Abstract
Colour patterns (e.g. irregular, spotted or barred forms) are widespread in the animal kingdom, yet their potential role as signals of quality has been mostly neglected. However, a review of the published literature reveals that pattern itself (irrespective of its size or colour intensity) is a promising signal of individual quality across species of many different taxa. We propose at least four main pathways whereby patterns may reliably reflect individual quality: (i) as conventional signals of status, (ii) as indices of developmental homeostasis, (iii) by amplifying cues of somatic integrity and (iv) by amplifying individual investment in maintenance activities. Methodological constraints have traditionally hampered research on the signalling potential of colour patterns. To overcome this, we report a series of tools (e.g. colour adjacency and pattern regularity analyses, Fourier and granularity approaches, fractal geometry, geometric morphometrics) that allow objective quantification of pattern variability. We discuss how information provided by these methods should consider the visual system of the model species and behavioural responses to pattern metrics, in order to allow biologically meaningful conclusions. Finally, we propose future challenges in this research area that will require a multidisciplinary approach, bringing together inputs from genetics, physiology, behavioural ecology and evolutionary-developmental biology.
Keywords: amplifier, badge of status, coloration, developmental stability, honest signalling, melanin
1. Introduction
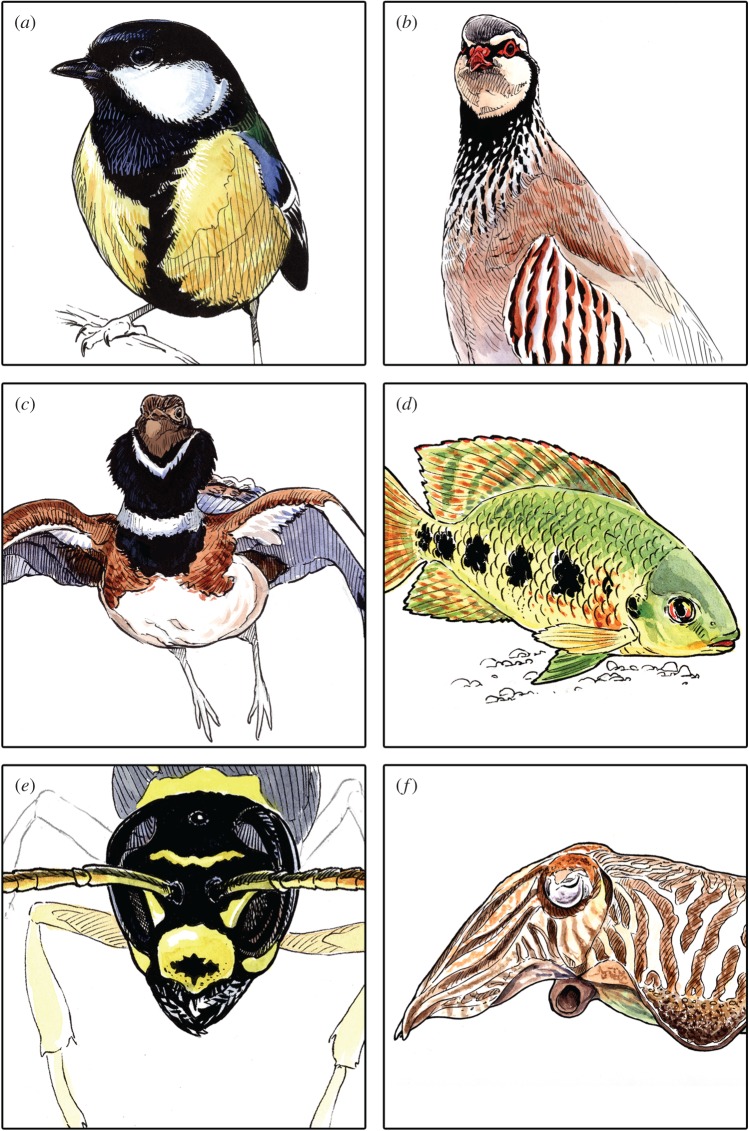
Animal coloration is widely involved in mate choice, intra-sexual competition, dominance relationships and other social interactions, playing a central role in quality signalling [1]. Most research on colour-based signals of quality has focused on pigment-based traits (especially carotenoids, but also melanins). Under the assumption that pigment bioavailability is the main constraint in colour expression, most emphasis has been placed on the acquisition, metabolism and allocation trade-offs of each pigment or its precursors [1]. Derived from this marked interest in ‘quantity-dependent’ colour expression, most studies have focused on measuring the size or colour intensity/hue of colour patches as proxies of individual quality, irrespective of their production mechanism. However, coloured patches often vary among conspecifics in the shape, distribution and connectivity of their constituent units (e.g. spots, stripes and other heterogeneous markings; figure 1). That is, the actual two- or three-dimensional pattern of a colour trait can be highly variable among individuals. Such variability in patterning is largely independent of the area or colour intensity of the patch, and may therefore be subject to other functional constraints, allowing alternative—but not mutually exclusive—signalling pathways and reliability mechanisms for visual traits. Understanding the quality-signalling potential of visual patterns requires a conceptual and empirical change to our approach to animal coloration, addressing how they can be linked to individual quality, how animals perceive them, and what specific tools can be used to quantify these patterns.
Figure 1.
Six examples of colour patterns for which empirical evidence supporting quality-signalling potential has been reported: (a) white cheek patch of great tit (Parus major), (b) black spotted bib of the red-legged partridge (Alectoris rufa), (c) black V-shaped foreneck collar of little bustard (Tetrax tetrax), (d) black body patterning of tilapia (Tilapia mariae), (e) black clypeal spot of female paper wasps (Polistes dominula) and (f) body patterning of common cuttlefish (Sepia officinalis). Further details are given in table 1. Illustrations courtesy of Francisco J. Hernández. (Online version in colour.)
Here, we review the main examples of quality-signalling colour patterns across animal taxa (§2); propose potential mechanisms by which patterns may reliably signal several aspects of individual quality (§3); summarize relevant analytical tools for objective quantitative descriptions of colour patterns (§4); identify aspects of pattern perception mechanisms that must be considered to interpret the biological relevance of pattern features (§5); and identify future challenges in this research area (§6).
2. Empirical evidence of quality-signalling colour patterns
Several studies using more holistic pattern descriptions than simply measuring the area or the number of constituent elements (e.g. number of spots or stripes) support the relevance of visual patterns in signalling contexts (figure 1 and table 1; see the electronic supplementary material, table S1, for a more complete list). Most evidence comes from birds, but a foremost example is the clypeal black patch of paper wasps (Polistes dominula; table 1 and figure 1), where shape is used in dominance signalling and is linked to developmental condition and other individual physiological variables. Cichlids provide among the best fish examples, with fast changes among discrete colour patterns reflecting the motivational state of the bearer or the outcome of social interactions (electronic supplementary material, table S1). The same applies to cephalopods, which also rely on skin chromophores to display variable colour patterns that can change within seconds and may reflect dominance relationships in agonistic interactions (electronic supplementary material, table S1). Scant evidence is available for mammals and reptiles, where camouflage, predator–prey communication, thermoregulation or warning signalling are the most commonly suggested adaptive functions attributed to colour patterning. However, interspecific studies suggest that quality signalling is also a likely function in these taxa [20–22], thus encouraging empirical studies at the specific level in candidate mammal and reptile species.
Table 1.
Illustrative examples of colour patterns of species from different taxa for which evidence compatible with quality signalling role has been provided (further examples are provided in the electronic supplementary material, table S1). Patterns are shown in figure 1.
| species | colour pattern feature | evidence of its role as quality signal | ref. |
|---|---|---|---|
| great tit (Parus major) | regularity of the white cheek patch borders | Assortative mating according to the pattern, which also determines social status and survival. The pattern is also positively related to breeding investment and offspring quality | [2–5] |
| red-legged partridge (Alectoris rufa) | fractal dimension of the black bib | the pattern reflects male condition and immunocompetence | [6] |
| little bustard (Tetrax tetrax) | symmetry of the V-shaped foreneck collar | males with more symmetric patterns occupy more competitive leks and are preferred by females | [7,8] |
| tilapia (Tilapia mariae) | variable black patterning across the body | pattern expression mediates male's behavioural responses to opponents and reflects motivational state | [9,10] |
| paper wasp (Polistes dominula) | irregularity of the black clypeal spot | pattern reflects dominance status, nutrition during early development and juvenile hormone levels at adulthood; it also predicts overwinter survival and breeding success; it is negatively related to parasite prevalence at the population level | [11–17] |
| common cuttlefish (Sepia officinalis) | variable colour patterning across the body | certain colour patterns reflect motivational state and mediate agonistic interactions | [18,19] |
3. Quality-dependent expression of colour patterns
Assessing the quality-signalling role of colour patterns requires an understanding of the factors determining differential expression between high- and low-quality individuals; that is, addressing the information a receiver can extract from the signal and the factors ensuring signal reliability. Different colour patterns probably entail different reliability mechanisms according to their own architecture, complexity, production mechanism, stage of ontogeny when they are generated, and the particular life history and ecology of the species. Below we suggest four non-mutually exclusive main pathways that may link individual quality to the expression of colour patterns. While melanin is responsible of most colour patterning found in animals, these pathways are potentially applicable to patterns resulting from any production mechanism (either pigmentary or structural).
(a). Conventional signals of social status
Traits mediating intraspecific social interactions often evolve as conventional signals, or badges of status [23,24]. These traits do not necessarily involve significant production costs, but target receivers penalize the mismatch between sender quality and its signal level through agonistic interactions [24]. Given that the reliability of conventional signals is based on a consensus among senders and receivers, signal form is not necessarily constrained by a linkage between production mechanism and information conveyed [24]. Thus, any colour pattern could, in principle, evolve as a badge of status. However, for reasons of signalling efficiency, we would expect patterns used as badges of status to be simple and thus easily discriminable by the receiver (see §4). In fact, most examples of this kind of trait consist of simple colour patches that mainly vary in size between high- and low-quality individuals [23]. However, there are some examples where more complex pattern features work as a badge of status, like the uniformity of the cheek patch of great tits, the black spots of paper wasps, or the rapidly variable and state-dependent patterns displayed by cichlids and certain marine taxa (table 1; electronic supplementary material, table S1).
Nevertheless, some degree of condition-dependence can also be expected in badges of status because signal expression, agonistic behaviour and condition are likely interrelated, and displaying a certain level of the signal implies a prime physiological state to face the social costs associated with it [25]. The mechanisms invoked to link physiological state and dominance signalling via colour intensity or size of badges of status often involve endocrine or energetic constraints [23], but the potential effects of these factors on the spatial features of a colour patch are yet to be elucidated. For instance, evidence from paper wasps indicates that the shape of the clypeal spot is subject to social control by conspecifics, but is also influenced by body condition during early development and is correlated to juvenile hormone levels (an invertebrate analogous to testosterone), thus supporting the existence of such links. In any case, carefully designed experimental set-ups [23] are required to tease apart the relative importance of social costs and physiological constraints on the production of colour patterns used as badges of status.
(b). Indices of developmental homeostasis
Developmental homeostasis (including developmental stability and canalization) buffers small perturbations that can cause alterations in the normal developmental process of individuals, leading to fitness reductions [26–28]. In organisms with bilateral symmetry, fluctuating asymmetry is the most commonly used estimate of this phenomenon [26,28] and is often assumed to behave as a reliable index of individual quality [28]. However, most studies on fluctuating asymmetry have focused on morphological traits, but rarely on colour pattern features (but see the electronic supplementary material, table S1, for exceptions). This is surprising since morphological traits are likely under strong stabilizing selection, as even subtle asymmetries in any structural traits would entail significant viability costs [28]. By contrast, fluctuating asymmetry of colour traits is less likely to entail viability costs. This probably relaxes the selection for tight control over their symmetry, increasing sensitivity to environmental and genetic stress, and allowing them to better reflect developmental stability.
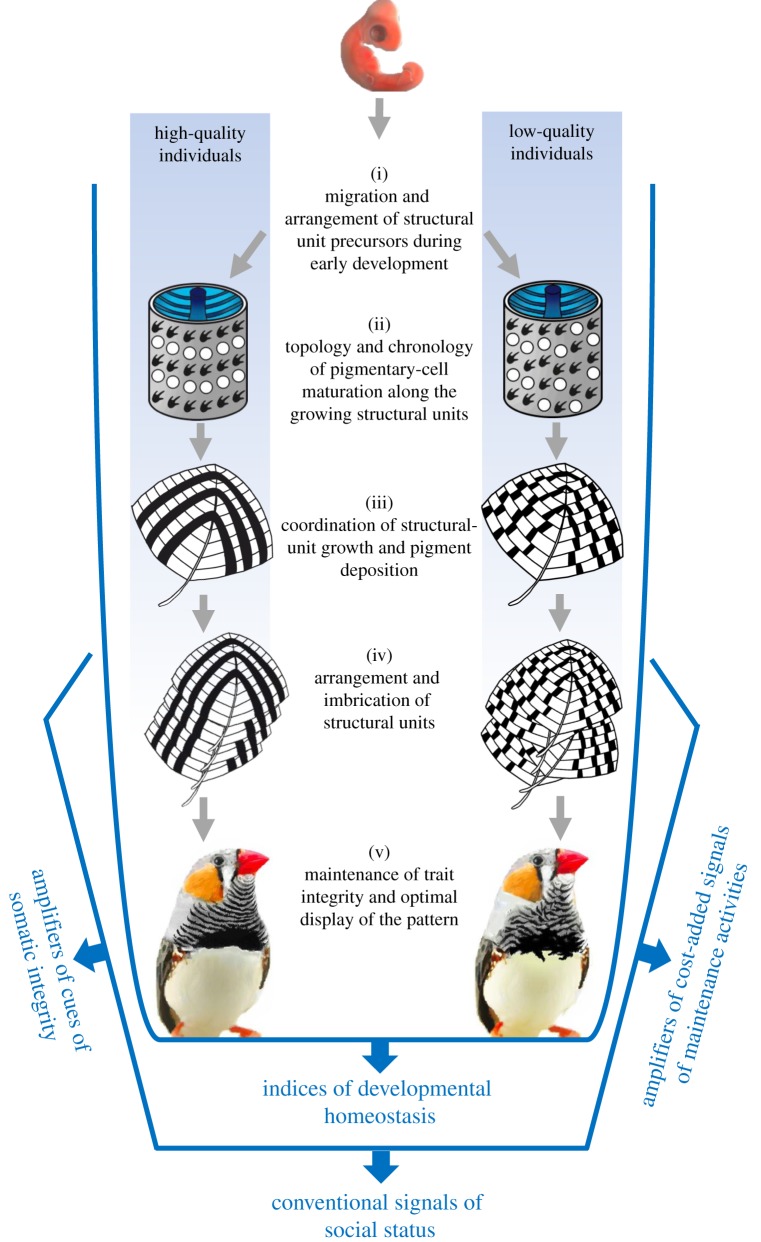
Beyond fluctuating asymmetry of symmetric traits, the capacity of individuals to express a given pattern can also reveal an individual's developmental homeostasis [26]. The pathway to produce a colour pattern form involves many different steps that must be synchronized at very different temporal and spatial scales (figure 2; e.g. [29,30]). Genetic and environmental perturbations can affect this process at different levels, causing cumulative deviations from the target pattern, which can be reliable indicators of the incapacity of the individual to buffer the developmental process. This is highlighted by a trait architecture that involves the imbrication of different units, like feathers, hairs or scales (figure 2). However, the same basic mechanism applies to taxa lacking these structures (e.g. invertebrates and amphibians), as the maturation, migration and arrangement across the body of their main coloration units—chromatophores—are equally sensitive to the same stressors [31].
Figure 2.
Schematic of the developmental process of colour pattern formation and how this relates to the four reliability mechanisms discussed in §3. A melanin-based pattern expressed in a plumage trait has been selected as an illustrative example, although the general scheme can be easily translated to other types of traits (skin-, hair- or scale-based). Pattern expression depends on the developmental control of processes that take place at different scales and that require a tight spatio-temporal coordination. These include, for instance, the arrangement during early embryonic development of structural units (feather germs) and pigmentary cell precursors (melanocytes) across the body according to the general pattern layout (i). During structural unit growth, the topology and maturation of undifferentiated (white circles) into differentiated melanocytes (black symbols) must be coordinated with structural unit growth (ii). A correct synchronization between melanosome production by differentiated melanocytes and their transfer to proliferating keratinocytes is required to elaborate the within-unit pattern adequately (iii). Furthermore, these structural units must be developed, arranged and perfectly imbricated to fully display the composite pattern resulting from their combined effect (iv). Stressors altering all these steps will exert cumulative effects on the final pattern, which would be gradually deviated from its optimum. Individual capacity to buffer such deleterious effects will differ among high- and low-quality individuals, making colour pattern expression a reliable index of developmental homeostasis (§3b). Beyond these factors affecting pattern development, individual wearing an undamaged, immaculate and well-groomed plumage, coat or skin will be able to better display their colour pattern (v), which would then act as an amplifier of somatic integrity (§3c) and investment on maintenance activities (§3d). Finally, overall pattern appearance would elicit variable responses from conspecifics, mediating the reliability of colour pattern features as conventional signals of status (§3a). (Online version in colour.)
Sharp and uniform borders, as well as regular repetition of elements (i.e. bars and spots) probably represent challenges for developmental buffering mechanisms, particularly in complex forms. Thus, uniformity, regularity and complexity are likely candidates as signals of developmental homeostasis. However, in most cases, identifying the optimum display a priori would be difficult. To avoid using arbitrary criteria, the best approach would be to rely on behavioural data (e.g. mate choice or dominance tests) to identify the pattern features positively selected under signalling scenarios. Identifying the factors deviating patterns from these optima would then be the next step.
Trait sensitivity to alterations of developmental homeostasis varies across ontogeny [26,28], and this is probably the case for pattern capacity to mirror individual quality. This implies that stressful conditions will only impact pattern expression at certain developmental windows that will vary among species or traits. This is particularly relevant for traits in animals that undergo one or multiple moulting processes. In these cases, pattern sensitivity to individual physiological state during moult can be restricted to early development or remain open at every moulting event, depending on the lability of the precise mechanisms implicated in the expression of each pattern feature. Colour patterns fixed during early development, even though insensitive to physiological state afterwards, are indeed good candidate indices of quality, as stressful conditions early in life often have long-lasting effects on individual viability [31].
(c). Amplifiers of cues of somatic integrity
The wear of plumage, skin or pelage is often related to suboptimal performance, senescence or overall somatic deterioration [2,32,33]. Parasites impose significant fitness costs to the individual, and in the case of ectoparasites, their action often damages external host appearance. In addition, damage, scars and broken or missing feathers or scales are usually the result of close encounters with predators or outcomes of agonistic interactions from which the individual did not escape unscathed. It is therefore not surprising that all these alterations of somatic integrity can be used as cues for individual quality assessment (e.g. [32,34]).
Cues of somatic integrity would be amplified by certain colour patterns [35]. In fact, this potential role of some plumage decorations was originally selected by Hasson to illustrate the concept of an ‘amplifier’ (i.e. a trait that increases the resolution of a signal, enhancing the discrimination power of the receiver) [36], and some empirical evidence supports this. For instance, in great tits (Parus major), cheek patch irregularities often reveal the presence of ectoparasites or injuries caused by conspecifics [2,5]. Similarly, the lateral barred pattern of the red-legged partridge (Alectoris rufa), resulting from the perfect alignment of flank feathers (figure 1b), is conspicuously altered by feather loss [37]; interestingly, replacement feathers do not perfectly fill these gaps, leaving traces of traumatic events [37].
This somatic integrity role of colour patterns should not be confounded with the handicapping role of certain markings that increase the risk of damage, abrasion or degradation by bacteria or ectoparasites, as typically proposed for some plumage traits [33]. Whereas the latter role is dependent on the size or location of the markings, the amplifying function of somatic integrity is mostly based on the shape of the pattern and the particular architecture of the trait. We propose that traits composed of multiple units and whose imbrication produces a regular pattern of repeated elements (bars and spots) evenly distributed across a given body region are particularly prone to evolve under this amplifying function.
(d). Amplifiers of cost-added signals of maintenance activities
Preening and grooming activities are essential to remove ectoparasites and maintain the insulating and signalling properties of external teguments. Animals, and particularly vertebrates, spend considerable time and energy in maintenance behaviour [38,39], trading off with other behaviours, such as feeding and vigilance. Given that these grooming and preening activities are particularly important to enhance the conspicuousness of ornamental traits [39], it has been suggested that they function as cost-added signals of individual quality revealing that the bearer can afford a high day-to-day investment [39].
The effective execution of these maintenance activities would be amplified by certain colour patterns. As in the previous case, composite patterns whose correct display involves an optimal arrangement of multiple units probably require higher investment. Combinations of highly contrasting colours, and predominance of white markings, may be particularly used by receivers to assess the signaller's ability to keep their pelage, skin or plumage in good shape.
4. Methods for quantifying colour patterning
One of the main factors to have hampered our understanding of the functions of colour patterns is the difficulty in quantifying overall pattern appearance. As a simple holistic solution, some studies have relied on qualitative classifications of the patterns (electronic supplementary material, table S1). This is a reasonable method for clearly distinguishable and discrete forms, such as the state-dependent patterns of many cichlid fishes (table 1). However, this is not advisable for more continuous traits, or when discrete categories are in fact a summary of different independent traits that may signal different aspects of individual quality (§3). In recent years, an increasing number of analytical tools and methodological approaches for capturing different aspects of colour patterns have become available. Although in some cases their application to animal colour markings is still pending, they constitute promising venues for objectively describing patterns and thus exploring their potential biological function.
(a). Barred patterns and regularity analysis
Barred patterns (i.e. those composed by lines or stripes) are widespread across taxa. In those groups where the barred pattern results from lighter and darker elements placed side by side, pattern regularity is one of the most evident trait features. The freely accessible software developed by Gluckman & Cardoso [40] analyses barred patterns by aligning the coloured bars, and then quantifying deviations from an ideal pattern, where all bars are uninterrupted, of constant widths and with smooth borders between colours. This measure only allows comparisons between equivalent patches among individuals of the same species, but not among different patches or species, because this measure is affected by the gross morphology of the pattern (e.g. the width of the bars) [41]. So far, this method has only been applied to the plumages of a few bird species [40]. In common waxbills (Estrilda astrild), it has provided compelling evidence that the regularity of their barred plumage would serve as a quality signal, as revealed by its sex-by-age variability and its link to body condition [41].
(b). Colour adjacency analysis
Built on the basis of colour analyses and visual modelling, the colour adjacency method [42] provides a framework based upon transitions between colour patches that make it possible to estimate pattern parameters like colour diversity, complexity or aspect ratio. The adjacency analysis relies on collecting colour characteristics—either by spectrophotometric methods or digital photography—as in conventional coloration studies. Instead of collecting colour samples in a single patch, they are collected in a large number of points ordered in a grid covering the entire body of the animal or the body region of interest. This grid is aligned with a reference body axis, so that colour measures are encoded into a zone map that allows subsequent adjacency analyses. These allow quantifying patch size and the number and orientation of transitions across colour patches, thus providing indices of pattern elongation, regularity and complexity, while also considering the particularities of the visual system of the study species. No specific software has been released for this method, although all procedures can be carried out in R or MATLAB (functions are available from the author) [42].
Adjacency analyses have been used to address the study of the highly variable colour patterning of poison frogs [43,44]. This approach allowed summarizing frogs' dorsal patterns in a few descriptive variables, like the relative contribution of each colour to the forms or pattern complexity and elongation, which have been shown to be useful to understand their biological function [43,44]. However, while this approach is a statistically useful avenue to analyse patterns, it does not resemble the way that visual systems process pattern information.
(c). Spotted patterns and Fourier and granularity analyses
Substantial earlier work revealed a number of properties of early spatial vision processing across a range of animals, including the presence of receptive fields that respond to contrast, edges and shape information, including in particular orientations (e.g. [45,46]). Such features are often processed at different spatial frequencies (e.g. pattern sizes) [47]. The advent of image analysis tools opened up a wide range of avenues with regards to quantifying patterns, many of them based on spatial frequency techniques, especially Fourier analysis. Here, a given pattern can be quantified in terms of its contrast, spatial frequency, phase and orientation.
In nature, many patterns are not lines and gratings (e.g. stripes), but rather composed of spots and ‘blobs’ of different sizes. A comparatively recent approach to quantify these has been through ‘granularity’ analysis, whereby images of a given object pattern or scene are Fourier-processed followed by bandpass filtering to create a subset of images containing information at a number of spatial frequency bands, ranging from high (small markings) to low spatial frequency (large markings). Following this, the amount of ‘energy’ at each band can be measured, with higher energy corresponding to more prominent markings. A plot of energy versus spatial frequency produces a ‘granularity spectrum’, from which a number of descriptive metrics can be obtained, including marking size, contrast and diversity. This approach has successfully been implemented in various studies describing types of cuttlefish camouflage markings [48,49], as well as pattern mimicry and rejection behaviour of cuckoo-host eggs [50]. It is likely that animals respond to multiple metrics derived from such analyses, but that the specific features used vary with species and context. For example, hosts of brood parasites base their egg rejection behaviour on assessing mimicry with regard to egg marking size, contrast, variability and dispersion, but the specific features used and their relative importance varies with species [50,51]. However, to our knowledge, the application of these approaches to the study of quality-signalling patterns is still pending. These granularity approaches are freely available in a recently released image calibration and analyses toolbox [52].
There are at least two other approaches here to quantify spot-type patterns. First, recent work has used an approach called Scale Invariant Feature Transform (SIFT), which is essentially a computer vision approach for object and feature recognition at different angles and scales. This has been successfully applied to analysing cuckoo-host egg markings [53]. Another complementary approach used by some authors is to threshold patterns into binary black and white images, and then measure the distribution and coverage of markings over different regions of an object [54]. A recent set of functions called ‘SpotEgg’ [55] have also been published that allow adaptive thresholding of images to cope with differences in illumination and object shape, while providing information about spot size, distribution, shape and other features such as fractal dimension (see below).
(d). Fractal geometry
Fractals are mathematical objects that are self-similar across scales and whose shape is too complex to be described by Euclidean geometry [56]. Many natural objects are not strictly self-similar, but can be considered ‘statistical fractals’ and their shape can be successfully described by fractal geometry [56].
There are several types of fractal analyses, but all of them rely on some type of ’fractal dimension’, which estimates pattern complexity as a scaling rule comparing how a pattern's detail changes with the scale at which it is considered. Fractal dimension is often calculated by box-counting methods, which proceed by overlaying the studied pattern by meshes of different cell side lengths, subsequently counting the number cells occupied by the pattern for each mesh size. The scaling rule of cell size over the inverse of the number of cells occupied by the pattern (both in logarithmic scale) determines its fractal dimension [56]. Fractal dimension can be calculated on lines, surfaces or volumes, capturing the space-filling capacity of the pattern, which is closely related to different properties such as the number, length, tortuosity and connectivity of its elements. Importantly, fractal dimension may be sensitive to different trait features for different types of patterns. Therefore, understanding the meaning of the fractal dimension for each pattern requires a case-by-case exploration [6]. However, irrespective of the particular pattern studied, it should be noted that the applicability of this method does not imply that animals are able to detect fractal dimension itself; rather, this measure captures variations in certain pattern features that animals can detect, but that are difficult to quantify objectively by other methods.
Fractal dimension is the simplest and most popular fractal analysis, but not the only one. Multifractal analysis provides a much more detailed description of a pattern, where the arrangement (mass distribution) of the pattern is analysed at different scales by the ‘singularity spectrum’. ‘Lacunarity’ is another useful concept from fractal geometry that quantifies the gappiness and heterogeneity of a given pattern, as well as its rotational invariance. Performing most of these analyses is relatively straightforward by using freely available software (e.g. Fractaldim, HarFa, FracLac).
Fractal geometry techniques are particularly suitable for addressing intricate, complex and heterogeneous patterns. By measuring the continuity of a pattern through scales, it somehow mirrors the inherent architecture of many animal colour traits composed by different units (scales, feathers and hairs), and is thus an interesting tool to capture the variability resulting from such multi-scaled construction of the trait. However, to date, their application to animal colour patterning has been limited [57]. In a recent experimental study, fractal dimension of the black bib of the red-legged partridge (figure 1b) was particularly useful to distinguish between individuals with a smooth or a sharp transition from the plain black to the spotted areas of the bib. This trait feature predicted individual body condition and immune responsiveness, a relationship that remained unnoticed when using simpler measures of the trait [6]. Also, although not from the perspective of quality-signalling, fractal geometry has proved useful to describe butterfly wing patterns [58], and the cranial and shell sutures in mammals and ammonoid taxa [59,60]. The latter examples support the usefulness of fractal dimension to quantify the integrity and regularity of whole colour patches or their borders.
(e). Geometric morphometrics
Geometric morphometrics is the analysis of morphological structures using Cartesian geometric coordinates rather than linear, areal or volumetric variables. One of the main advantages of this tool is that it allows capturing the shape of an object independent of its size, position and orientation. Object shape is translated into a series of derived coordinates that are easy to interpret and represent, and amenable to a wide array of statistical approaches [61,62].
Geometric morphometrics is based on ‘landmarks’ (i.e. homologous points that represent the same biological location across specimens). Once identified and digitized, landmarks can be processed by different geometric approaches, of which the Procrustes superimposition method is the most widespread [61,62]. The homology requisite of landmarks is usually fulfilled by using clearly identifiable points like cusps, invaginations or intersections.
There are several issues that make geometric morphometrics a particularly interesting tool for colour patterning analysis. For instance, it allows describing both in two- and three-dimensional shapes. This is particularly useful to capture colour patterns displayed in tridimensional structures, like legs, wings or non-flat areas of the body, providing these are digitized in a natural display manner. Also, geometric morphometrics allow controlling for potential allometric effects, or for a covariance between body and pattern shape. Another interesting feature of geometric morphometrics is its unique potential to capture several types of symmetry, from matching or object symmetry to more complex configurations, like reflection, rotational, translational or spiral symmetries [27]. It also addresses what specific pattern features are contributing most to symmetry deviations, which is of great interest for understanding the subjacent mechanisms linking pattern expression and individual quality (§5).
There are several freely available software tools and R packages for data digitalization, conversion, visualization and analysis of geometric morphometric data (see life.bio.sunysb.edu/morph). Despite the advantages of geometric morphometrics and availability of free and user-friendly software, to our knowledge no study has applied this set of powerful tools to the study of colour patterns in the context discussed here.
5. The need to consider the visual properties of the receiver
In recent years, the study of animal coloration has advanced considerably with the widespread use of objective measures of colour and models of animal vision. Unlike colour perception, which varies considerably across and even within species [63], many of the general features determining pattern vision seem to be similar even across taxa (at least in low-level vision [64]). This makes modelling certain aspects of pattern vision and producing widely relevant techniques potentially highly tractable.
A number of approaches to quantifying animal patterns have been based on the idea of approximately resembling visual processing, most notably Fourier and granularity analyses (see above; though note that these algorithms do not mimic real visual systems exactly, but rather broad principles). Other approaches include techniques for quantifying the edges of objects and patterns (e.g. [65]), although again how exactly edge detection is undertaken by real visual systems is unclear and many models exist [64]. Ultimately, any model used needs to be validated with behavioural data to determine its relevance. In theory, it is possible to come up with a highly sophisticated model of high-level pattern vision, yet if this misses some key step or process found in real visual systems then this may produce inaccurate results. By contrast, comparatively simple models of pattern assessment could produce very effective metrics. The latter is broadly the case for granularity and edge detection approaches, whereby derived pattern metrics do effectively predict behavioural responses [50]. Other models may not mimic visual processing pathways closely (e.g. fractal analysis) but still derive information that is closely akin to that acquired and used by the receiver.
Ultimately, just as with metrics of colour, we need to test that the values and variation among individuals in pattern metrics coincide with a response by the receiver; that is, that the receiver actually sees and responds to that information. This is the key consideration for any pattern quantification tool. If on top of that the model is aimed at mimicking principles of visual processing, then this also allows us to potentially understand the visual mechanisms involved. Note, however, that to make more realistic models of pattern vision, further precise information on pattern processing is needed, as well as more information on things such as display behaviour and angle and distance of viewing by the receiver to the signaller. For example, visual acuity (the ability to resolve features of a given sized object) varies considerably with species' visual system and observation distance, and this will affect how well a receiver can see aspects of pattern. Furthermore, owing to features of receptive fields and spatial frequency processing (see above), animals differ in their ability to detect different spatial frequencies at different contrast levels, which can be characterized with a so-called ‘contrast sensitivity function’ (CSF) [47]. CSFs can describe and compare visual performance at different levels of pattern scale and contrast among species. As such, incorporating CSF and acuity information (which are available for a range of species) into models of pattern vision should, in principle, provide a more accurate approach to determining the information available from animal markings to the receiver, and can allow other information to be considered (such as viewing distances). At present, information on acuity and CSF are rarely incorporated into analyses of animal colour patterns, yet this information could be valuable in determining receiver responses to patterns of different contrast and size from different viewing distances.
6. Concluding remarks and future research directions
In this review, we have highlighted the potential of animal colour patterning, beyond size and colour intensity, to play a relevant role as reliable signals of individual quality. Available evidence supports this signalling role in a wide array of taxa. The reliability of quality-signalling colour patterns might involve several mechanisms, like social control, impaired developmental homeostasis, somatic deterioration or reduced investment on self-maintenance. Although methodological limitations have hampered the research of these patterns for a long time, these are no longer a major constraint, as currently available analytical methods allow an objective and accurate quantification of different pattern features. However, the use of these tools must consider relevant aspects of the visual system of the model species and (crucially) behavioural responses, in order to allow biologically meaningful conclusions.
The different reliability mechanisms proposed here are not mutually exclusive. Indeed, colour patterns may (and probably do) act as ‘multicomponent signals’, where different features of a given pattern may inform about different aspects of the bearer or act as back-ups [66]. For instance, the symmetry and uniformity of the markings composing a given plumage pattern could indicate the stress levels suffered by the individual during early development. But once developed, the ability of the individual to keep undamaged and perfectly arranged all the feathers composing the display would amplify its capacity to keep its soma in prime conditions and allocate resources to self-maintenance. If the pattern behaves as a badge of status, the social costs derived from agonistic interactions can also be added to the system. The specific weight of each reliability pathway will depend on the ecology of the species and the particular architecture of the trait. Experiments would be needed to disentangle the relative importance of each signalling pathway.
A quality-signalling capacity does not preclude the same colour patterns from simultaneously being used in other functions, such as thermoregulation, anti-glare, concealment, individual recognition or aposematism [67]. This would be the case, for instance, of the facial masks and other common head markings of birds often assumed to provide anti-glare protection or gaze concealment to predators or opponents [67], but whose expression (regularity of borders, symmetry) might also reflect individual quality (e.g. [2,8]). This polyvalent conception of colour patterns can conciliate, for instance, a signalling function and a role in predation avoidance via camouflage or aposematism for a single trait. In the latter case, this would help to explain the apparently paradoxical variability in aposematic patterns observed within a given species [43].
Understanding the information content of colour patterns requires a deep knowledge of the main factors constraining its expression. This is particularly pertinent in the case of amplifiers of developmental homeostasis (§3b), as the reliability of the colour pattern is intrinsically linked to its production mechanism. Understanding how each pattern feature can reflect the homeostasis of the developmental process is therefore a major challenge. In this sense, reaction–diffusion models provide a useful framework for studying pattern formation, both within structural units and across the whole body (e.g. [68,69]). According to these models, increasing pattern complexity requires controlling a higher number of morphogens and regulatory parameters to achieve the target pattern [68,69]. Such complexity derived from theoretical models predicts the evolutionary pathways and key ecological aspects of patterns in different taxa [70,71]; however, addressing whether increased pattern complexity implies higher lability during development is still pending. This task requires a multidisciplinary approach, combining inputs from genetics, biochemistry, physiology and evolutionary developmental biology. Incorporating other reliability mechanisms into the equation (§3) would require behavioural tests and comparative approaches. Unfortunately, to date, evidence of the different reliability mechanisms discussed here is fragmentary, and comes from very different study models. Bringing together behavioural and mechanistic approaches in the same study system is a pressing task to fully assess the quality-signalling role of colour patterns.
Supplementary Material
Acknowledgements
We are grateful to Tomás Redondo for some discussions on signalling theory, to Gonçalo Cardoso for advice on pattern regularity analysis, and to P. Ferns and an anonymous referee for useful comments. Francisco J. Hernández and Beatriz Gómez Pérez kindly provided illustrations for figures 1 and 2, respectively.
Authors' contributions
L.P.-R. conceived the study; all authors contributed to manuscript writing.
Competing interests
The authors declare no competing interests.
Funding
During manuscript writing, L.P.-R. was supported by a postdoctoral contract from MINECO through the Severo Ochoa Programme for Centres of Excellence in Research, Development, and Innovation (SEV-2012-0262).
References
- 1.Espmark Y, Amundsen T, Rosenqvist G (eds) 2000. Animal signals: signalling and signal design in animal communication. Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- 2.Ferns PN, Hinsley SA. 2004. Immaculate tits: head plumage pattern as an indicator of quality in birds. Anim. Behav. 67, 261–272. ( 10.1016/j.anbehav.2003.05.006) [DOI] [Google Scholar]
- 3.Remes V, Matysiokova B, Klejdus B. 2011. Egg yolk antioxidant deposition as a function of parental ornamentation, age, and environment in great tits Parus major. J. Avian Biol. 42, 387–396. ( 10.1111/j.1600-048X.2011.05402.x) [DOI] [Google Scholar]
- 4.Remes V, Matysiokova B. 2013. More ornamented females produce higher-quality offspring in a socially monogamous bird: an experimental study in the great tit (Parus major). Front. Zool. 10, 10 ( 10.1186/1742-9994-10-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galván I, Sanz JJ. 2009. Cheek plumage uniformity as a social status signal in great tits. Ann. Zool. Fenn. 46, 271–282. ( 10.5735/086.046.0404) [DOI] [Google Scholar]
- 6.Pérez-Rodríguez L, Jovani R, Mougeot F. 2013. Fractal geometry of a complex plumage trait reveals bird's quality. Proc. R. Soc. B 280, 20122783 ( 10.1098/rspb.2012.2783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiguet F, Bretagnolle V. 2006. Manipulating lek size and composition using decoys: an experimental investigation of lek evolution models. Am. Nat. 168, 758–768. ( 10.1086/508808) [DOI] [PubMed] [Google Scholar]
- 8.Jiguet F, Bretagnolle V. 2014. Sexy males and choosy females on exploded leks: correlates of male attractiveness in the Little Bustard. Behav. Processes 103, 246–255. ( 10.1016/j.beproc.2014.01.008) [DOI] [PubMed] [Google Scholar]
- 9.Baldaccini NE. 1973. An ethological study of reproductive behaviour including the colour patterns of the cichlid fish Tilapia mariae (Boulanger). Monitore Zoologico Italiano 7, 247–290. [Google Scholar]
- 10.Slovin M, Rowland WJ. 1978. Effects of color patterns on aggressive behavior of Tilapia mariae (Boulanger). Behav. Biol. 24, 378–386. ( 10.1016/S0091-6773(79)90236-0) [DOI] [Google Scholar]
- 11.Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in a paper wasp. Nature 432, 218–222. ( 10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- 12.Tibbetts EA. 2006. Badges of status in worker and gyne Polistes dominulus wasps. Ann. Zool. Fenn. 43, 575–582. [Google Scholar]
- 13.Tibbetts EA, Curtis TR. 2007. Rearing conditions influence quality signals but not individual identity signals in Polistes wasps. Behav. Ecol. 18, 602–607. ( 10.1093/beheco/arm013) [DOI] [Google Scholar]
- 14.Tibbetts EA, Lindsay R. 2008. Visual signals of status and rival assessment in Polistes dominulus paper wasps. Biol. Lett. 4, 237–239. ( 10.1098/rsbl.2008.0048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibbetts EA, Izzo A, Huang ZY. 2010. Behavioral and physiological factors associated with juvenile hormone in Polistes wasp foundresses. Behav. Ecol. Sociobiol. 65, 1123–1131. ( 10.1007/s00265-010-1126-6) [DOI] [Google Scholar]
- 16.Tibbetts EA, Skaldina O, Zhao V, Toth AL, Skaldin M, Beani L, Dale J. 2011. Geographic variation in the status signals of Polistes dominulus paper wasps. PLoS ONE 6, 8 ( 10.1371/journal.pone.0028173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibbetts EA, Forrest T, Vernier C, Jinn J, Madagame A. 2015. Socially selected ornaments and fitness: signals of fighting ability in paper wasps are positively associated with survival, reproductive success, and rank. Evolution 69, 2917–2926. ( 10.1111/evo.12793) [DOI] [PubMed] [Google Scholar]
- 18.Adamo SA, Hanlon RT. 1996. Do cuttlefish (Cephalopoda) signal their intentions to conspecifics during agonistic encounters? Anim. Behav. 52, 73–81. ( 10.1006/anbe.1996.0153) [DOI] [Google Scholar]
- 19.Palmer ME, Calve MR, Adamo SA. 2006. Response of female cuttlefish Sepia officinalis (Cephalopoda) to mirrors and conspecifics: evidence for signaling in female cuttlefish. Anim. Cogn. 9, 151–155. ( 10.1007/s10071-005-0009-0) [DOI] [PubMed] [Google Scholar]
- 20.Caro T, Stankowich T. 2009. The function of contrasting pelage markings in artiodactyls. Behav. Ecol. 21, 78–84. ( 10.1093/beheco/arp165) [DOI] [Google Scholar]
- 21.Caro T, Stankowich T, Mesnick SL, Costa DP, Beeman K. 2012. Pelage coloration in pinnipeds: functional considerations. Behav. Ecol. 23, 765–774. ( 10.1093/beheco/ars025) [DOI] [Google Scholar]
- 22.Chen IP, Stuart-Fox D, Hugall AF, Symonds MRE. 2012. Sexual selection and the evolution of complex color patterns in dragon lizards. Evolution 66, 3605–3614. ( 10.1111/j.1558-5646.2012.01698.x) [DOI] [PubMed] [Google Scholar]
- 23.Senar JC. 2006. Color displays as intrasexual signals of aggression and dominance. In Bird coloration, vol. II: function and evolution (eds Hill GE, McGraw KJ), pp. 87–136. Cambridge, MA: Harvard University Press. [Google Scholar]
- 24.Guilford T, Dawkins MS. 1995. What are conventional signals? Anim. Behav. 49, 1689–1695. ( 10.1016/0003-3472(95)90090-X) [DOI] [Google Scholar]
- 25.Tibbetts EA. 2014. The evolution of honest communication: integrating social and physiological costs of ornamentation. Integr. Comp. Biol. 54, 578–590. ( 10.1093/icb/icu083) [DOI] [PubMed] [Google Scholar]
- 26.Debat V, David P. 2001. Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 16, 555–561. ( 10.1016/S0169-5347(01)02266-2) [DOI] [Google Scholar]
- 27.Klingenberg CP. 2015. Analyzing fluctuating asymmetry with geometric morphometrics: concepts, methods, and applications. Symmetry-Basel 7, 843–934. ( 10.3390/sym7020843) [DOI] [Google Scholar]
- 28.Van Dongen S. 2006. Fluctuating asymmetry and developmental instability in evolutionary biology: past, present and future. J. Evol. Biol. 19, 1727–1743. ( 10.1111/j.1420-9101.2006.01175.x) [DOI] [PubMed] [Google Scholar]
- 29.Lin SJ, et al. 2013. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science 340, 1442–1445. ( 10.1126/science.1230374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata M, Otaki JM. 2016. Spatial patterns of correlated scale size and scale color in relation to color pattern elements in butterfly wings. J. Insect Physiol. 85, 32–45. ( 10.1016/j.jinsphys.2015.11.013) [DOI] [PubMed] [Google Scholar]
- 31.Lindstrom J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick S, Price P. 1997. Magpies’ tails: damage as an indicator of quality. Behav. Ecol. Sociobiol. 40, 209–212. ( 10.1007/s002650050334) [DOI] [Google Scholar]
- 33.Fitzpatrick S. 1998. Birds’ tails as signaling devices: markings, shape, length, and feather quality. Am. Nat. 151, 157–173. ( 10.1086/286109) [DOI] [PubMed] [Google Scholar]
- 34.Hoglund J, Alatalo RV, Lundberg A, Ratti O. 1994. Context-dependent effects of tail-ornament damage on mating success in black grouse. Behav. Ecol. 5, 182–187. ( 10.1093/beheco/5.2.182) [DOI] [Google Scholar]
- 35.Hasson O. 1989. Amplifiers and the handicap principle in sexual selection: a different emphasis. Proc. R. Soc. Lond. B 235, 383–406. ( 10.1098/rspb.1989.0006) [DOI] [PubMed] [Google Scholar]
- 36.Hasson O. 1991. Sexual displays as amplifiers: practical examples with an emphasis on feather decorations. Behav. Ecol. 2, 189–197. ( 10.1093/beheco/2.3.189) [DOI] [Google Scholar]
- 37.Bortolotti GR, Blas J, Negro JJ, Tella JL. 2006. A complex plumage pattern as an honest social signal. Anim. Behav. 72, 423–430. ( 10.1016/j.anbehav.2006.01.016) [DOI] [Google Scholar]
- 38.Goldstein DL. 1988. Estimates of daily energy expenditure in birds: the time-energy budget as an integrator of laboratory and field studies. Am. Zool. 28, 829–844. ( 10.1093/icb/28.3.829) [DOI] [Google Scholar]
- 39.Walther BA, Clayton DH. 2005. Elaborate ornaments are costly to maintain: evidence for high maintenance handicaps. Behav. Ecol. 16, 89–95. ( 10.1093/beheco/arh135) [DOI] [Google Scholar]
- 40.Gluckman T-L, Cardoso GC. 2009. A method to quantify the regularity of barred plumage patterns. Behav. Ecol. Sociobiol. 63, 1837–1844. ( 10.1007/s00265-009-0823-5) [DOI] [Google Scholar]
- 41.Marques CIJ, Batalha HR, Cardoso GC. 2016. Signalling with a cryptic trait: the regularity of barred plumage in common waxbills. R. Soc. open sci. 3, 9 ( 10.1098/rsos.160195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endler JA. 2012. A framework for analysing colour pattern geometry: adjacent colours. Biol. J. Linn. Soc. 107, 233–253. ( 10.1111/j.1095-8312.2012.01937.x) [DOI] [Google Scholar]
- 43.Rojas B, Endler JA. 2013. Sexual dimorphism and intra-populational colour pattern variation in the aposematic frog Dendrobates tinctorius. Evol. Ecol. 27, 739–753. ( 10.1007/s10682-013-9640-4) [DOI] [Google Scholar]
- 44.Rojas B, Devillechabrolle J, Endler JA. 2014. Paradox lost: variable colour-pattern geometry is associated with differences in movement in aposematic frogs. Biol. Lett. 10, 20140193 ( 10.1098/rsbl.2014.0193) [DOI] [Google Scholar]
- 45.Barlow HB. 1953. Summation and inhibition in the frogs retina. J. Physiol. 119, 69–88. ( 10.1113/jphysiol.1953.sp004829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubel DH, Wiesel TN. 1962. Receptive fields, binocular interaction and functional architecture in cats visual cortex. J. Physiol. 160, 106–154. ( 10.1113/jphysiol.1962.sp006837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell FW, Robson JG. 1968. Application of Fourier analysis to visibility of gratings. J. Physiol. 197, 551–566. ( 10.1113/jphysiol.1968.sp008574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbosa A, Mäthger LM, Buresh KC, Kelly J, Chubb C, Chiao C-C, Hanlon RT. 2008. Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res. 48, 1242–1253. ( 10.1016/j.visres.2008.02.011) [DOI] [PubMed] [Google Scholar]
- 49.Chiao CC, Chubb C, Buresch K, Siemann L, Hanlon RT. 2009. The scaling effects of substrate texture on camouflage patterning in cuttlefish. Vision Res.. 49, 1647–1656. ( 10.1016/j.visres.2009.04.002) [DOI] [PubMed] [Google Scholar]
- 50.Spottiswoode CN, Stevens M. 2010. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676. ( 10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spottiswoode CN, Stevens M. 2011. How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. R. Soc. B 278, 3566–3573. ( 10.1098/rspb.2011.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Troscianko J, Stevens M, Rands S. 2015. Image calibration and analysis toolbox: a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol. Evol. 6, 1320–1331. ( 10.1111/2041-210X.12439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoddard MC, Kilner RM, Town C. 2014. Pattern recognition algorithm reveals how birds evolve individual egg pattern signatures. Nat. Comm. 5, 10 ( 10.1038/ncomms5117) [DOI] [PubMed] [Google Scholar]
- 54.Stoddard MC, Stevens M. 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc. R. Soc. B 277, 1387–1393. ( 10.1098/rspb.2009.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gómez J, Liñán-Cembrano G. 2016. SpotEgg: an image-processing tool for automatised analysis of colouration and spottiness. J. Avian Biol. 47, 1–11. ( 10.1111/jav.00736) [DOI] [Google Scholar]
- 56.Mandelbrot BB. 1983. The fractal geometry of nature. New York, NY: W. H. Freeman. [Google Scholar]
- 57.Jovani R, Pérez-Rodríguez L, Mougeot F. 2013. Fractal geometry for animal biometrics: a response to Kuhl and Burghardt. Trends Ecol. Evol. 28, 499–500. ( 10.1016/j.tree.2013.06.004) [DOI] [PubMed] [Google Scholar]
- 58.Castrejon-Pita AA, Sarmiento-Galan A, Castrejon-Pita JR, Castrejon-Garcia R. 2005. Fractal dimension in butterflies’ wings: a novel approach to understanding wing patterns? J. Math. Biol. 50, 584–594. ( 10.1007/s00285-004-0302-6) [DOI] [PubMed] [Google Scholar]
- 59.Gibert J, Palmqvist P. 1995. Fractal analysis of the Orce skull sutures. J. Hum. Evol. 28, 561–575. ( 10.1006/jhev.1995.1042) [DOI] [Google Scholar]
- 60.Perez-Claros JA, Palmqvist P, Oloriz F. 2002. First and second orders of suture complexity in ammonites: a new methodological approach using fractal analysis. Math. Geol. 34, 323–343. ( 10.1023/A:1014847007351) [DOI] [Google Scholar]
- 61.Zelditch ML, Swiderski DL, Sheets HD, Fink WL. 2004. Geometric morphometrics for biologists: a primer. London, UK: Elsevier. [Google Scholar]
- 62.Mitteroecker P, Gunz P. 2009. Advances in geometric morphometrics. Evol. Biol. 36, 235–247. ( 10.1007/s11692-009-9055-x) [DOI] [Google Scholar]
- 63.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 64.Bruce V, Georgeson MA, Green PR. 2003. Visual perception: physiology, psychology and ecology, 4th edn Hove, UK: Psychology Press. [Google Scholar]
- 65.Marr D, Hildreth E. 1980. Theory of edge-detection. Proc. R. Soc. Lond. B 207, 187–217. ( 10.1098/rspb.1980.0020) [DOI] [PubMed] [Google Scholar]
- 66.Harper DGC. 2006. Maynard Smith: amplifying the reasons for signal reliability. J. Theor. Biol. 239, 203–209. ( 10.1016/j.jtbi.2005.08.034) [DOI] [PubMed] [Google Scholar]
- 67.Bortolotti GR. 2006. Natural selection and coloration: protection, concealment, advertisement or deception? In Bird coloration, vol. II: function and evolution (eds Hill GE, McGraw KJ), pp. 3–35. Cambridge, MA: Harvard University Press. [Google Scholar]
- 68.Murray JD. 1981. On pattern-formation mechanisms for lepidopteran wing patterns and mammalian coat markings. Phil. Trans. R. Soc. Lond. B 295, 473–496. ( 10.1098/rstb.1981.0155) [DOI] [PubMed] [Google Scholar]
- 69.Prum RO, Williamson S. 2002. Reaction–diffusion models of within-feather pigmentation patterning. Proc. R. Soc. Lond. B 269, 781–792. ( 10.1098/rspb.2001.1896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gluckman T-L, Mundy NI. 2016. Evolutionary pathways to convergence in plumage patterns. BMC Evol. Biol. 16, 172 ( 10.1186/s12862-016-0741-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen WL, Cuthill IC, Scott-Samuel NE, Baddeley R. 2011. Why the leopard got its spots: relating pattern development to ecology in felids. Proc. R. Soc. B 278, 1373–1380. ( 10.1098/rspb.2010.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.