Abstract
Differences in the limits and range of aerobic activity levels between endotherms and ectotherms remain poorly understood, though such differences help explain basic differences in species' lifestyles (e.g. movement patterns, feeding modes, and interaction rates). We compare the limits and range of aerobic activity in endotherms (birds and mammals) and ectotherms (fishes, reptiles, and amphibians) by evaluating the body mass-dependence of VO2 max, aerobic scope, and heart mass in a phylogenetic context based on a newly constructed vertebrate supertree. Contrary to previous work, results show no significant differences in the body mass scaling of minimum and maximum oxygen consumption rates with body mass within endotherms or ectotherms. For a given body mass, resting rates and maximum rates were 24-fold and 30-fold lower, respectively, in ectotherms than endotherms. Factorial aerobic scope ranged from five to eight in both groups, with scope in endotherms showing a modest body mass-dependence. Finally, maximum consumption rates and aerobic scope were positively correlated with residual heart mass. Together, these results quantify similarities and differences in the potential for aerobic activity among ectotherms and endotherms from diverse environments. They provide insights into the models and mechanisms that may underlie the body mass-dependence of oxygen consumption.
Keywords: metabolic rate, scaling, respiration, VO2 max, metabolic theory
1. Introduction
The tremendous variation in aerobic activity levels among vertebrates is reflected in their lifestyles, from highly active to sedentary. Differences in aerobic activity underlie basic differences in the ecology and behaviour of species (e.g. movement patterns, feeding modes, interaction rates) [1–4] that may affect survival, growth, and reproduction (i.e. fitness) [5–7]. Such differences also reflect patterns in species' energy expenditure given the fixed relationship between oxygen consumption rate and metabolic rate [3,8]. For these reasons, biologists have long sought to understand variation in aerobic activity levels among vertebrates [9–12].
Attempts to quantify aspects of the aerobic activity or ‘athleticism’ of vertebrates typically rely on one of three related measures: a species' maximum rate of oxygen consumption (i.e. VO2 max), often induced through strenuous exercise, is used as a measure of the upper limit of aerobic activity [13]. Heart mass, too, is thought to reflect this limit, in part because endothermic species that engage in intense bouts of aerobic activity (e.g. hummingbirds and greyhounds) may possess relatively large hearts [14,15]. Finally, factorial aerobic scope, or maximum oxygen consumption divided by minimum consumption, is used to assess the potential range in aerobic activity [16,17].
Each of the three related measures used to characterize aerobic activity levels has been shown to increase nonlinearly with body mass in vertebrates. Both heart mass and VO2 max are thought to scale to the 0.83–0.90 power of body mass and thus more steeply than the 2/3–3/4 power scaling of resting consumption rates [15,18–20]. This is relevant since many have argued that selection has acted to match the design of respiratory systems to maximum rather than minimum rates of consumption, and because maximum rates are thought by some to be more indicative of natural activity levels [18,21]. On the basis of the difference in the scaling of maximum and minimum rate of consumption, aerobic scope is also then expected to scale positively with body mass [18]. This has led some to suggest that minimum and maximum rates of oxygen consumption are not functionally linked [22–24], which contradicts a central tenet of Bennett and Ruben's explanation for the evolution of endothermy (i.e. the aerobic capacity model) [10]. However, these conclusions are often based on research in mammals. VO2 max, aerobic scope, and the relationship of heart mass to aerobic capacity have scarcely been investigated across ectothermic vertebrates [12].
Here, we provide a broad-scale comparison of aerobic activity levels in endotherms (birds, mammals) and ectotherms (fishes, amphibians, reptiles). We assess the body mass-dependence of VO2 max, aerobic scope, and heart mass for each group in a phylogenetic context, and after accounting for any effects of temperature. A comparison of the body mass-dependence of these three measures in these two groups provides a step towards a more synthetic understanding of aerobic activity across vertebrates.
2. Material and methods
(a). Data
Published data were compiled for each analysis with an emphasis on broadly representing the taxonomic diversity, body size range, and range in aerobic capacity/athleticism found in each class of vertebrates. Species included here span all major biomes (marine, freshwater, and terrestrial) and show a diverse range of life histories. Data collection was restricted to subadult or adult individuals (electronic supplementary material, Appendices S1–S3).
(i). Oxygen consumption rates
Resting oxygen consumption rates of endotherms were obtained primarily from two recent compilations of data (e.g. [25,26]), which were then supplemented with additional data to provide a more complete taxonomic representation. Resting oxygen consumption rates of ectotherms were taken from the compilation of [26] and electronic supplementary material, Appendix S3. For each species listed in this latter dataset, only the largest individual(s) at the highest constant temperature was included to facilitate phylogenetic analyses. Maximum oxygen consumption rates of endotherms were obtained from a large number of sources, including [20,27] for endotherms and [28,29] for ectotherms. For birds, oxygen consumption rates during flight were treated as maximum rates because, even at optimally efficient flight speeds, oxygen consumption during flight represents 60–85% of VO2 max [30]. For all endotherms, efforts were made to exclude data collected in cold rooms at temperatures well below ambient temperatures because maximum rates are increased under these conditions [31].
(ii). Heart mass
Heart mass measures were typically estimated in the original studies by simply excising the heart and weighing it. Additionally, for a small number of species, heart mass was estimated from measures of ventricle mass by assuming that ventricle mass constitutes 70% of total heart mass [32,33].
(iii). Body mass and temperature estimates
Body mass values for all analyses were taken from the original study when available, and if not, estimates of adult mass from other published sources were used. For endotherms, resting body temperatures were used in the analysis of oxygen consumption rates and assumed to be equivalent to body temperatures during activity. When estimates for individual species were not available, body temperatures were assumed for birds or mammals based on the average temperature reported for these groups (birds: 41.5; mammals: 36.5°C) [34]. For ectotherms, the constant temperatures at which oxygen consumption rates were measured by the original authors were used in analyses. Body temperatures of ectotherms and endotherms were then used to standardize all oxygen consumption rates to 38°C for endotherms and to 25°C for ectotherms by assuming a Q10 of 2 [26].
(b). Statistical analyses
Both ordinary least-squares (OLS) and phylogenetic generalized least-squares (PGLS; [35]) regression were performed using R v. 3.0 [36]. Analyses of the residuals of these regression models are provided in the electronic supplementary material. To perform PGLS, a phylogenetic supertree was constructed using the ‘matrix representation using parsimony’ (MRP) approach [37,38], and the Baum–Ragan coding procedure [39]. The tree was constructed based on recent phylogenies from each vertebrate class (fishes [40], mammals [41], amphibians [42], birds [43], and reptiles [44–46]; see also [47]). To do so, trees were converted to a common format using Mesquite v. 2.75 [48], and the final MRP matrix was performed in Tree Analysis Using New Technology (TNT) using the defaults for ‘traditional search’ [49]. These methods of supertree construction are widely used and effective [41].
For PGLS, polytomies were treated as soft [50], and branch lengths were assumed to be equal given the uncertainty in divergence time estimates among vertebrates [51]. A Brownian motion model of trait evolution [52,53] was used to estimate the expected covariance between species with the APE package for R [54]. Because a significant number of species in each analysis were not present in the phylogeny, results from both OLS and PGLS are presented in tables 1–3.
Table 1.
Statistics describing the relationships between resting and maximum oxygen consumption rates (ml O2 h−1) with body mass (g) in endothermic (birds, mammals) and ectothermic (fishes, amphibians, reptiles) vertebrates. Rates for endotherms are normalized to 38°C, and those for ectotherms to 25°C, assuming a Q10 of 2. Models were fitted using both ordinary least-squares (OLS) and phylogenetic generalized least-squares (PGLS) regression (i.e. ‘Phy’), where ‘σ2’ represents the variance in Brownian motion (see the electronic supplementary material for residual analyses).
| group | n | σ2 | intercept | scaling exponent | R2 |
|---|---|---|---|---|---|
| resting consumption rate versus body mass | |||||
| endotherms | 617 | n.a. | 1.47 (1.4, 1.53)** | 0.70 (0.69, 0.72)** | 0.96 |
| endotherms (Phy) | 591 | 0.01 | 1.21 (0.68, 1.75)** | 0.74 (0.72, 0.77)** | 0.97 |
| ectotherms | 386 | n.a. | −2.05 (−2.17, −1.93)** | 0.85 (0.83, 0.88)** | 0.91 |
| ectotherms (Phy) | 249 | 0.07 | −1.95 (−3.3, −0.61)** | 0.80 (0.74, 0.86)** | 0.88 |
| maximum consumption rate versus body mass | |||||
| endotherms | 155 | n.a. | 3.16 (3.02, 3.3)** | 0.83 (0.8, 0.85)** | 0.97 |
| endotherms (Phy) | 152 | 0.006 | 3.27 (2.78, 3.77)** | 0.82 (0.77, 0.86)** | 0.96 |
| ectotherms | 160 | n.a. | −0.31 (−0.53, −0.1)* | 0.91 (0.86, 0.96)** | 0.89 |
| ectotherms (Phy) | 119 | 0.024 | −0.03 (−0.88, 0.82) | 0.78 (0.71, 0.85)** | 0.80 |
**p < 0.001, *p < 0.02.
Table 2.
Statistical models describing the relationship between heart mass (g) and body mass (g) in endothermic (birds, mammals) and ectothermic (fishes, amphibians, and reptiles) vertebrates. Models were fitted using both OLS and PGLS regression (i.e. ‘Phy’), where ‘σ2’ represents the variance in Brownian motion. Slopes and intercepts show a significance level of p < 0.001 in all cases (see electronic supplementary material for residual analyses).
| group | n | σ2 | intercept | scaling exponent | R2 |
|---|---|---|---|---|---|
| endotherms | 96 | n.a. | −4.23 (−4.38, −4.07) | 0.9 (0.88, 0.93) | 0.99 |
| endotherms (Phy) | 95 | 0.003 | −4.39 (−4.74, −4.05) | 0.94 (0.91, 0.96) | 0.99 |
| ectotherms | 74 | n.a. | −5.62 (−5.96, −5.28) | 0.91 (0.86, 0.97) | 0.93 |
| ectotherms (Phy) | 57 | 0.011 | −5.76 (−6.43, −5.09) | 0.93 (0.87, 1) | 0.93 |
Table 3.
Statistics describing the relationships shown in figure 3a,b. Models were fitted using both OLS and PGLS regression (i.e. ‘Phy’), where ‘σ2’ represents the variance in Brownian motion.
| group | n | σ2 | intercept | scaling exponent | R2 |
|---|---|---|---|---|---|
| resting consumption rate versus residuals (figure 3a) | |||||
| OLS | 25 | 4.5 (3.48, 5.53)** | 1.52 (0.36, 2.68)* | 0.21 | |
| PGLS | 24 | 0.08 | 4.29 (2.81, 5.77)** | 0.38 (−1, 1.77) | 0.02 |
| maximum consumption rate versus residuals (figure 3a) | |||||
| OLS | 25 | 6.92 (5.89, 7.95)** | 2.38 (1.22, 3.55)** | 0.42 | |
| PGLS | 24 | 0.08 | 6.61 (5.15, 8.07)** | 1.31 (−0.06, 2.68) | 0.17 |
| aerobic scope versus residuals (figure 3b) | |||||
| OLS | 25 | 2.4 (2.2, 2.61)** | 0.85 (0.61, 1.08)** | 0.70 | |
| PGLS | 24 | 0.007 | 2.31 (1.9, 2.73)** | 0.92 (0.54, 1.31)** | 0.75 |
**p < 0.001, *p < 0.02.
3. Results
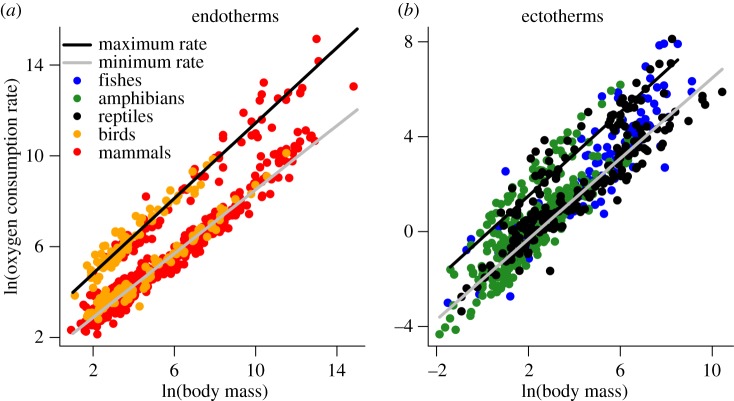
The body mass scaling of maximum oxygen consumption rates (i.e. VO2 max) in ectotherms was statistically indistinguishable from that of endotherms (endotherms: 3.27M0.82; ectotherms: −0.03M0.78) based on the 95% CI of the scaling exponents from PGLS regression analyses (endotherms: n = 152, CI: 0.77–0.86; ectotherms: n = 119, CI: 0.71–0.85; figure 1a,b). In the case of ectotherms, the confidence intervals include the often cited value of 3/4. Moreover, within both endotherms and ectotherms, the body mass scaling of VO2 max was statistically indistinguishable from that of resting rates based on the 95% CI—albeit just barely so in the case of endotherms (table 1 and figure 1a,b). Yet, the intercepts of these relationships show that maximum rates for ectotherms were on average about 30-fold lower than those of endotherms. Only a small fraction of this difference (i.e. approx. 2.5-fold) can be attributed to differences in temperature between groups (38°C versus 25°C) assuming a Q10 of 2. The relationship for ectotherms was also more variable than that of endotherms (R2: 0.80 for ectotherms versus 0.96 for endotherms).
Figure 1.
(a,b) Relationships of maximum and resting oxygen consumption rates to body mass (g) in (a) endotherms (birds and mammals) and (b) ectotherms (fishes, amphibians, and reptiles) based on OLS regression analyses (see table 1 for statistics, including results of PGLS regression analyses). Oxygen consumption rates (ml O2 h−1) are normalized to 38°C in endotherms, and 25°C in ectotherms by assuming a Q10 of 2.
The body mass scaling of resting oxygen consumption rates in ectotherms was also statistically indistinguishable from that of endotherms (endotherms, 1.21M0.74; ectotherms, −1.95M0.80). And here, in both cases, the 95% CI included the value of 3/4, though not 2/3 (95% CI: endotherms, 0.72–0.77; ectotherms, 0.74–0.86; figure 1a,b). On average, for a given body mass, resting rates were about 24-fold lower based on the intercepts of the PGLS regressions (n = 591 for endotherms; n = 249 for ectotherms; table 1). In both cases, the fitted models explained a substantial portion of the variation (R2: 0.88 for ectotherms; 0.97 for endotherms).
The relationships of minimum and maximum oxygen consumption rates to body mass yield estimates for the average aerobic scope of endotherms and ectotherms. The difference in the fitted lines of these relationships for ectotherms gives a factorial scope of 6.82 (PGLS; CI: 4.18, 11.25), with a slight but significant body mass-dependence (M−0.02; CI: −0.03, −0.01). For endotherms, the difference between lines gives a factorial scope of 7.84 (CI: 7.54, 8.2). However, unlike in ectotherms, scope in endotherms increased modestly with body mass (i.e. M0.08; CI: 0.05, 0.09). Results from OLS regression, which included significantly more species, yielded somewhat different estimates for aerobic scope in both groups. Factorial scope based on these analyses was more similar between groups ((5.41; CI: 5.05, 5.87) for endotherms versus 5.69 (CI: 5.15, 6.23) for ectotherms), and scope in both groups showed a positive relationship with body mass (M0.13 for endotherms (CI: 0.11, 0.13) versus M0.06 for ectotherms (CI: 0.03, 0.08)).
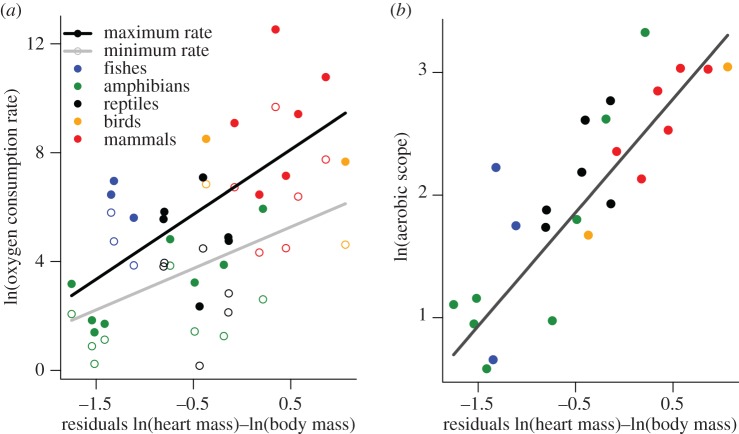
The body mass scaling of heart mass differed from minimum and maximum oxygen consumption rates in endotherms and ectotherms based on PGLS analyses (figure 2 and table 2). In both cases, heart mass scaled roughly linearly with body mass (endotherms: 0.94, 95% CI: 0.91–0.96; ectotherms: 0.95, 95% CI: 0.88–1.01). Moreover, the 4.2-fold difference in intercepts between these relationships for ectotherms and endotherms was small compared with the 20–30-fold difference for that of oxygen consumption rates between groups (figures 1a,b and 2). Still, maximum oxygen consumption rates (but not minimum rates) were positively correlated with the residuals of the heart mass–body mass relationships shown in figure 2 (figure 3a and table 3). Consequently, a strong positive relationship was observed between factorial aerobic scope and relative heart mass across all vertebrates (figure 3b and table 3).
Figure 2.
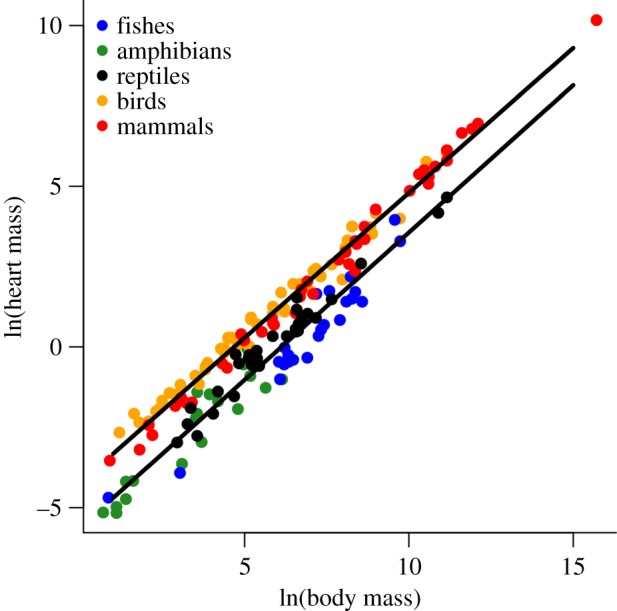
Relationship between heart mass (g) and body mass (g) in endotherms (birds and mammals) and ectotherms (reptiles, amphibians, and fishes) based on OLS regression analyses (see table 2 for statistics, including results of PGLS regression analyses).
Figure 3.
(a) Relationship between minimum and maximum oxygen consumption rates and residual heart mass in vertebrates. Solid points represent maximum rates, and hollow points represent minimum rates. (b) Relationship between aerobic scope and residual heart mass in vertebrates. Residual heart mass values for endotherms and ectotherms were determined based on the relationships shown in figure 2. The fitted lines represent the results of our OLS regression models, but statistics for PGLS models are also presented (table 3).
4. Discussion
Results show that the scaling of VO2 max with body mass (i.e. the slopes) was statistically indistinguishable for endotherms and ectotherms. For ectotherms, maximum rates were also statistically indistinguishable from the often cited value scaling exponent of 3/4 for oxygen consumption rates [3,25,55]. Similarly, the body mass scaling of minimum consumption rates did not differ significantly between endotherms and ectotherms. Previous work has concluded that the scaling of minimum oxygen consumption rate with body mass is significantly steeper in ectotherms than in endotherms [26,56]. This discrepancy is perhaps attributed to (i) our taking into account phylogenetic relatedness and (ii) our limiting of analyses to subadult or adult individuals. Note, too, that the scaling of minimum rates in both groups differed significantly from the value of 2/3 observed in some analyses of particular taxonomic groups [57], but not the 3/4 value observed by others [55]. The debate on the exponent of these relationships has not been previously evaluated for endotherms (birds and mammals) and ectotherms (fishes, amphibians, and reptiles) in a phylogenetic context. Previous work has focused largely on individual classes of vertebrates, particularly mammals [12,15,18–20,28,29]. Our results provide some support for models that argue for a value of 3/4 (e.g. [58]), but results were mixed (table 1). Our results provide little or no support for a 2/3 scaling of oxygen consumption based on other hypotheses [59].
For aerobic scope, results show that ectotherms and endotherms are similar. In both groups, scope varied by fivefold to eightfold depending on analyses. Aerobic scope was nearly invariant with respect to body mass in ectotherms based on PGLS analyses, though not in endotherms (figure 1a,b and table 1). This reflects the observation that the body mass scaling of maximum oxygen consumption rates was not significantly different from minimum rates in ectotherms (0.78 versus 0.80, table 1) and that the scaling of maximum rates was modestly higher than minimum rates in endotherms (0.74 for minimum versus 0.82 for maximum; table 1). Previous work in mammals has shown that the majority of this difference may be explained by larger species having higher core body temperatures during strenuous exercise [60]—an observation that we could not account for in our analyses. If temperature were accounted for in this way, both ectotherms and endotherms may show no difference in the body mass scaling of minimum and maximum consumption rates. This observation raises questions about the argument that vertebrate respiratory systems have evolved to optimize performance at maximum rather than minimum rates of oxygen consumption [18]. It also raises questions about the argument that maximum and minimum rates of oxygen consumption are decoupled and controlled by different processes [22–24]. Both arguments require that maximum rates scale significantly more steeply with body mass than minimum rates. Our results suggest otherwise at least for ectotherms, in agreement with Bennett & Ruben [10]. Note that even a modest scaling of aerobic scope with body mass would imply that animals, the size of elephants or whales, would have aerobic scopes well beyond those of any species measured to date. This seems unlikely. Previous work comparing the scaling of minimum and maximum rates of cold-induced oxygen consumption found no difference in the scaling of these two rates in both mammals and birds [17]. Previous work has also suggested that observed differences between minimum and maximum rates of oxygen consumption in vertebrates can be explained by dynamical changes to respiratory systems, namely the partial pressure gradient of oxygen [47].
However, our analyses do not account for the many factors aside from body mass or temperature that may affect aerobic respiration (e.g. climate, resource availability, behavioural thermoregulation, and body shape) [61–65]. As such, the distribution of points shown in figures 1–3 could reasonably be viewed as constraint envelopes. The location of an individual species within these envelopes may point to species-specific differences in aerobic activity that arise due to differences in life history, habitat, or climate. For example, we found a strong, positive association between aerobic scope and residual heart mass (figure 3a,b and table 3). These results show that species with relatively large hearts for a given body mass, species such as hummingbirds and greyhound dogs, reach higher levels of aerobic respiration and exhibit broader aerobic scope. Heart mass itself though was found to scale almost linearly with body mass (unlike VO2 max) and thus similar to many other major organs (e.g. liver; [66], figure 2). Thus, the scaling of heart mass does not appear to be a metric or indicator of the scaling of VO2 max, contrary to previous reports [14,15].
Together, these results reveal both similarities and differences in the limits and range of aerobic capacity for endotherms and ectotherms from diverse environments (i.e. terrestrial, aquatic). In endotherms, the scaling of minimum and maximum rates of oxygen consumption was more similar to each other than that has previously been reported for birds or mammals [20,27]. In ectotherms, we found no significant differences in the scaling of these two rates (table 1). In comparing between the two groups, both the scaling of oxygen consumption rates and aerobic scope were remarkably similar—though differences did exist (table 1 and figure 1a,b). Yet, the absolute differences in minimum and maximum rates were substantial between endotherms and ectotherms, based on the observed differences in intercepts (i.e. 20–30-fold).
To better understand these similarities and differences in aerobic function between endotherms and ectotherms requires a better comparative understanding of respiratory system structure. In some cases, structural differences between endotherms and ectotherms appear to correspond well to observed differences in function. For example, differences in the intercepts of the relationships between oxygen consumption and body mass shown in figure 1a,b correspond to differences in the area and thickness of respiratory surface areas governing passive oxygen diffusion [47]. In other cases, the relationship between structure and function is less clear. For example, aerobic scope is quite similar between the two groups despite many basic structural differences (e.g. mitochondrial density) [1,66–68]. Furthermore, we show here that heart mass is not directly proportional to either minimum or maximum rates of oxygen consumption across vertebrates (table 2). Thus, our hope is that these results will provide impetus to revisit models and mechanisms that may underlie similarities and differences in aerobic capacity among vertebrates.
Supplementary Material
Acknowledgements
We thank the NETI working group for valuable feedback at different stages of this project. We also thank A. Clarke for graciously providing us with his dataset on endotherm body temperatures.
Data accessibility
All data used in this manuscript are provided as the electronic supplementary material.
Authors' contributions
J.F.G. designed the study, gathered the data, contributed to analysis of the data, and wrote the manuscript. J.P.G. analysed the data, constructed figures and tables, and edited the manuscript. E.M. constructed the phylogenetic tree used in analyses and provided assistance in other analyses. All authors approved of the submission of the final manuscript.
Competing interests
We have no competing interests.
Funding
This work was not supported by funding.
References
- 1.Bennett AF. 1980. The metabolic foundations of vertebrate behavior. Bioscience 30, 452–456. ( 10.2307/1307946) [DOI] [Google Scholar]
- 2.Hein AM, Hou C, Gillooly JF. 2012. Energetic and biomechanical constraints on animal migration distance. Ecol. Lett. 15, 104–110. ( 10.1111/j.1461-0248.2011.01714.x) [DOI] [PubMed] [Google Scholar]
- 3.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Towards a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 4.Auer SK, Salin K, Anderson GJ, Metcalfe NB. 2015. Aerobic scope explains individual variation in feeding capacity. Biol. Lett. 11, 20150793 ( 10.1098/rsbl.2015.0793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold SJ. 1983. Morphology, performance, and fitness. Am. Zool. 23, 347–361. ( 10.1093/icb/23.2.347) [DOI] [Google Scholar]
- 6.Kingsolver J, Huey RB. 2008. Size, temperature, and fitness: three rules. Evol. Ecol. Res. 10, 251–268. [Google Scholar]
- 7.Angilletta MJJ, Huey RB, Frazier MR. 2010. Thermodynamic effects on organismal performance: is hotter better? Physiol. Biochem. Zool. 83, 197–206. ( 10.1086/648567) [DOI] [PubMed] [Google Scholar]
- 8.Gillooly JF, Brown JH, West GB, Savage VM, Brown JH. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251. ( 10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 9.Norin T, Clark T. 2016. Measurement and relevance of maximum metabolic rate in fishes. J. Fish Biol. 88, 122–151. ( 10.1111/jfb.12796) [DOI] [PubMed] [Google Scholar]
- 10.Bennett AF, Ruben JA. 1979. Endothermy and activity in vertebrates. Science 206, 649–654. ( 10.1126/science.493968) [DOI] [PubMed] [Google Scholar]
- 11.Bennett AF. 1978. Activity metabolism of the lower vertebrates. Ann. Rev. Physiol. 40, 447–469. ( 10.1146/annurev.ph.40.030178.002311) [DOI] [PubMed] [Google Scholar]
- 12.Hillman SS, Hancock TV, Hedrick MS. 2013. A comparative meta-analysis of maximal aerobic metabolism of vertebrates: implications for respiratory and cardiovascular limits to gas exchange. J. Comp. Physiol. B 183, 167–179. ( 10.1007/s00360-012-0688-1) [DOI] [PubMed] [Google Scholar]
- 13.Seeherman HJ, Taylor CR, Maloiy GM, Armstrong RB. 1981. Design of the mammalian respiratory system. II. Measuring maximum aerobic capacity. Respir. Physiol. 44, 11–23. ( 10.1016/0034-5687(81)90074-8) [DOI] [PubMed] [Google Scholar]
- 14.Bishop CM. 1997. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Phil. Trans. R. Soc. Lond. B 352, 447–456. ( 10.1098/rstb.1997.0032) [DOI] [Google Scholar]
- 15.Bishop CM. 1999. The maximum oxygen consumption and aerobic scope of birds and mammals: getting to the heart of the matter. Proc. R. Soc. Lond. B 266, 2275–2281. ( 10.1098/rspb.1999.0919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priede IG. 1985. Metabolic scope in fishes. In Fish energetics (eds Tytler P, Calow P), pp. 33–64. Berlin, Germany: Springer. [Google Scholar]
- 17.Hinds DS, Baudinette R, Macmillen RE, Halpern EA. 1993. Maximum metabolism and the aerobic factorial scope of endotherms. J. Exp. Biol. 182, 41–56. [DOI] [PubMed] [Google Scholar]
- 18.Weibel ER, Hoppeler H. 2005. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J. Exp. Biol. 208, 1635–1644. ( 10.1242/jeb.01548) [DOI] [PubMed] [Google Scholar]
- 19.Withers P, Hillman S. 2001. Allometric and ecological relationships of ventricle and liver mass in anuran amphibians. Funct. Ecol. 15, 60–69. ( 10.1046/j.1365-2435.2001.00495.x) [DOI] [Google Scholar]
- 20.Dlugosz EM, et al. 2013. Phylogenetic analysis of mammalian maximal oxygen consumption during exercise. J. Exp. Biol. 216, 4712–4721. ( 10.1242/jeb.088914) [DOI] [PubMed] [Google Scholar]
- 21.Hayes JP, Garland T Jr. 1995. The evolution of endothermy: testing the aerobic capacity model. Evolution 49, 836–847. ( 10.2307/2410407) [DOI] [PubMed] [Google Scholar]
- 22.Taigen TL. 1983. Activity metabolism of anuran amphibians: implications for the origin of endothermy. Am. Nat. 121, 94–109. ( 10.1086/284041) [DOI] [PubMed] [Google Scholar]
- 23.Koteja P. 1987. On the relation between basal and maximum metabolic rate in mammals. Comp. Biochem. Physiol. A Comp. Physiol. 87, 205–208. ( 10.1016/0300-9629(87)90447-6) [DOI] [PubMed] [Google Scholar]
- 24.Pough FH, Andrews RM. 1984. Individual and sibling-group variation in metabolism of lizards: the aerobic capacity model for the origin of endothermy. Comp. Biochem. Physiol. A Comp. Physiol. 79, 415–419. ( 10.1016/0300-9629(84)90537-1) [DOI] [Google Scholar]
- 25.Savage VM, et al. 2004. The predominance of quarter power scaling in biology. Funct. Ecol. 18, 257–282. ( 10.1111/j.0269-8463.2004.00856.x) [DOI] [Google Scholar]
- 26.White C, Phillips N, Seymour R. 2006. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2, 125–127. ( 10.1098/rsbl.2005.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiersma P, Muñoz-Garcia A, Walker A, Williams JB. 2007. Tropical birds have a slow pace of life. Proc. Natl Acad. Sci. USA 104, 9340–9345. ( 10.1073/pnas.0702212104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatten REJ, Miller K, Full R. 1992. Energetics at rest and during locomotion. In Environmental physiology of the amphibians (eds Feder ME, Burggren WW), pp. 314–377. Chicago, IL: University of Chicago Press. [Google Scholar]
- 29.Bennett AF. 1982. The energetics of reptilian activity. In Biology of the Reptilia, vol. 13 (eds Gans C, Dawson WR), pp. 155–199. New York, NY: Academic Press. [Google Scholar]
- 30.McWilliams SR, Guglielmo C, Pierce B, Klaassen M. 2004. Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J. Avian Biol. 35, 377–393. ( 10.1111/j.0908-8857.2004.03378.x) [DOI] [Google Scholar]
- 31.Hayes JP, Chappell MA. 1986. Effects of cold acclimation on maximum oxygen consumption during cold exposure and treadmill exercise in deer mice, Peromyscus maniculatus. Physiol. Zool. 59, 473–481. ( 10.1086/physzool.59.4.30158600) [DOI] [Google Scholar]
- 32.Brill RW, Bushnell PG. 1991. Metabolic and cardiac scope of high energy demand teleosts, the tunas. Can. J. Zool. 69, 2002–2009. ( 10.1139/z91-279) [DOI] [Google Scholar]
- 33.Seymour RS. 2009. Raising the sauropod neck: it costs more to get less. Biol. Lett. 5, 317–319. ( 10.1098/rsbl.2009.0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke A, Rothery P. 2008. Scaling of body temperature in mammals and birds. Funct. Ecol. 22, 58–67. [Google Scholar]
- 35.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. Lond. B 326, 119–157. ( 10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 36.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing. [Google Scholar]
- 37.Baum BR. 1992. Combining trees as a way of combining data sets for phylogenetic inference, and the desirability of combining gene trees. Taxon 41, 3–10. ( 10.2307/1222480) [DOI] [Google Scholar]
- 38.Ragan MA. 1992. Phylogenetic inference based on matrix representation of trees. Mol. Phylogenet. Evol. 1, 53–58. ( 10.1016/1055-7903(92)90035-F) [DOI] [PubMed] [Google Scholar]
- 39.Creevey CJ, McInerney JO. 2009. Trees from trees: construction of phylogenetic supertrees using clann. Methods Mol. Biol. 537, 139–161. ( 10.1007/978-1-59745-251-9_7) [DOI] [PubMed] [Google Scholar]
- 40.Betancur RR, et al. 2013. The tree of life and a new classification of bony fishes. PLoS Curr. 5, ii. ( 10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bininda-Emonds OR. 2014. An introduction to supertree construction (and partitioned phylogenetic analyses) with a view toward the distinction between gene trees and species trees. In Modern phylogenetic comparative methods and their application in evolutionary biology (ed. Garamszegi LZ.), pp. 49–76. Berlin, Germany: Springer. [Google Scholar]
- 42.Isaac NJ, Redding DW, Meredith HM, Safi K. 2012. Phylogenetically-informed priorities for amphibian conservation. PLoS ONE 7, e43912 ( 10.1371/journal.pone.0043912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jetz W, Thomas G, Joy J, Hartmann K, Mooers A. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 44.Bergmann PJ, Irschick DJ. 2012. Vertebral evolution and the diversification of squamate reptiles. Evolution 66, 1044–1058. ( 10.1111/j.1558-5646.2011.01491.x) [DOI] [PubMed] [Google Scholar]
- 45.Pyron RA, et al. 2013. Genus-level phylogeny of snakes reveals the origins of species richness in Sri Lanka. Mol. Phylogenet. Evol. 66, 969–978. ( 10.1016/j.ympev.2012.12.004) [DOI] [PubMed] [Google Scholar]
- 46.Jaffe AL, Slater GJ, Alfaro ME. 2011. The evolution of island gigantism and body size variation in tortoises and turtles. Biol. Lett. 7, 558–561. ( 10.1098/rsbl.2010.1084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillooly JF, Gomez JP, Mavrodiev EV, Rong Y, McLamore ES. 2016. Body mass scaling of passive oxygen diffusion in endotherms and ectotherms. Proc. Natl Acad. Sci. USA 113, 5340–5345. ( 10.1073/pnas.1519617113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maddison W, Maddison D. 2015. Mesquite: a modular system for evolutionary analysis. Version 2.75. See http://mesquiteproject org.
- 49.Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786. ( 10.1111/j.1096-0031.2008.00217.x) [DOI] [Google Scholar]
- 50.Purvis A, Garland T Jr. 1993. Polytomies in comparative analyses of continuous characters. Syst. Biol. 42, 569–575. ( 10.1093/sysbio/42.4.569) [DOI] [Google Scholar]
- 51.Tamura K, et al. 2012. Estimating divergence times in large molecular phylogenies. Proc. Natl Acad. Sci. USA 109, 19 333–19 338. ( 10.1073/pnas.1213199109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 53.Martins EP, Hansen TF. 1997. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667. ( 10.1086/286013) [DOI] [Google Scholar]
- 54.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 55.Farrell-Gray CC, Gotelli NJ. 2005. Allometric exponents support a 3/4-power scaling law. Ecology 86, 2083–2087. ( 10.1890/04-1618) [DOI] [Google Scholar]
- 56.White CR, Cassey P, Blackburn TM. 2007. Allometric exponents do not support a universal metabolic allometry. Ecology 88, 315–323. ( 10.1890/05-1883) [DOI] [PubMed] [Google Scholar]
- 57.White CR, Seymour RS. 2003. Mammalian basal metabolic rate is proportional to body mass 2/3. Proc. Natl Acad. Sci. USA 100, 4046–4049. ( 10.1073/pnas.0436428100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West GB, Brown JH, Enquist BJ. 1997. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126. ( 10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]
- 59.Glazier DS. 2005. Beyond the ‘3/4-power law’: variation in the intra-and interspecific scaling of metabolic rate in animals. Biol. Rev. 80, 611–662. ( 10.1017/S1464793105006834) [DOI] [PubMed] [Google Scholar]
- 60.Gillooly JF, Allen AP. 2007. Changes in body temperature influence the scaling of and aerobic scope in mammals. Biol. Lett. 3, 100–103. ( 10.1098/rsbl.2006.0576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lovegrove BG. 2000. The zoogeography of mammalian basal metabolic rate. Am. Nat. 156, 201–219. ( 10.1086/303383) [DOI] [PubMed] [Google Scholar]
- 62.Dillon ME, Wang G, Huey RB. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706. ( 10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 63.Nagy KA. 2005. Field metabolic rate and body size. J. Exp. Biol. 208, 1621–1625. ( 10.1242/jeb.01553) [DOI] [PubMed] [Google Scholar]
- 64.Hirst AG, Glazier DS, Atkinson D. 2014. Body shape shifting during growth permits tests that distinguish between competing geometric theories of metabolic scaling. Ecol. Lett. 17, 1274–1281. ( 10.1111/ele.12334) [DOI] [PubMed] [Google Scholar]
- 65.Glazier DS, Hirst AG, Atkinson D. 2015. Shape shifting predicts ontogenetic changes in metabolic scaling in diverse aquatic invertebrates. Proc. R. Soc. B 282, 20142302 ( 10.1098/rspb.2014.2302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters RH. 1983. The ecological implications of body size. New York, NY: Cambridge University Press. [Google Scholar]
- 67.Suarez RK, Darveau C-A, Childress JJ. 2004. Metabolic scaling: a many-splendoured thing. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 139, 531–541. ( 10.1016/j.cbpc.2004.05.001) [DOI] [PubMed] [Google Scholar]
- 68.Else P, Hulbert A. 1985. An allometric comparison of the mitochondria of mammalian and reptilian tissues: the implications for the evolution of endothermy. J. Comp. Physiol. B 156, 3–11. ( 10.1007/BF00692920) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this manuscript are provided as the electronic supplementary material.