicke S. 2013. The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Phil. Trans. R. Soc. B 368, 20120439 ( 10.1098/rstb.2012.0439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stumpp M, Wren J, Melzner F, Thorndyke M, Dupont S. 2011. CO2 induced seawater acidification impacts sea urchin larval development I: elevated metabolic rates decrease scope for growth and induce developmental delay. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 160, 331–340. ( 10.1016/j.cbpa.2011.06.022) [DOI] [PubMed] [Google Scholar]
- 20.Futuyma D, Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233. ( 10.1146/annurev.es.19.110188.001231) [DOI] [Google Scholar]
- 21.Pespeni M, et al. 2013. Evolutionary change during experimental ocean acidification. Proc. Natl Acad. Sci. USA 110, 6937–6942 10.1073/pnas.1220673110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly M, Padilla-Gamiño J, Hofmann G. 2013. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob. Chang. Biol. 19, 2536–2546. ( 10.1111/gcb.12251) [DOI] [PubMed] [Google Scholar]
- 23.Marshall D, Monro K, Bode M, Keough M, Swearer S. 2010. Phenotype–environment mismatches reduce connectivity in the sea. Ecol. Lett. 13, 128–140. ( 10.1111/j.1461-0248.2009.01408.x) [DOI] [PubMed] [Google Scholar]
- 24.Chan K, Grünbaum D, O'Donnell M. 2011. Effects of ocean-acidification-induced morphological changes on larval swimming and feeding. J. Exp. Biol. 214, 3857–3867. ( 10.1242/jeb.054809) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as the electronic supplementary material.