Abstract
Parental environment can widely influence offspring phenotype, but paternal effects in the absence of parental care remain poorly understood. We asked if protein content in the larval diet of fathers affected paternity success and gene expression in their sons. We found that males reared on high-protein diet had sons that fared better during sperm competition, suggesting that postcopulatory sexual selection is subject to transgenerational paternal effects. Moreover, immune response genes were downregulated in sons of low-protein fathers, while genes involved in metabolic and reproductive processes were upregulated.
Keywords: transgenerational effects, gene expression, parental effects, postcopulatory sexual selection, RNAseq, transcriptomics
1. Introduction
Parental effects can be triggered by diverse factors and describe non-genetic contributions of parents to offspring developmental phenotypes. Maternal effects are well documented, but less-understood paternal effects can also significantly impact offspring phenotypes [1,2], including sexually selected traits [3–5], even when males contribute only sperm [1,5,6]. Paternal diet, in particular, can influence offspring traits, if females choosing sperm from males adapted to the local nutritional environment produce offspring with higher fitness [7]. Molecular mechanisms of transgenerational paternal diet effects remain poorly understood but include altered methylation in metabolism-linked loci (reviewed in [8]), perturbed glucose–insulin homeostasis [9], altered cholesterol biosynthesis [10], and modified chromatin states related to obesity [6]. Here, we examine how high- and low-protein paternal larval diet influences postcopulatory sexual selection and gene expression in sons of Drosophila melanogaster.
2. Material and methods
Experimental D. melanogaster expressed green fluorescent protein (GFP) in sperm heads and ubiquitously in somatic cells for paternity assignment (focal males) or red fluorescent protein (RFP; females and competitor males) in sperm heads [11]. GFP larvae were reared on high- (HP; 200 g yeast : 50 g sugar) or low-protein (LP; 50 g yeast : 50 g sugar) diet known to yield 80–96% survival [12]. For each treatment, 10 vials were prepared upon eclosion, each with five CO2-collected males and five same-stock females reared on standard diet (SD; 100 g yeast : 50 g sugar), housed in SD vials (see electronic supplementary material for more detailed methods). Virgin focal sons were transferred to SD until mating. Three-day-old virgin SD RFP females were first mated with SD RFP competitor males (day 0) in individual vials and provided 6 h opportunities to remate with a focal son for 4 subsequent days (days 1–4) under continuous observation. After remating, females oviposited on fresh SD food vials for 4 days. Paternity of adult offspring [13] was determined using a Nikon SMZ18 fluorescent stereoscope. P2 was calculated as the proportion of GFP-sired progeny, and data were analysed with logistic regressions with binomial error structure (glm in R v. 3.2.0 [14]).
RNA was extracted from two replicates of 20 7-day-old focal sons per treatment using an RNeasy kit (Qiagen) and quantified using Agilent Bioanalyzer. Illumina TruSeq mRNA stranded libraries were constructed, and 76 bp paired-end sequences were obtained on an Illumina NextSeq 500, replicated across two flow cells, with within-sample replicates pooled for further analysis [15]. We performed RNASeq data analysis using the Tuxedo Protocol in the DNA Subway online platform [16] with quality control using FastX-Toolkit (v. 0.0.13.2). Reads were mapped to the D. melanogaster transcriptome and genome (Ensembl r76, BDPG5) using TopHat (v. 2.0.11, [17]). Differentially expressed (DE) genes were identified using CUFFDIFF (v. 2.1.1, [16]) at a q-value < 0.05 after false discovery rate correction [18]. Results were visualized with CummeRbund and Cytoscape (for biological networks, [19]) in R.
3. Results
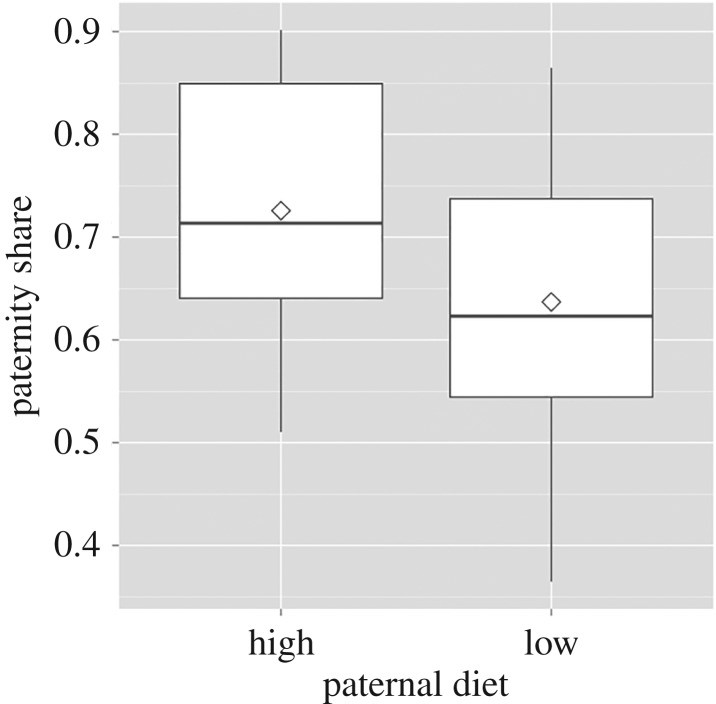
P2 of sons from fathers on high larval diet was higher than that of sons from low larval diet fathers (estimate ± s.e. = −0.216 ± 0.077, Z = –2.80, p = 0.005; figure 1). Of 69 DE genes (q ≤ 0.05; fold change > 1.5), 58 were downregulated (fold change 1.54–10.6; mean ± s.e. = 2.30 ± 1.46) in LP sons related to immune response, specifically antimicrobial humoral response and response to insecticides and other toxins (figure 2a). Eleven genes were upregulated primarily in reproductive and metabolic functions (fold change 1.66–6.2; mean ± s.e. = 2.83 ± 1.54; see table 1, electronic supplementary material table S1 and figure 2b).
Figure 1.
Paternity share (P2) of sons from fathers on either high or low larval diet.
Figure 2.
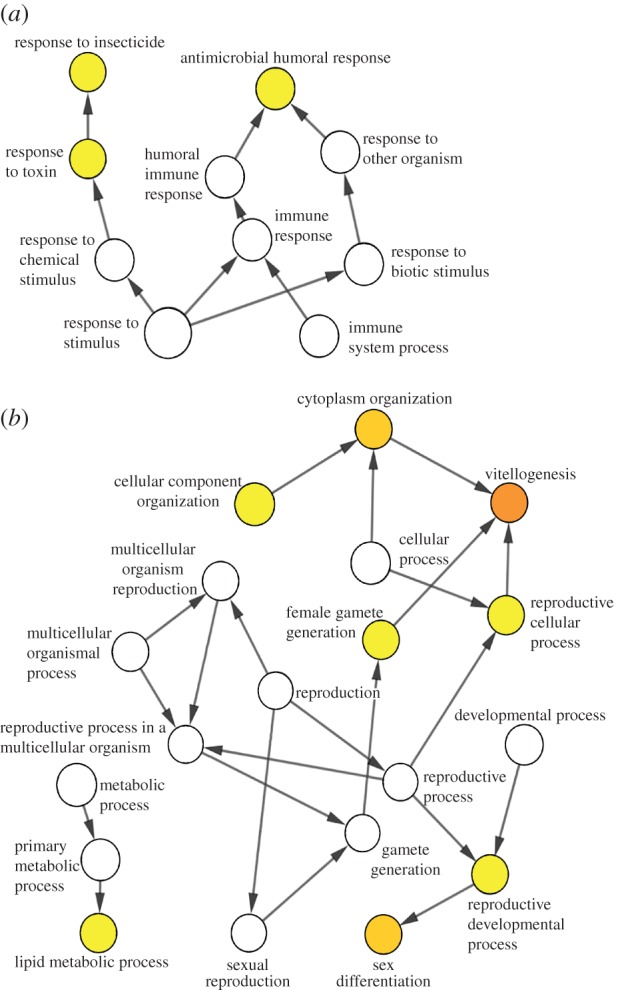
(a) Downregulated gene clusters with GO (gene ontology) terms in sons of fathers on low diet. (b) Upregulated gene clusters with GO terms in sons of fathers on low diet. Nodes with significantly enriched GO terms are shown in colour.
Table 1.
Differentially expressed genes in sons (q ≤ 0.05) at ≥ 2-fold. FPKM, fragments per kilobase of transcript per million reads mapped. If no further information on a gene is available, cells have been left blank.
| gene | fold change | direction (low diet) | high diet (FPKM) | low diet (FPMK) | q-value | description (gene product) | biological function |
|---|---|---|---|---|---|---|---|
| AttD | 10.6 | down | 62.98 | 5.94 | 0.0171 | attacin-D | antimicrobial |
| CG8534 | 6.45 | down | 3.34 | 0.52 | 0.0171 | fatty acid elongation | |
| para | 6.31 | down | 1.54 | 0.24 | 0.0171 | paralytic | courtship song |
| Yp1 | 6.2 | up | 0.73 | 4.55 | 0.0171 | yolk protein 1 | seminal vesicle protein |
| Yp2 | 5.49 | up | 0.88 | 4.84 | 0.0171 | yolk protein 2 | seminal vesicle protein |
| CG11873 | 5 | down | 3.31 | 0.66 | 0.0171 | response to endoplasmic reticulum stress | |
| CG42795 | 3.62 | down | 1.97 | 0.55 | 0.0171 | regulation of GTPase activity | |
| Cpr92F | 2.8 | down | 3.47 | 1.24 | 0.0171 | cuticular protein 92F | chitin-based cuticle development |
| Jeb | 2.73 | down | 1.84 | 0.67 | 0.0171 | jelly belly | various |
| CG9377 | 2.67 | up | 3.03 | 8.09 | 0.0171 | proteolysis | |
| Dp | 2.62 | down | 1.77 | 0.68 | 0.0171 | dumpy | chitin-based embryonic cuticle biosynthetic process |
| CG40472 | 2.55 | up | 10.91 | 27.87 | 0.0171 | mitochondrial respiratory chain complex I | |
| mei-P26 | 2.47 | down | 1.75 | 0.71 | 0.0171 | mei-P26 | gamete generation |
| DopR | 2.31 | down | 0.62 | 0.27 | 0.0171 | dopamine receptor | learning |
| Ace | 2.29 | down | 11.17 | 4.87 | 0.0171 | acetylcholine esterase | catabolic process |
| zfh2 | 2.27 | down | 2.02 | 0.89 | 0.0171 | Zn finger homeodomain 2 | nervous system development |
| Ca-alpha1T | 2.16 | down | 2.26 | 1.04 | 0.03 | Ca2+-channel protein alpha 1 subunit T | calcium ion import |
| CG30069 | 2.13 | down | 6.38 | 3.00 | 0.0171 | ||
| CR40685 | 2.13 | down | 6.81 | 3.20 | 0.03 | ||
| Scrt | 2.11 | down | 2.46 | 1.16 | 0.0171 | scratch | dendrite morphogenesis |
| CR40469 | 2.11 | up | 274.25 | 577.54 | 0.0171 | ||
| Corin | 2.1 | down | 1.23 | 0.59 | 0.0171 | Corin | proteolysis |
| Dp | 2.1 | down | 1.25 | 0.59 | 0.0171 | dumpy | |
| Ac3 | 2.09 | down | 1.58 | 0.75 | 0.0171 | Ac3 | cAMP biosynthetic process |
| CG13185 | 2.08 | down | 1.71 | 0.82 | 0.0171 | cellular response to starvation | |
| Kst | 2.02 | down | 19.76 | 9.78 | 0.0171 | karst | microtubule binding |
| Yp3 | 2 | up | 4.68 | 9.38 | 0.0171 | yolk protein 3 | neurogenesis |
4. Discussion
Sons of fathers reared on LP diet fared worse in sperm competition, with associated downregulation of immune response genes and upregulation of genes involved in metabolism and reproduction. Non-mutually exclusive mechanisms of paternal effects on paternity success include seminal fluid and other ejaculate effects [20] and cryptic female choice [21]. Females may have been able to detect treatment-induced variation in male behaviour and may have allocated more resources into reproduction with descendants of high-diet males. It is well known that high-quality diet positively affects male sexual characters [22], fitness [23] and subsequent female choice [24]. Indeed, the gene paralytic (para) affects courtship song [25] and male olfaction in response to female pheromones [26] and was downregulated in sons of LP fathers. As downregulation of para reduces neuronal excitability [27], it is conceivable that negative fitness effects include lower-quality courtship song and reduced olfaction ability, which are very important factors in female precopulatory choice [28]. However, while higher latency (willingness) to mate and reduced mating duration for males with low-quality courtship song and reduced olfactory ability may be expected, we did not find an effect of paternal diet regime on mating duration, and we did not investigate more detailed behavioural traits to confirm correlational outcomes with the expression of para. Only few studies have so far reported transgenerational effects in relation to diet quality [29,30]. To our knowledge, this is the first study reporting on postcopulatory advantages conferred by parental diet.
Importantly, DE genes confirm the existence of differences between sons of fathers reared on different diets, enabling further investigations of transgenerationally affected sexually selected traits. Antimicrobial peptides (AMPs) are upregulated by D. melanogaster when challenged by Gram-negative bacteria [31,32]. Downregulation of these AMPs in sons of LP fathers in our study might therefore be a form of immunosuppression, which, according to theory, trades off against sexually selected traits [33]. Thus, reproductive fitness of LP sons might have been even lower if immunosuppression had not occurred. Indeed, sexually selected male D. melanogaster that showed higher competitive mating ability had lowered immune function, compared with control males [34].
The two most upregulated genes in sons of low-diet fathers are YP1 and YP2. While the suggested functional annotation, vitellogenesis, is clearly a female-limited function, effects of YP1 and YP2 in male D. melanogaster [35] and the moth Spodoptora littoralis [36] include yolk protein precursors, which directly interact with spermatozoa. YP2 coats the spermatozoa and might provide protection or aid in gamete recognition. However, the functional significance of these proteins has not been established, and we have no knowledge about how upregulation of YP1 and YP2 may influence reproductive fitness in male fruit flies.
The direction of regulation of proteolysis (CG9377), biosynthesis of chitin-based cuticle (Cpr92F and dp) and gamete generation (mei-P26) is consistent with organismal preparation for a suboptimal nutritional environment, investing less and recycling more. Intriguingly, CG9377 has been also found to be upregulated in brains of male D. melanogaster courting females, compared with non-courting males [37], establishing another link of our paternal diet treatment to precopulatory sexual selection (although the direction of the effect seems to promote courtship, rather than reduce it, as discussed above). Valtonen et al. [38] found substantial transgenerational effects of larval diet on development time and adult body size in D. melanogaster, but not on pathogen resistance. The different findings in immune response between [38] and the presented study may be due to the efficiency of the manipulated media. Diet components and protein : carbohydrate ratios are difficult to compare between studies, owing to use of different protein (P) and carbohydrate (C) sources. Crude estimates of P : C ratios and the within-study difference between ratios were much higher in our study (low = 0.4, high = 8; [38] assuming 100 g of sugar/litre diet: low = 0.07, standard = 0.14), illustrating the need to employ a more exact nutritional framework to determine high-resolution reaction norms of traits of interest [39].
Supplementary Material
Acknowledgements
Sarah Josway assisted with data collection. We thank an anonymous reviewer and Juliano Morimoto for helpful comments.
Data accessibility
Data on paternity success are archived in Dryad (http://dx.doi.org/10.5061/dryad.9qs53) [13]; sequencing reads are deposited in NCBI under BioProject number PRJNA360276.
Authors' contributions
F.Z., S.Z. and M.M. designed and performed the experiment; F.Z. and S.Z. analysed data; F.Z., S.Z. and M.M. wrote the manuscript. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We declare no competing interests.
Funding
The research was supported by NSF (DEB-1257859) and GW CCFF awards to M.M., and the Andalucía Talent Hub and the Spanish Severa Ochoa Programme to S.Z.
References
- 1.Crean AJ, Bonduriansky R. 2014. What is a paternal effect? Trends Ecol. Evol 29, 554–559. ( 10.1016/j.tree.2014.07.009) [DOI] [PubMed] [Google Scholar]
- 2.Soubry A, Hoyo C, Jirtle RL, Murphy SK. 2014. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. Bioessays 36, 359–371. ( 10.1002/bies.201300113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Gonzalez F, Dowling DK. 2015. Transgenerational effects of sexual interactions and sexual conflict: non-sires boost the fecundity of females in the following generation. Biol. Lett. 11, 20150067 ( 10.1098/rsbl.2015.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qvarnstrom A, Price TD. 2001. Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 16, 95–100. ( 10.1016/s0169-5347(00)02063-2) [DOI] [PubMed] [Google Scholar]
- 5.Zajitschek S, Hotzy C, Zajitschek F, Immler S. 2014. Short-term variation in sperm competition causes sperm-mediated epigenetic effects on early offspring performance in the zebrafish. Proc. R. Soc. B 281, 20140422 ( 10.1098/rspb.2014.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Öst A, et al. 2014. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 159, 1352–1364. ( 10.1016/j.cell.2014.11.005) [DOI] [PubMed] [Google Scholar]
- 7.Holman L, Kokko H. 2014. The evolution of genomic imprinting: costs, benefits and long-term consequences. Biol. Rev. 89, 568–587. ( 10.1111/brv.12069) [DOI] [PubMed] [Google Scholar]
- 8.Soubry A. 2015. Epigenetic inheritance and evolution: a paternal perspective on dietary influences. Prog. Biophys. Mol. Biol. 118, 79–85. ( 10.1016/j.pbiomolbio.2015.02.008) [DOI] [PubMed] [Google Scholar]
- 9.Ng SF, Lin RCY, Laybutt DR, Barres R, Owens JA, Morris MJ. 2010. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 467, 963–966. ( 10.1038/nature09491) [DOI] [PubMed] [Google Scholar]
- 10.Carone BR, et al. 2010. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096. ( 10.1016/j.cell.2010.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 328, 354–357. ( 10.1126/science.1187096) [DOI] [PubMed] [Google Scholar]
- 12.Matzkin LM, Johnson S, Paight C, Bozinovic G, Markow TA. 2011. Dietary protein and sugar differentially affect development and metabolic pools in ecologically diverse Drosophila. J. Nutr. 141, 1127–1133. ( 10.3945/jn.111.138438) [DOI] [PubMed] [Google Scholar]
- 13.Zajitschek F, Zajitschek S, Manier M. 2017. Data from: High protein paternal diet confers an advantage to sons in sperm competition. Dryad Digital Repository. ( 10.5061/dryad.9qs53) [DOI] [PMC free article] [PubMed]
- 14.R Development Core Team. 2008 R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org.
- 15. NCBI. 2017 Drosophila melanogaster (fruit fly). Dietary transgenerational effects on sperm competition. NCBI BioProject no. PRJNA360276. https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA360276 .
- 16.Hilgert U, McKay S, Khalfan M, Williams J, Ghiban C, Micklos D. 2014. DNA Subway: making genome analysis egalitarian. In Proc. 2014 Annual Conference on Extreme Science and Engineering Discovery Environment. XSEDE ’14, Atlanta, GA, USA70, pp. 1–3. New York, NY: ACM.
- 17.Trapnell C, et al. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578. ( 10.1038/nprot.2012.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300. ( 10.2307/2346101) [DOI] [Google Scholar]
- 19.Smoot ME, Ono K, Ruscheinski J, Wang P-L, Ideker T. 2011. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432. ( 10.1093/bioinformatics/btq675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crean AJ, Adler MI, Bonduriansky R. 2016. Seminal fluid and mate choice: new predictions. Trends Ecol. Evol. 31, 253–255. ( 10.1016/j.tree.2016.02.004) [DOI] [PubMed] [Google Scholar]
- 21.Eberhard WG. 1996. Cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 22.Sentinella AT, Crean AJ, Bonduriansky R. 2013. Dietary protein mediates a trade-off between larval survival and the development of male secondary sexual traits. Funct. Ecol. 27, 1134–1144. ( 10.1111/1365-2435.12104) [DOI] [Google Scholar]
- 23.Maklakov AA, et al. 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1066. ( 10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- 24.Xie J, De Clercq P, Zhang Y, Wu H, Pan C, Pang H. 2015. Nutrition-dependent phenotypes affect sexual selection in a ladybird. Sci. Rep. 5, 13111 ( 10.1038/srep13111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peixoto AA, Hall JC. 1998. Analysis of temperature-sensitive mutants reveals new genes involved in the courtship song of Drosophila. Genetics 148, 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gailey DA, Lacaillade RC, Hall JC. 1986. Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav. Genet. 16, 375–405. ( 10.1007/BF01071319) [DOI] [PubMed] [Google Scholar]
- 27.Stern M, Kreber R, Ganetzky B. 1990. Dosage effects of a Drosophila sodium channel gene on behavior and axonal excitability. Genetics 124, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markow TA. 1987. Behavioural and sensory basis of courtship success in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 84, 6200–6204. ( 10.1073/pnas.84.17.6200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonduriansky R, Head ML. 2007. Maternal and paternal condition effects on offspring phenotype in Telostylinus angusticollis (Diptera: Neriidae). J. Evol. Biol. 20, 2379–2388. ( 10.1111/j.1420-9101.2007.01419.x) [DOI] [PubMed] [Google Scholar]
- 30.Fricke C, Bretman A, Chapman T. 2008. Adult male nutrition and reproductive success in Drosophila melanogaster. Evolution 62, 3170–3177. ( 10.1111/j.1558-5646.2008.00515.x) [DOI] [PubMed] [Google Scholar]
- 31.Castillo JC, Creasy T, Kumari P, Shetty A, Shokal U, Tallon LJ, Eleftherianos I. 2015. Drosophila anti-nematode and antibacterial immune regulators revealed by RNA-Seq. BMC Genomics 16, 519 ( 10.1186/s12864-015-1690-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imler J-L, Bulet P. 2005. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem. Immunol. Allergy 86, 1–21. ( 10.1159/000086648) [DOI] [PubMed] [Google Scholar]
- 33.McKean KA, Nunney L. 2001. Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 7904–7909. ( 10.1073/pnas.131216398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKean KA, Nunney L. 2008. Sexual selection and immune function in Drosophila melanogaster. Evolution 62, 386–400. ( 10.1111/j.1558-5646.2007.00286.x) [DOI] [PubMed] [Google Scholar]
- 35.Majewska MM, Suszczynska A, Kotwica-Rolinska J, Czerwik T, Paterczyk B, Polanska MA, Bernatowicz P, Bebas P. 2014. Yolk proteins in the male reproductive system of the fruit fly Drosophila melanogaster: spatial and temporal patterns of expression. Insect Biochem. Mol. Biol. 47, 23–35. ( 10.1016/j.ibmb.2014.02.001) [DOI] [PubMed] [Google Scholar]
- 36.Bebas P, Kotwica J, Joachimiak E, Giebultowicz JM. 2008. Yolk protein is expressed in the insect testis and interacts with sperm. BMC Dev. Biol. 8, 64 ( 10.1186/1471-213X-8-64) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis LL, Carney GE. 2011. Socially-responsive gene expression in male Drosophila melanogaster is influenced by the sex of the interacting partner. Genetics 187, 157–169. ( 10.1534/genetics.110.122754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valtonen TM, Kangassalo K, Pölkki M, Rantala MJ. 2012. Transgenerational effects of parental larval diet on offspring development time, adult body size and pathogen resistance in Drosophila melanogaster. PLoS ONE 7, e31611 ( 10.1371/journal.pone.0031611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto J, Wigby S. 2016. Differential effects of male nutrient balance on pre- and post-copulatory traits, and consequences for female reproduction in Drosophila melanogaster. Sci. Rep. 6, 27673 ( 10.1038/srep27673) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zajitschek F, Zajitschek S, Manier M. 2017. Data from: High protein paternal diet confers an advantage to sons in sperm competition. Dryad Digital Repository. ( 10.5061/dryad.9qs53) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data on paternity success are archived in Dryad (http://dx.doi.org/10.5061/dryad.9qs53) [13]; sequencing reads are deposited in NCBI under BioProject number PRJNA360276.