Abstract
In some arctic areas, marine-derived nutrients (MDN) resulting from fish migrations fuel freshwater and terrestrial ecosystems, increasing primary production and biodiversity. Less is known, however, about the role of seabird-MDN in shaping ecosystems. Here, we examine how the most abundant seabird in the North Atlantic, the little auk (Alle alle), alters freshwater and terrestrial ecosystems around the North Water Polynya (NOW) in Greenland. We compare stable isotope ratios (δ15N and δ13C) of freshwater and terrestrial biota, terrestrial vegetation indices and physical–chemical properties, productivity and community structure of fresh waters in catchments with and without little auk colonies. The presence of colonies profoundly alters freshwater and terrestrial ecosystems by providing nutrients and massively enhancing primary production. Based on elevated δ15N in MDN, we estimate that MDN fuels more than 85% of terrestrial and aquatic biomass in bird influenced systems. Furthermore, by using different proxies of bird impact (colony distance, algal δ15N) it is possible to identify a gradient in ecosystem response to increasing bird impact. Little auk impact acidifies the freshwater systems, reducing taxonomic richness of macroinvertebrates and truncating food webs. These results demonstrate that the little auk acts as an ecosystem engineer, transforming ecosystems across a vast region of Northwest Greenland.
Keywords: marine-derived nutrients, nutrient subsidies, stable isotopes, arctic food webs, ecosystem engineer, seabird colonies
1. Introduction
Migratory animals translocate energy and nutrients between ecosystems and may support productivity and biomass in otherwise unproductive systems [1]. Species responsible for such translocation of nutrients are often termed ‘ecosystem engineers’ as they may profoundly change the recipient ecosystem (e.g. [2–4]). For example, Pacific salmon species are responsible for large-scale transport of marine-derived nutrients (MDN) to freshwater and terrestrial ecosystems in temperate and arctic regions of North America and Asia [5–8]. Productivity and biodiversity increase in systems with Pacific salmon as MDN are assimilated into stream biofilms and terrestrial vegetation [7,9].
In many locations around the globe, seabirds feeding at sea and breeding in colonies in terrestrial systems are known to bring nutrients to land [9–13]. However, evidence of the extent to which the seabird-MDN subsidy alters ecosystems is sparse and what exists is limited to detecting the presence of MDN in terrestrial soil, vegetation and a few soil invertebrates [14–17]. These studies rely on nitrogen stable isotope ratios (δ15N), exploiting the fact that marine δ15N is higher than terrestrial δ15N and thus easily traceable in freshwater and terrestrial biota (e.g. [17]). For example, δ15N was found to be higher in soil and vegetation at bird-affected sites compared with bird-free sites for a range of colonial seabird species in Antarctica [16], Florida, USA [18], New Zealand [15,19] and Svalbard [20].
The few studies considering change in ecosystem structure induced by seabirds (e.g. [10,11,13]) suggest that seabird colonies alter terrestrial vegetation structure, increase primary productivity and fuel terrestrial food webs both above and below ground [13,14]. Seabird guano may also alter the chemical properties of fresh waters by changing nutrient concentrations and pH [21]. However, these findings apply to relatively small catchments and are mostly valid only for ecosystems located immediately adjacent to specific bird colonies on islands [13,18,19].
Here, we sought to elucidate the nature and extent of the marine nutrient subsidy from the extensive seabird colonies along the shores of the North Water Polynya (NOW) in Northwest Greenland (figure 1). This area is the main breeding ground of the little auk (Alle alle), a small (approx. 160 g), zooplanktivorous alcid, which is the most abundant seabird in the North Atlantic [22,23]. Within a range of approx. 400 km, approximately 80% of the global little auk population, or 33 million breeding pairs, have their nesting sites in dense colonies on talus slopes up to 10 km inland from the coast [24–26]. Colonies are attended from early-May to mid-August when parents, performing round-trips to at-sea foraging areas up to 100 km from the breeding site, raise a single chick on a diet of lipid-rich Arctic copepods [27]. In the NOW, little auks are estimated to be capable of consuming up to 24% of the copepod standing stock [28], bringing vast quantities of MDN to land.
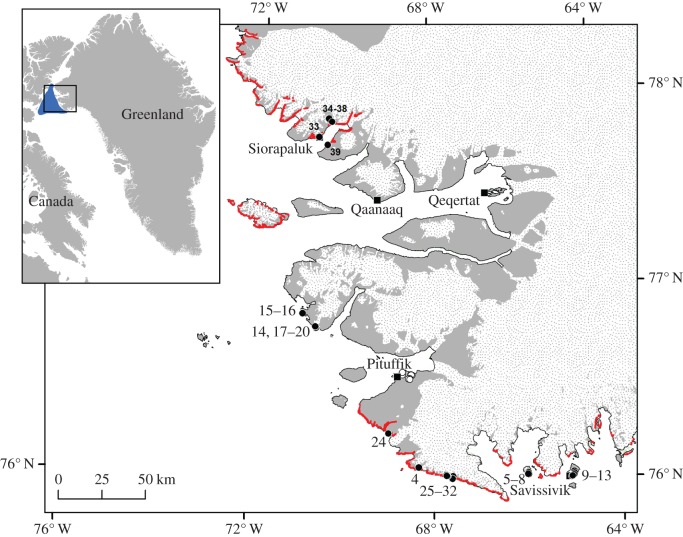
Figure 1.
The investigation area in Northwest Greenland encompassing the entire range of little auk breeding colonies associated with the North Water Polynya (NOW). Red areas: little auk breeding colonies after (24). Black dots: sites sampled in 2014–2015. White dots: sites sampled in 2001 and used for comparison. Black squares: permanently inhabited settlements. On the overview map, the solid blue area represents the approximate late winter/early spring extent of the NOW.
The overall aim of the study was to determine the contribution of seabird-MDN to the biomass of primary producers and consumers in both terrestrial and aquatic habitats. Specifically, we sought to identify the effect of bird colonies on terrestrial and freshwater primary productivity, freshwater physical–chemical characteristics and biological community composition and also to investigate the potential mechanisms behind any differences between affected and unaffected systems. We hypothesize that: (i) a very large proportion of the biomass of aquatic and terrestrial primary producers and consumers in bird colony areas is fuelled by MDN; (ii) little auk colonies increase terrestrial and aquatic primary production, and alter physical–chemical properties and community structure in recipient freshwater ecosystems, resulting in increased nutrient concentrations, algal biomass and taxa richness.
2. Material and methods
(a). Study area
The NOW polynya in Smith Sound, Northern Baffin Bay lies between Greenland and Canada. It covers around 85 000 km2 and is the largest polynya in the Arctic [29] (figure 1). The combination of year-round nutrient rich, open waters and constant light in the summer makes the NOW one of the most productive marine areas in the Arctic [30].
(b). Sampling campaigns
In late-July and early-August 2014 and 2015, terrestrial and freshwater ecosystems were sampled along the Greenlandic coastline of NOW from Savissivik in the south to Siorapaluk in the north (figure 1). In the field, sampling sites were classified as either ‘colony’ or ‘control’ sites. Sites located in the drainage catchment of a little auk colony or under a flight corridor of birds commuting between a colony and at-sea foraging areas (areas receiving bird droppings) were classified as colony sites. Sites located in catchments without colonies or overflying little auks were classified as control sites. Additionally, we used data on stable isotopes and nutrient concentrations in lakes sampled in an area without little auks near Pituffik in 2001 (electronic supplementary material, Methods S1; figure 1).
(c). Stable isotope sampling
At each site, samples were collected for analysis of C and N stable isotopes (δ15N and δ13C). Samples were obtained from soil, terrestrial mosses, pooled terrestrial plant leaves, excrement (little auk, geese, arctic hare (Lepus arcticus) and musk ox (Ovibos moschatus)), hair from arctic hare, and a skull from an arctic fox (Vulpes lagopus). In freshwater habitats, filamentous algae, aquatic mosses, debris (conditioned leaf litter), benthic biofilm, macroinvertebrates, seston, zooplankton, profundal lake sediments and fish were sampled. Samples were analysed at UC Davis Stable Isotope Facilities, California, USA (http://stableisotopefacility.ucdavis.edu). Isotopic data from animals were lipid-corrected based on their C : N ratio following eqn 3 of Post et al. [31]. Details of the stable isotope sampling and corrections are given in the electronic supplementary material, Methods S1.
(d). Freshwater consumer taxa richness and physical–chemical properties
The richness of consumer taxa was measured as the number of aquatic consumer taxa obtained during the sampling for stable isotopes, taxa being defined at family level. Family level richness was used as species-level identification of macroinvertebrates was not possible in the field. Family-level richness generally correlates well with species richness, thus constituting a valid measure of richness (e.g. [32]). Sampling effort was similar at the different sites, allowing comparison between sites (see electronic supplementary material, Methods S1 for details). In each aquatic system, we also took water samples for measuring nutrient concentrations and algal biomass samples (chlorophyll-a). Key physical–chemical variables were recorded using a multi-parameter probe (for details, see electronic supplementary material, Methods S1). Information about the physical–chemical characteristics of sites is available in electronic supplementary material, table S2 and details of taxa collected are given in electronic supplementary material, table S3.
(e). Terrestrial productivity
As a measure of terrestrial productivity, we used an enhanced vegetation index (EVI) image from MODIS Terra, 28/7–12/8 2015 [33,34]. Owing to the coarse resolution of the image (250 × 250 m), and the heterogeneous nature of the landscape in the vicinity of the sampling sites (e.g. patches of vegetation in places where soil formation is possible, interspaced with areas of bare rock and water), the maximum EVI value within a 500 m radius of each sampling site was used in the statistical analyses (for details, see electronic supplementary material, Methods S1).
(f). Comparison of colony and control sites
To avoid making type II errors, univariate tests of differences between colony and control sites were preceded by two PERMANOVAs [35], one including δ15N and δ13C of terrestrial and aquatic primary producer and consumer groups, and another including grouped freshwater physical–chemical parameters. In the univariate tests, variance was often heterogeneous and generalized least squares models (GLS, α = 0.05, [36]) were used with the appropriate error structures to account for this. Residual plots were checked for remaining heterogeneity and for spatial autocorrelation [36]. Where residual spatial autocorrelation was detected, a spatial weights matrix was integrated into the model and residuals were re-checked. Full details of the models can be found in electronic supplementary material, Methods S1.
In relation to aquatic habitats, comparisons of parameters between colony and control sites were made for all system types (lakes, ponds and streams) pooled. However, in the case of algal biomass, separate comparisons were made for lotic (streams) and lentic (lakes + ponds) systems due to different units being used: in lotic systems benthic algal biomass was in micrograms per square centimetre whereas in lentic systems phytoplankton biomass was expressed in micrograms per litre.
(g). Estimation of the contribution of seabird-derived nutrient to biomass
Enhanced δ15N at colony sites relative to control sites provides an unequivocal marker of the presence of seabird-MDN. Following the procedure of Harding et al. [11], the contribution of seabird-MDN to the biomass of different terrestrial and aquatic primary producer and consumer groups at colony sites was estimated by means of mass balance models for N. Details of methodology are provided in electronic supplementary material, Methods S1.
(h). Changes along a gradient of bird impact
To study how terrestrial and freshwater ecosystems were affected along a gradient of bird impact, we investigated the relationships between distance to nearest little auk colony and EVI, aquatic algal biomass (lotic and lentic Chl-a) and δ15N of freshwater benthic algae. Further, in a detailed case study of Savissivik Island, where GPS tracking of breeding little auks was conducted, we examined how EVI and freshwater benthic algal δ15N varied in relation to overflight intensity of little auks (proxy of bird dropping intensity) at five sample sites at varying distances from the tracking colony. We also modelled the drainage pattern from the Savissivik colony to evaluate its effect on the spread of nutrients in the landscape. All details are provided in electronic supplementary material, Methods S1.
The combined results of these analyses strongly indicated that benthic algal δ15N is a good proxy of the relative magnitude of bird nutrient input in fresh waters, reflecting true impact much better than distance to nearest little auk colony (see Results and Discussion). We therefore used benthic algal δ15N to detect changes in freshwater physical–chemical and community characteristics (pH, total nitrogen, total phosphorus, algal biomass and consumer taxa richness) along a gradient of bird impact. The use of δ15N as an indicator of MDN input has been supported in diverse studies (e.g. [7,12,21,37]). Details of the models used to test the relationships are provided in electronic supplementary material, Methods S1.
(i). Potential drivers of changes in freshwater ecosystems along a gradient of bird impact
Finally, in order to identify potential mechanisms behind changes in freshwater community structure along a gradient of bird impact, we tested for relationships between environmental variables changing with bird impact, i.e. nutrient concentrations and pH, versus algal biomass (phytoplankton Chl-a in lentic and benthic algal Chl-a in lotic systems) and consumer taxa richness. Statistical procedures are described in electronic supplementary material, Methods S1.
3. Results and discussion
(a). Comparison of colony and control sites
Unequivocal evidence of fertilization by seabird-derived nutrients was reflected in the different isotopic fingerprints of C and N in terrestrial and aquatic primary producers and consumers at little auk colony sites compared with control sites (PERMANOVA F = 9.9; d.f.res = 11, p < 0.01), in particular by the approximate 10-fold difference in their δ15N (GLS: p < 0.05, table 1; electronic supplementary material, figure S1). The greater statistical significance of the difference in δ15N compared with δ13C between colony and control sites reflects the fact that while marine-derived nitrogen is incorporated in both terrestrial and freshwater ecosystems, carbon of marine origin is only incorporated in freshwater systems (e.g. [15]). Specifically aquatic mosses and chironomids were enriched in δ13C at colony sites. The pattern of elevated δ15N at colony sites was significant in terrestrial systems and across all fresh waters—lakes, ponds and streams (table 1; electronic supplementary material, figure S1). Lake sediment, seston, zooplankton and hair from arctic hare (Lepus arcticus) also had higher δ15N values at colony sites, although this could not be tested statistically due to lack of replicates (table 1; electronic supplementary material, figure S1). The observed 10-fold increase in δ15N is larger than that recorded in systems where migratory fish transfer MDN to terrestrial and aquatic ecosystems (i.e. a threefold to fourfold δ15N increase at fish impacted versus control sites [7,37]).
Table 1.
Nitrogen and carbon isotopic signatures (δ15N and δ13C, ‰, mean ± s.d.) at colony and control sites, and estimated contribution of marine-derived nitrogen (MDN) to the biomass of primary producers and consumers at colony sites. Calculations of the contribution of MDN to biomass were performed with mass balance models using as marine nitrogen source δ15N of peat at colony sites, adding 2‰ enrichment in the case of aquatic resources and consumers. This 2‰ enrichment represents the hydrolysable proportion of nitrogen reaching aquatic ecosystems. This estimate is tentative as the variability in δ15N reaching freshwaters is high due to diverse microbial processes affecting guano and soil δ15N, implying that values more than 100% may occur. Significant differences in isotopic fingerprints are marked in bold and marginal p-values are given in italics.
| no. samples analysed |
δ13C (mean ± s.d.) |
δ15N (mean ± s.d.) |
GLS test parameters (t; d.f.res; p-value) |
mass balance model. mean contribution of MDN to biomass (%) | ||||
|---|---|---|---|---|---|---|---|---|
| colony, control | colony | control | colony | control | test for δ13C | test for δ15N | colony | |
| PERMANOVA (all isotopic signatures) | 4; 9 | δ13C and δ15N: F = 9.9; d.f.res = 11; p < 0.01 | ||||||
| freshwater environment | ||||||||
| terrestrial debris | 3; 6 | −27.2 ± 2.9 | −29.8 ± 1.0 | 8.4 ± 6.5 | −1.3 ± 2.7 | 2.0; 7; p = 0.08 | 2.8; 7; p < 0.05 | nt |
| profundal lake sediment | 2; 5 | −24.2 ± 0.3 | −22.4 ± 5.2 | 20.7 ± 2.4 | 1.9 ± 0.4 | nt | nt | nt |
| aquatic moss | 15; 14 | −23.9 ± 1.7 | −28.7 ± 4.9 | 17.3 ± 5.8 | 1.8 ± 2.5 | 3.4; 26; p < 0.01 | 8.7; 26; p < 0.0001 | 116.5 |
| benthic algae | 39; 25 | −19.9 ± 4.4 | −21.7 ± 6.6 | 17.9 ± 8.8 | 1.1 ± 2.4 | 0.87; 33; p > 0.1 | 4.8; 33; p < 0.0001 | 127 |
| chironomids | 33; 42 | −17.5 ± 3.3 | −23.9 ± 4.7 | 16.2 ± 7.1 | 4.4 ± 3.2 | 4.5; 31; p < 0.0001 | 3.9; 31; p < 0.001 | 87.9 |
| other invertebrates | 4; 31 | −21.5 ± 1.8 | −21.3 ± 3.8 | 15.9 ± 5.0 | 4.6 ± 1.9 | −0.11; 12; p > 0.1 | 4.3; 12; p < 0.0001 | 86.1 |
| seston | 1; 3 | −19 | −24.6 ± 2.6 | 20.8 | 3.5 ± 0.7 | nt | nt | 128.7 |
| zooplankton | 1; 9 | −16.8 | −25.3 ± 3.4 | 34.2 | 4.2 ± 2.3 | nt | nt | 229.5 |
| terrestrial environment | ||||||||
| peat soil | 5; 6 | −26.1 ± 1.9 | −25.3 ± 0.9 | 11.1 ± 3.5 | 0.4 ± 1.5 | −0.9; 9; p > 0.1 | 6.8; 9; p < 0.001 | nt |
| terrestrial vegetation | 9; 9 | −28.7 ± 1.1 | −28.5 ± 1.4 | 16.5 ± 6.7 | 5.3 ± 6.6 | −1.0; 16; p > 0.1 | 5.5; 16; p < 0.001 | 97.8 |
| Arctic hare | 1; 2 | −25.2 | −25.7 ± 0.5 | 14.5 | 5.2 ± 2.9 | nt | nt | nt |
| Arctic fox | 1 | −16.7 | 13.5 | nt | nt | nt | ||
In agreement with our first hypothesis, the modelling of the MDN subsidy indicated that an overwhelming majority of both terrestrial and freshwater primary producer and consumer biomass was fuelled by MDN at little auk colony sites (table 1). The mass balance models always yielded values larger than 85% at colony sites. While directly comparable with other studies (e.g. [5,11]), there are uncertainties associated with these estimates due to possible variation in the fractionation of marine nitrogen from guano to its final uptake product, which is dependent on various microbial processes [11]. For example, the process of conversion of uric acid to ammonia involves the volatilization of ammonia, a powerful fractionation process leaving the remaining substrate enriched by approximately 40‰ in δ15N [38]. By contrast, the fractionation of nitrogen during nitrification depletes δ15N of the substrate with about −25‰ [39]. For seston and zooplankton, the uncertainty is higher due to low sample size from colony sites (table 1). Notwithstanding these uncertainties, the data provide strong evidence of a very large MDN subsidy of terrestrial and aquatic ecosystem production at colony sites. This is highlighted by the fact that the proportions of MDN assimilated into freshwater organisms described here (always more than 85%) are much higher than those reported for biota in New Zealand streams related to seabird colonies using directly comparable methods (28 to 38% of biomass generated from MDN) [11]. Our proportions are also much higher than those estimated in MDN subsidy studies of Pacific salmon (e.g. 23 and 25% of marine-derived biomass in aquatic organisms and terrestrial vegetation, respectively; [7]). The higher values compared with other studies probably reflect both the large quantity of the MDN input in little auk colonies and the paucity of other nutrient sources at these high latitudes (76–78 deg. N).
In agreement with our second hypothesis, we found the physical–chemical characteristics of freshwater systems differed significantly between colony and control sites (PERMANOVA F = 8.8, d.f.res = 26; p < 0.01; table 2). Nutrient concentrations were significantly higher at colony sites, the only exception being the marginal significance of phosphate concentrations (GLS: p = 0.07; table 2). Algal biomass was also significantly higher at colony sites—approximately 20-fold for phytoplankton biomass in lentic systems and approximately 10-fold for benthic algal biomass in lotic systems (GLS: p < 0.05; table 2). The nutrient levels and algal biomass observed in the aquatic systems at colony sites are the highest reported for Greenland, where most systems are characterized by nutrient limitation (e.g. [40,41]). Correspondingly, in terrestrial systems, there were significantly higher EVI values (approximately twofold higher) at colony sites (GLS: p < 0.01; table 2). However, contrary to expectations, freshwater consumer taxa richness was lowest at the nutrient enriched, bird-impacted sites (GLS: p < 0.05; table 2; electronic supplementary material, table 3 in supplementary appendix S2).
Table 2.
Terrestrial vegetation, physical–chemical and biotic characteristics of freshwater systems in catchments with and without little auk colonies (colony versus control sites). Values are given as mean ± s.d. PERMANOVA and pairwise GLS test parameters are given to allow comparison of each parameter between colony and control sites. Significant differences are marked in bold and marginal p-values are given in italics. nt = not tested due to lack of replicates. Generally, the GLS tests were conducted for all system types pooled (lakes, ponds streams). However, differences in algal biomasses were tested separately for lentic and lotic systems due to different measurement methods (see Material and methods).
| lentic systems |
lotic systems |
all systems |
GLS test parameters |
||||||
|---|---|---|---|---|---|---|---|---|---|
| colony | control | colony | control | colony | control | all systems (t; d.f.res; p-value) | lentic(t; d.f.res; p-value) | lotic (t; d.f.res; p-value) | |
| water physical–chemical parameters | |||||||||
| PERMANOVA (all physical–chemical parameters) | F = 8.87; d.f.res = 26; p < 0.01 | ||||||||
| pH | 5.7 ± 1.8 | 7.2 ± 1.4 | 5.1 ± 1.4 | 6.9 ± 0.8 | 5.3 ± 1.5 | 7.1 ± 1.1 | −2.59; 30; p < 0.05 | ||
| conductivity (ms cm−2) | 0.1 ± 0.2 | 0.07 ± 0.06 | 0.04 ± 0.03 | 0.08 ± 0.08 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.08; 29; p > 0.1 | ||
| dissolved oxygen (mg l−1) | 13.1 ± 1.6 | 12.0 ± 0.6 | 13.2 ± 1.1 | 12.1 ± 1.5 | 13.2 ± 1.1 | 12.1 ± 1.1 | 2.49; 29; p < 0.05 | ||
| total nitrogen (mg l−1) | 1.5 ± 1.3 | 0.4 ± 0.2 | 1.7 ± 1.6 | 0.3 ± 0.09 | 1.7 ± 1.5 | 0.4 ± 0.2 | 2.35; 32; p < 0.05 | ||
| NO2 + NO3 (mg N l−1) | 0.7 ± 0.8 | 0.05 ± 0.09 | 1.3 ± 1.2 | 0.1 ± 0.09 | 1.1 ± 1.1 | 0.1 ± 0.1 | 3.55; 32; p < 0.01 | ||
| total phosphorus (mg l−1) | 0.2 ± 0.2 | 0.01 ± 0.005 | 0.1 ± 0.1 | 0.007 ± 0.004 | 0.12 ± 0.14 | 0.009 ± 0.005 | 3.3; 32; p < 0.05 | ||
| PO4 (mg l−1) | 0.08 ± 0.1 | 0.01 ± 0.01 | 0.08 ± 0.1 | 0.002 ± 0.001 | 0.088 ± 0.11 | 0.004 ± 0.003 | 1.8; 32; p = 0.07 | ||
| biotic structure | |||||||||
| enhanced vegetation index (EVI) | 0.27 ± 0.07 | 0.16 ± 0.06 | 3.5; 33; p < 0.01 | ||||||
| algal biomass (lentic Chla (µg l−1); lotic Chla (µg cm−2) | 41.5 ± 34.9 | 2.2 ± 0.9 | 3.4 ± 2.8 | 0.4 ± 0.09 | nt | 2.3; 11; p < 0.05 | 3.3; 15; p < 0.01 | ||
| richness of aquatic consumer taxa (no. of taxa) | 1.3 ± 0.5 | 2.7 ± 1.4 | 1 ± 0.6 | 1.8 ± 1.2 | 1.1 ± 0.6 | 2.4 ± 1.3 | −3.29; 31; p < 0.01 | ||
Among the chemical properties of the fresh waters, pH differed significantly with colony sites being more acidic than control sites (GLS: p < 0.05; table 2). This effect appears to be a particular characteristic of little auk colonies, contrasting with findings from Devon Island, Canada, where pools under a northern fulmar (Fulmarus glacialis) colony exhibited increased pH values relative to control sites without birds [21]. In Svalbard, it has been observed that zooplanktivorous seabirds, such as the little auk, promote soil acidification, whereas piscivorous seabirds do not [42].
(b). Changes along a gradient of bird impact
At a broad scale, a decrease in the distance to the nearest little auk colony was associated with an increase in EVI (GLS: t = −2.6 p < 0.05, pseudo r2 = 0.06, n = 29), benthic algae biomass (lotic Chl-a) (GLS: t = −4.7 p < 0.0001, pseudo r2 = 0.07, n = 17) and δ15N of freshwater benthic algae (GLS: t = −5.6 p < 0.00001, pseudo r2 = 0.29, n = 27), whereas the relationship with phytoplankton biomass (lentic Chl-a) was not significant (electronic supplementary material, figure S2). When considering only sites closer than 2500 m from colonies the relationships became more clear: EVI increased strongly with proximity to colony (GLS: t = −5.1 p < 0.001, pseudo r2 = 0.52, n = 27) as did benthic algal biomass (GLS: t = −4.5 p < 0.0001, pseudo r2 = 0.12, n = 15) and benthic algal δ15N (GLS: t = −4.6 p < 0.0001, pseudo r2 = 0.17, n = 21) (electronic supplementary material, figure S2). However, from the scatterplots it is evident that distance to colony is a relatively poor predictor as a site can be proximal to a colony and remain relatively unaffected by MDN.
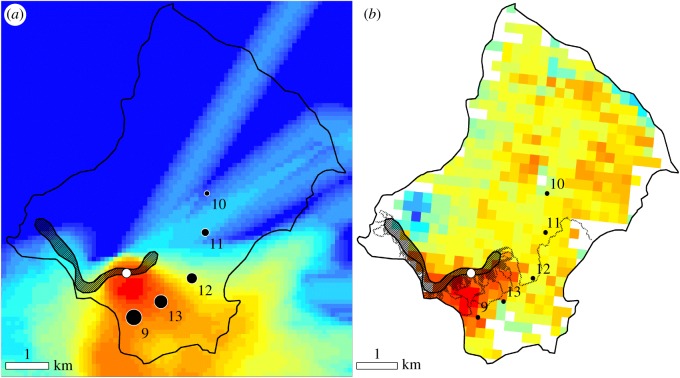
An explanation for this is provided by a case study of Savissivik Island, where, due to GPS tracking, we were in the unique position of being able to relate EVI and δ15N of benthic algae with an estimate of overflight intensity of birds (proxy of bird dropping intensity) (figure 2). Acknowledging that we only have five sample sites on Savissivik Island, these indicate strong, positive correlations between overflight intensity of little auks and both EVI (LM: p < 0.01; r2 = 0.88; n = 5) and δ15N of freshwater benthic algae (LM: p < 0.01; r2 = 0.88; n = 5). Further, benthic algal δ15N and EVI were strongly, positively correlated at the sample sites (LM: p < 0.001; r2 = 0.94; n = 5). The drainage pattern from the colony seemingly also corresponds with the EVI and benthic algal δ15N values at the sample sites (figure 2). Thus, while it is clear that the little auk colony is the main source of the nutrients on Savissivik Island, the relative importance of overflight versus drainage in the spread of nutrients could not be discerned.
Figure 2.
Relationships between flight intensity of little auks, enhanced vegetation index (EVI) and δ15N of freshwater benthic algae at Savissivik Island where GPS tracking of little auks was conducted. (a) Relative flight intensity of little auks (kilometre of track line per square kilometre) on a colour scale from blue (low) to red (high). Coastline depicted as black line, little auk colony as hatched area, and deployment site used during GPS tracking as a white dot. Freshwater sampling sites from Savissivik Island (NOW 9–13) are labelled on the map and the symbol size is scaled according to the benthic algal δ15N value of the site. (b) EVI from MODIS Terra, 28/7–12/8 2015 (33), on a colour scale from blue (low) to red (high). The black, hatched region extending from the little auk colony indicates drainage from the colony, modelled on the basis of a digital elevation model of Savissivik Island (see electronic supplementary material, Methods S1, for details).
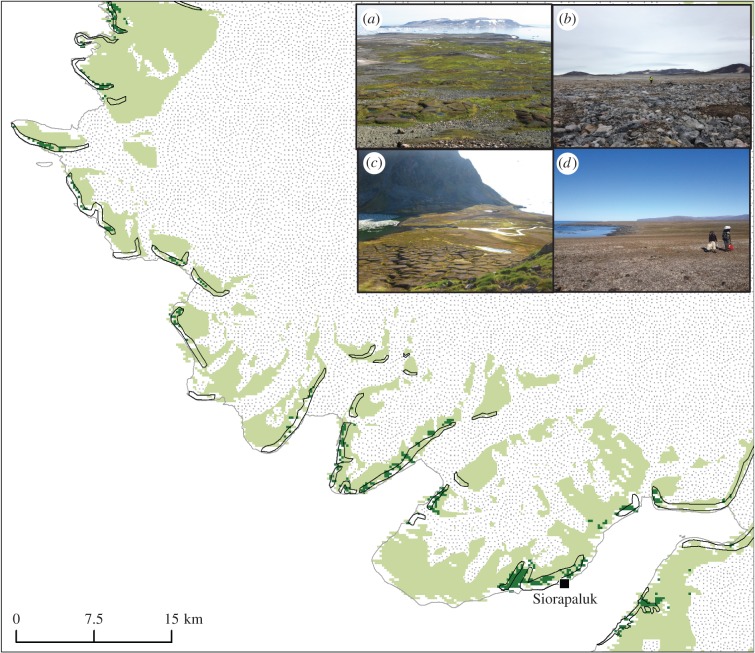
The Savissivik case clearly demonstrates that little auks use distinct flight paths when commuting between their breeding colony and offshore foraging areas, and that local drainage systems transport the nutrients in particular directions. Thus, it is possible to have a low impact site close to a large colony, if the site is located outside the flight path and upstream of the drainage from the colony/flight path. This appears to be the main reason for the inadequacy of distance to colony as a predictor of aquatic and terrestrial primary producer biomass. The EVI evidence suggests that relatively fertile terrestrial areas do exist outside the influence of little auk colonies, primarily in conjunction with meadows. However, EVI values more than 0.25 are almost exclusively found in association with little auk colonies, and terrestrial productivity is clearly elevated over large areas of the Greenlandic coastal forelands of the NOW due to the extensive little auk colonies (figure 3).
Figure 3.
Terrestrial productivity is elevated in little auk colonies. Little auk colonies (black polygons; after (24)) and enhanced vegetation index (EVI) values below 0.25 (light green) and above 0.25 (dark green) in the northern part of the investigation area. The EVI image is from MODIS Terra, 28/7–12/8 2015 (33). Despite many EVI values missing along the coastline due to mixed pixels, the map clearly shows that EVI values above 0.25 are almost exclusively found in association with little auk colonies. In inserts (a–d), landscape photos illustrate the contrasting terrestrial productivity at colony and control sites. (a) Productive landscape in the catchment of the little auk colony on Savissivik Island (site 9); (c) productive landscape in the catchment of the little auk colony at Annikitsoq on the south coast of Cape York (sites 25–32); (b) barren landscape at the control site on Savissivik Island (site 10); (d) barren landscape at the control site close to Booth Sound (site 15–16). See figure 1 and electronic supplementary material, table S2 for exact positions.
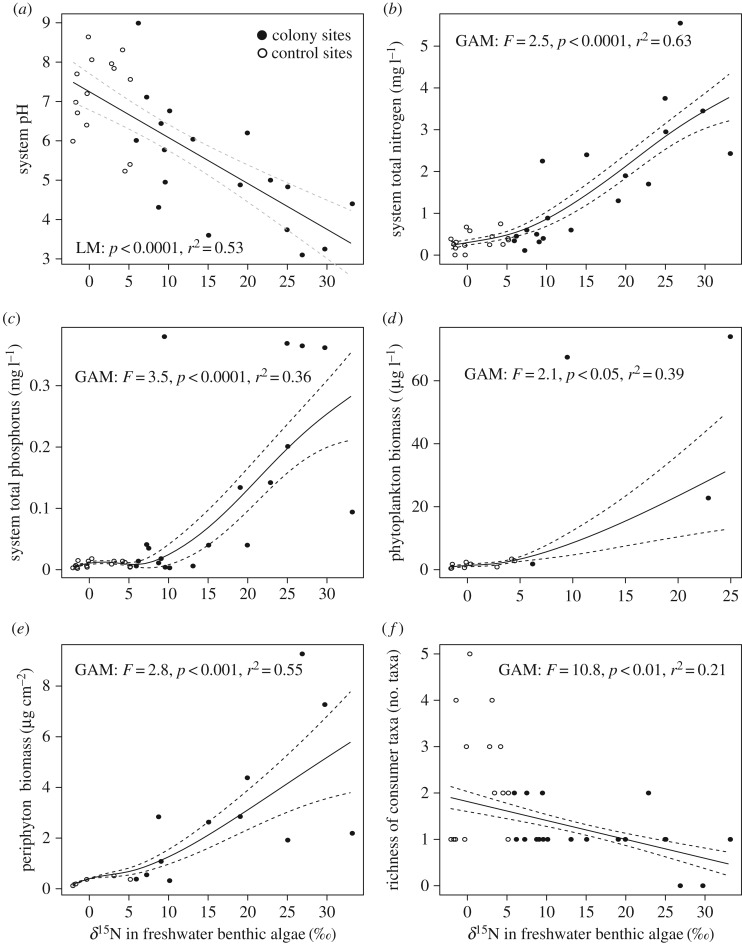
The fact that freshwater benthic algal δ15N values were approximately 15-fold higher at colony sites compared with control sites, decrease with distance to colony, and, in the case of Savissivik island, are tightly coupled to overflight intensity and drainage input from the colony, indicated that it is a robust indicator of the intensity of MDN impact. This is in line with findings in Canadian seabird colonies [12,21] and relationships observed between δ15N of terrestrial vegetation and distance to a salmon stream [7,37] or relative salmon carcass density [37]. Thus, benthic algal δ15N was employed as a proxy of little auk impact to investigate changes of freshwater physical–chemical properties and biotic community structure along a gradient of impact. As δ15N of benthic algae rose there was a significant increase in concentrations of total nitrogen (GAM: F = 2.5, p < 0.0001, r2 = 0.63, n = 34) and phosphorus (GAM: F = 3.5, p < 0.0001, r2 = 0.36, n = 34) and system acidity (LM: p < 0.0001, r2 = 0.53, n = 32) (figure 4). With increasing benthic algal δ15N, algal biomass rose in both lentic (GAM: F = 2.1, p < 0.05, r2 = 0.39) and lotic systems (GAM: F = 2.8, p < 0.001, r2 = 0.55), whereas freshwater consumer taxa richness decreased (GAM: F = 10.8, p < 0.01, r2 = 0.21) (figure 4).
Figure 4.
Changes in environmental and biotic characteristics induced by increasing little auk impact (using δ15N of benthic algae as a proxy of impact). Sampling sites in catchments with little auk colonies are marked with black dots and sites in control areas with open circles. (a) Decrease in pH with increasing bird influence. (b) Increase in total nitrogen concentrations with increasing bird influence. (c) Increase in total phosphorus concentrations with increasing bird influence. (d) Increase in phytoplankton biomass in lentic systems with increasing bird influence. (e) Increase in stream benthic algal biomass with increasing bird influence. (f) Decrease in consumer taxa richness with increasing bird influence. Model details are shown in each panel figure.
(c). Potential drivers of changes in freshwater ecosystems along a gradient of bird impact
We attribute the increase in nutrients to be the main driver of increased algal biomass in lakes and streams (electronic supplementary material, figure S3). In both lotic and lentic systems, the increase in total nitrogen concentrations promoted increased algal biomass (LMs: p < 0.0001, r2 = 0.77, n = 16 and p < 0.0001, r2 = 0.89, n = 11 for lotic and lentic systems, respectively) as did the total phosphorus concentration, which was also strongly related to algal biomass in both the lentic (LM: p < 0.0001, r2 = 0.99, n = 16) and lotic systems (LM: p < 0.0001, r2 = 0.75, n = 11) (electronic supplementary material, figure S3). In nutrient-poor systems, increased nutrient concentrations enhance primary producer biomass, resulting in bottom-up effects [13,43] that increase the abundance and biomass of primary and secondary consumers [4], often creating higher taxonomic richness [44]. This matches our field observations of terrestrial systems where sites located below bird colonies were the most productive and greenest with most observations of foxes, hares, geese and muskoxen, whereas control sites were largely barren (figure 3). However, in the freshwater systems, the enhanced algal biomass with increasing bird impact was associated with decreasing taxonomic richness. The acidification associated with little auk impact is a potential driver as pH was found to be negatively correlated with consumer taxa richness in both lotic (GAM: p < 0.001, r2 = 0.44, n = 17) and lentic systems (LM: p = 0.06, r2 = 0.49, n = 12) (electronic supplementary material, figure S3). Some bird-impacted sites had extremely low pH, down to 3.4, and such low levels cause biodiversity loss in lakes and streams [45,46]. At two colony sites, the harsh environment with low pH values meant that neither fish nor macroinvertebrates were found during the sampling (electronic supplementary material, table S3).
4. Conclusion
In the study region, the approximately 60–70 million breeding little auks [26] alter both terrestrial and freshwater ecosystems by promoting primary and secondary production. While these findings are similar to studies of MDN subsidy produced by migrating Pacific salmon species, the magnitude of the MDN subsidy reported here is much higher and the consequences for freshwater consumers differ significantly. In association with little auk impact on freshwater systems, we found a decrease in species richness of higher consumers and truncated food webs without fish. This highlights the key relevance of the identity of the vector of nutrient subsidies in order to understand and predict ecosystem-wide consequences of engineer species that translocate nutrients between ecosystems.
As the total horizontal extent of breeding colonies is approximately 400 km [24], a significant proportion the coastal forelands around the NOW has been transformed by this single species: the little auk. Similar significant changes in the terrestrial environment related to the presence of seabird colonies have also been reported for islands in Alaska where in total more than 10 million birds nest [10]. Here, the introduction of arctic fox (Vulpes lagopus) on some islands in the late nineteenth century resulted in decreased bird abundance, reducing the nutrient subsidies by seabirds to terrestrial productivity, and consequently the landscape shifted from grasslands to tundra [10]. During the breeding season, little auks depend on lipid-rich copepods species associated with cold water, and consequently it has been suggested that little auk populations will decline in response to the current warming of the Arctic [47,48]. If so, a landscape shift comparable to the one observed in [10] may be expected for the NOW.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Anne Mette Poulsen for manuscript editing, and we wish to extend our warmest thanks for help during fieldwork to the local communities Siorapaluk, Savissivik and Qaanaaq, and, at Thule Air Base, to liaison officer Kim Mikkelsen, and Tony Rønne Pedersen and Erland Søndergaard of Greenland Contractors. We would also like to thank the crew of the ships Minna Martek, Blue Jay and Hot Totty.
Ethics
The procedures used conform to the legal requirements of the country and institutional guidelines.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
I.G.-B., A.M., K.L.J., E.J. and T.A.D. participated in the conception and design of the study. All authors carried out the fieldwork. I.G.-B., T.A.D. and K.L.J. analysed the data. I.G.-B. carried out laboratory work and drafted the manuscript. A.M., K.L.J., F.L., E.J. and T.A.D. helped draft the manuscript. T.A.D. coordinated the study. All authors gave final approval for publication.
Competing interests
We have no competing interests
Funding
This study is part of The North Water Project (NOW.KU.DK) funded by the Velux Foundation, the Villum Foundation and the Carlsberg Foundation of Denmark. E.J. was further supported by the MARS project (Managing Aquatic ecosystems and water Resources under multiple Stress) funded under the 7th EU Framework Programme, Theme 6 (Environment including Climate Change), contract no.: 603378 (http://www.mars-project.eu). I.G.-B. was supported by SNI (Agencia Nacional de Investigación e Innovación, ANII, Uruguay).
References
- 1.Polis GA, Anderson WB, Holt RD. 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Ann. Rev. Ecol. Sys. 28, 289–316. ( 10.2307/2952495) [DOI] [Google Scholar]
- 2.Flecker AS, McIntyre PB, Moore JW, Anderson JT, Taylor BW, Hall RO Jr. 2010. Migratory fishes as material and process subsidies in riverine ecosystems. In Community ecology of stream fishes: concepts, approaches, and techniques (eds Gido KB, Jackson D), pp. 559–592. Bethesda, MD: American Fisheries Society, Symposium. [Google Scholar]
- 3.Jones CG, Lawton JH, Shachak M. 1994. Organisms as ecosystem engineers. Oikos 69, 373–386. ( 10.2307/3545850) [DOI] [Google Scholar]
- 4.Polis GA, Power MA, Huxel GR. 2004. Food webs at the landscape level. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Chaloner DT, Wipfli MS. 2002. Influence of decomposing Pacific salmon carcasses on macroinvertebrate growth and standing stock in southeastern Alaska streams. J. N. Am. Bent. Soc. 21, 430–442. ( 10.2307/1468480) [DOI] [Google Scholar]
- 6.Gende SM, Edwards RT, Willson MF, Wipfli MS. 2002. Pacific salmon in aquatic and terrestrial ecosystems: Pacific salmon subsidize freshwater and terrestrial ecosystems through several pathways, which generates unique management and conservation issues but also provides valuable research opportunities. BioScience 52, 917–928. ( 10.1641/0006-3568(2002)052%5B0917:psiaat%5D2.0.co;2) [DOI] [Google Scholar]
- 7.Koshino Y, Kudo H, Kaeriyama M. 2013. Stable isotope evidence indicates the incorporation into Japanese catchments of marine-derived nutrients transported by spawning Pacific salmon. Freshw. Biol. 58, 1864–1877. ( 10.1111/fwb.12175) [DOI] [Google Scholar]
- 8.Zhang Y, Negishi JN, Richardson JS, Kolodziejczyk R. 2003. Impacts of marine-derived nutrients on stream ecosystem functioning. Proc. R. Soc. Lond. B 270, 2117–2123. ( 10.1098/rspb.2003.2478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field RD, Reynolds JD. 2011. Sea to sky: impacts of residual salmon-derived nutrients on estuarine breeding bird communities. Proc. R. Soc. B 278, 3081–3088. ( 10.1098/rspb.2010.2731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. 2005. Introduced predators transform subarctic islands from grassland to tundra. Science 307, 1959–1961. ( 10.1126/science.1108485) [DOI] [PubMed] [Google Scholar]
- 11.Harding JS, Hawke DJ, Holdaway RN, Winterbourn MJ. 2004. Incorporation of marine-derived nutrients from petrel breeding colonies into stream food webs. Freshw. Biol. 49, 576–586. ( 10.1111/j.1365-2427.2004.01210.x) [DOI] [Google Scholar]
- 12.Michelutti N, Keatley BE, Brimble S, Blais JM, Liu H, Douglas MSV, Mallory ML, Macdonald RW, Smol JP. 2009. Seabird-driven shifts in Arctic pond ecosystems. Proc. R. Soc. B 276, 591–596. ( 10.1098/rspb.2008.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez-Piñero F, Polis GA. 2000. Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81, 3117–3132. ( 10.1890/0012-9658(2000)081%5B3117:BUDOAI%5D2.0.CO;2) [DOI] [Google Scholar]
- 14.Callaham MA Jr, Butt KR, Lowe CN. 2012. Stable isotope evidence for marine-derived avian inputs of nitrogen into soil, vegetation, and earthworms on the isle of Rum, Scotland, UK. Eur. J. Soil Biol. 52, 78–83. ( 10.1016/j.ejsobi.2012.07.004) [DOI] [Google Scholar]
- 15.Hawke DJ, Newman J. 2007. Carbon-13 and nitrogen-15 enrichment in coastal forest foliage from nutrient-poor and seabird enriched sites in southern New Zealand, NZ. New Zealand J. Botany 45, 309–315. ( 10.1080/00288250709509719) [DOI] [Google Scholar]
- 16.Huang T, Sun L, Wang Y, Chu Z, Qin X, Yang L. 2014. Transport of nutrients and contaminants from ocean to island by emperor penguins from Amanda Bay, East Antarctic. Sci. Total Environ. 468–469, 578–583. ( 10.1016/j.scitotenv.2013.08.082) [DOI] [PubMed] [Google Scholar]
- 17.Wainright SC, Haney JC, Kerr C, Golovkin AN, Flint MV. 1998. Utilization of nitrogen derived from seabird guano by terrestrial and marine plants at St Paul, Pribilof Islands, Bering Sea, Alaska. Mar. Biol. 131, 63–71. ( 10.1007/s002270050297) [DOI] [Google Scholar]
- 18.Irick DL, Gu B, Li YC, Inglett PW, Frederick PC, Ross MS, Wright AL, Ewe SML. 2015. Wading bird guano enrichment of soil nutrients in tree islands of the Florida Everglades. Sci. Total Environ. 532, 40–47. ( 10.1016/j.scitotenv.2015.05.097) [DOI] [PubMed] [Google Scholar]
- 19.Markwell TJ, Daugherty CH. 2002. Invertebrate and lizard abundance is greater on seabird-inhabited islands than on seabird-free islands in the Marlborough Sounds, New Zealand. Ecoscience 9, 293–299. ( 10.1080/11956860.2002.11682715) [DOI] [Google Scholar]
- 20.Zmudczyńska-Skarbek K, Balazy P, Kuklinski P. 2015. An assessment of seabird influence on Arctic coastal benthic communities. J. Mar. Syst. 144, 48–56. ( 10.1016/j.jmarsys.2014.11.013) [DOI] [Google Scholar]
- 21.Keatley BE, Douglas MSV, Blais JM, Mallory ML, Smol JP. 2008. Impacts of seabird-derived nutrients on water quality and diatom assemblages from Cape Vera, Devon Island, Canadian High Arctic. Hydrobiologia 621, 191–205. ( 10.1007/s10750-008-9670-z) [DOI] [Google Scholar]
- 22.Barrett RB, Chapdelaine G, Anker-Nilssen T, Mosbech A, Montevecch IWA, Reid J, Veit R. 2006. Seabird numbers and prey consumption in the North Atlantic. ICES J. Mar. Sci. 63, 1145–1158. ( 10.1016/j.icesjms.2006.04.004) [DOI] [Google Scholar]
- 23.Stempniewicz L. 2001. Alle alle Little auk. BWP Update. Birds of the Western Palearctic 3, 175–201. [Google Scholar]
- 24.Boertmann D, Mosbech A. 1998. Distribution of little auk (Alle alle) breeding colonies in Thule District, Northwest Greenland. Polar Biol. 19, 206–210. ( 10.1007/s003000050236) [DOI] [Google Scholar]
- 25.Kampp K, Falk K, Pedersen CE. 2000. Breeding density and population of little auks (Alle alle) in a Northwest Greenland colony. Polar Biol. 23, 517–521. ( 10.1007/s003000000115) [DOI] [Google Scholar]
- 26.Egevang C, Boertmann D, Mosbech A, Tamstorf MP. 2003. Estimating colony area and population size of little auks (Alle alle) at Northumberland Island using aerial images. Polar Biol. 26, 8–13. ( 10.1525/pol.2003.26.2.8) [DOI] [Google Scholar]
- 27.Frandsen M, Fort J, Rigét FF, Galatius A, Mosbech A. 2014. Composition of chick meals from one of the main little auk (Alle alle) breeding colonies in Northwest Greenland. Polar Biol. 37, 1055–1060. ( 10.1007/s00300-014-1491-0) [DOI] [Google Scholar]
- 28.Karnovsky N, Hunt GL. 2002. Estimation of carbon flux to dovekies (Alle alle) in the North Water. Deep-Sea Res. PT II 49, 5117–5130. ( 10.1016/S0967-0645(02)00181-9) [DOI] [Google Scholar]
- 29.Stirling I. 1980. The biological importance of polynyas in the Canadian Arctic. Arctic 33, 303–315. ( 10.14430/arctic2563) [DOI] [Google Scholar]
- 30.Melling H, Gratton Y, Ingram G. 2001. Ocean circulation within the North Water Polynya of Baffin Bay. Atmos. Ocean 39, 301–325. ( 10.1080/07055900.2001.9649683) [DOI] [Google Scholar]
- 31.Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG. 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189. ( 10.1007/s00442-006-0630-x) [DOI] [PubMed] [Google Scholar]
- 32.Balmford A, Green MJB, Murray MG. 1996. Using higher-taxon richness as a surrogate for species richness: I. Regional tests. Proc. R. Soc. Lond. B 263, 1267–1274. ( 10.1098/rspb.1996.0186) [DOI] [Google Scholar]
- 33.Didan K.2015. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250 m SIN Grid V006. (NASA EOSDIS Land Processes DAAC, https://lpdaac.usgs.gov/dataset_discovery/modis/modis_products_table/mod13q1 .
- 34.Huete A, Didan K, Miura T, Rodriguez EP, Gao X, Ferreira LG. 2002. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sensing of Environment 83, 195–213. ( 10.1016/S0034-4257(02)00096-2) [DOI] [Google Scholar]
- 35.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austr. Ecol. 26, 32–46. [Google Scholar]
- 36.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 37.Reimchen TE, Mathewson D, Hocking MD, Moran J, Harris D. 2002. Isotopic evidence for nrichment of salmon- derived nutrients in vegetation, soil and insects in riparian zones in coastal British Columbia. In Nutrients in salmonid ecosystems: sustaining production and biodiversity (ed. Stockner J.), pp. 59–69. Bethesda, MD: American Fisheries Society Symposium. [Google Scholar]
- 38.Mizutani H, Hasegawa H, Wada E. 1986. High nitrogen isotope ratio for soils of seabird rookeries. Biogeochemistry 2, 221–247. ( 10.1007/BF02180160) [DOI] [Google Scholar]
- 39.Robinson D. 2001. δ15N as an integrator of the nitrogen cycle. TREE 16, 153–162. ( 10.1016/S0169-5347(00)02098-X) [DOI] [PubMed] [Google Scholar]
- 40.Anderson NJ, Bennike O, Christoffersen K, Jeppesen E, Markager S, Miller G, Renberg I. 1999. Limnological and palaeolimnological studies of lakes in South-western Greenland. Geol. Greenland Survey Bull. 183, 68–73. [Google Scholar]
- 41.González-Bergonzoni I, Landkildehus F, Meerhoff M, Lauridsen TL, Özkan K, Davidson TA, Mazzeo N, Jeppesen E. 2014. Fish determine macroinvertebrate food webs and assemblage structure in Greenland subarctic streams. Freshw. Biol. 59, 1830–1842. ( 10.1111/fwb.12386) [DOI] [Google Scholar]
- 42.Zwolicki A, Małgorzata K, Zmudczyńska-Skarbek K, Iliszko L, Stempniewicz L. 2013. Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biol. 36, 363–372. ( 10.1007/s00300-012-1265-5) [DOI] [Google Scholar]
- 43.Huryn AD. 1998. Ecosystem-level evidence for top-down and bottom-up control of production in a grassland stream system. Oecologia 115, 173–183. ( 10.1007/s004420050505) [DOI] [PubMed] [Google Scholar]
- 44.VanderMeulen MA, Hudson AJ, Scheiner SM. 2001. Three evolutionary hypotheses for the hump-shaped productivity–diversity curve. Evol. Ecol. Res. 3, 379–392. [Google Scholar]
- 45.Layer K, Hildrew AG, Jenkins GB, Riede J, Rossiter SJ, Townsend CR, Woodward G. 2011. Long-term dynamics of a well-characterised food web: four decades of acidification and recovery in the Broadstone Stream model system. Adv. Ecol. Res. 44, 69–117. ( 10.1016/B978-0-12-374794-5.00002-X) [DOI] [Google Scholar]
- 46.Schindler DW. 1990. Experimental perturbations of whole lakes as tests of hypotheses concerning ecosystem structure and function. Oikos 57, 25–41. ( 10.2307/3565733) [DOI] [Google Scholar]
- 47.Grémillet D, Fort J, Amélineau F, Zakharova E, Le Bot T, Sala E, Gavrilo M. 2015. Arctic warming: nonlinear impacts of sea-ice and glacier melt on seabird foraging. Glob. Change Biol. 21, 1116–1123. ( 10.1111/gcb.12811) [DOI] [PubMed] [Google Scholar]
- 48.Jakubas D, Trudnowska E, Wojczulanis-Jakubas K, Iliszko L, Kidawa D, Darecki M, Błachowiak-Samołyk K, Stempniewicz L. 2013. Foraging closer to the colony leads to faster growth in little auks. MEPS 489, 263–278. ( 10.3354/meps10414) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.