Abstract
Understanding how humans and other animals learn to perform an act from seeing it done has been a major challenge in the study of social learning. To determine whether this ability is based on ‘true imitation’, many studies have applied the two-action experimental paradigm, examining whether subjects learn to perform the specific action demonstrated to them. Here, we show that the insights gained from animals' success in two-action experiments may be limited, and that a better understanding is achieved by monitoring subjects' entire behavioural repertoire. Hand-reared house sparrows that followed a model of a mother demonstrator were successful in learning to find seeds hidden under a leaf, using the action demonstrated by the mother (either pushing the leaf or pecking it). However, they also produced behaviours that had not been demonstrated but were nevertheless related to the demonstrated act. This finding suggests that while the learners were clearly influenced by the demonstrator, they did not accurately imitate her. Rather, they used their own behavioural repertoire, gradually fitting it to the demonstrated task solution through trial and error. This process is consistent with recent views on how animals learn to imitate, and may contribute to a unified process-level analysis of social learning mechanisms.
Keywords: social learning, imitation, two-action experiments, trial-and-error learning, social learning mechanisms
1. Introduction
The mechanisms underlying the ability of humans and other animals to learn to perform an act from seeing it done by others have been a hotly debated topic in the study of social learning and a key aspect in understanding advanced cognition [1–4]. To determine whether this ability is based on ‘true imitation’ or on other, simpler social learning mechanisms, many studies have applied the two-action experimental paradigm, in which observers are allowed to watch a demonstrator solving a task in one of two distinct ways [5–10]. The rationale behind this set-up is that the tendency to subsequently solve the task by performing the demonstrated action (rather than by the alternative, non-demonstrated one) reflects the subject's ability to imitate the observed behaviour [11,12].
While two-action experiments yielded positive results in several species [5,7,8], their interpretation have nevertheless been debated, as alternative mechanisms could not always be ruled out [3,13,14]. According to the conventions of this paradigm, in order to prove that an animal is capable of imitating observed behaviours, the logical inference required is of proof by contradiction: alternative mechanisms (such as stimulus enhancement [15] or emulation [13,16]) should be carefully considered, and imitation is inferred only if all other potential explanations could be convincingly rejected [17]. However, achieving this has proven difficult in the past, especially for ecologically relevant behaviours [3], and even when successful, it still does not explain how imitation really works at a mechanistic level [18].
Recent advances in the study of social learning suggest that a shift in focus may be insightful and possibly more productive. First, there is mounting evidence for the role of associative learning in the formation of mirror neurons that couple perception and action, implying that imitation ability is developed gradually through experience [4,12,19]. Thus, trying to study imitation in isolation from other, simpler, mechanisms may not be useful, as other processes may be involved in its gradual shaping. Second, it has recently been suggested that imitation involves a process similar to song-learning in birds, in which a template of an observed behaviour is stored in memory and trial-and-error learning is used to produce a behaviour that matches this template ([18], see also [14]). Note that this type of trial-and-error learning is not reinforced by food but by the success in producing a behaviour that closely matches the template (like in song learning). This view predicts that imitation should not appear instantaneously, but may involve a process of trial-and-error learning. A strict analysis of successful performance in two-action experiments can therefore be misleading as it may fail to provide evidence for this trial-and-error process.
Here, we suggest and demonstrate an alternative approach. We used a variant of a two-action experiment to expose hand-reared house sparrows to one of two distinct actions that allow them to obtain seeds hidden under a leaf: either pushing the leaf or pecking it. However, rather than restricting our attention to the question of whether or not the subjects in each experimental group had learned the demonstrated act, we hypothesized that careful monitoring of their entire behavioural repertoire as they interact with the task, might provide evidence regarding how they had learned to perform these actions. To that aim, we conducted a detailed step-by-step analysis of the course of development of independent task solving, including both successful and unsuccessful as well as demonstrated and non-demonstrated behaviours. The focus of this approach is not to test whether the observed type of learning falls under the traditional definition of imitation but to reveal how imitation or imitation-like behaviours may develop through a trial-and-error process.
2. Material and methods
(a). Hand-rearing and experimental set-up
During the spring of 2013, we hand-reared two cohorts of 12 house sparrow (Passer domesticus) nestlings originating from the house sparrow breeding colony in I. Meier Segal's Garden for Zoological Research, Tel-Aviv University. The young sparrows were imprinted on a stuffed female sparrow that served as their artificial parent and as a model for social learning (hereafter: the ‘mother’). Hand-rearing and imprinting procedures followed previous studies in our research group [20–22] and are described in detail in the electronic supplementary material.
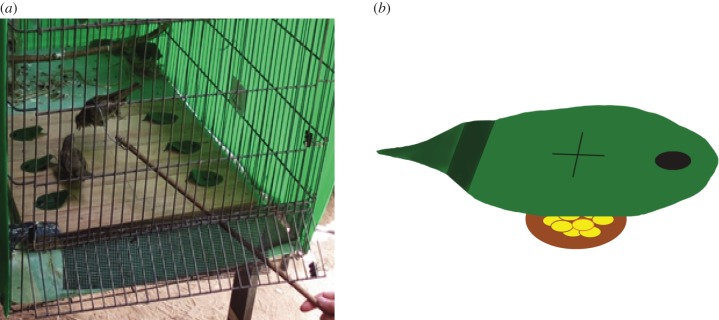
The experiment took place on a wooden grid with two columns of three foraging wells, containing 10 millet seeds each, and covered with artificial leaves attached to the grid with thumbtacks (figure 1). To reach the seeds, the ‘mother’ demonstrated one of two alternative actions: pushing the leaf's edge by inserting the beak below it or pecking through an X shape slit at the centre of the leaf (figure 1 and further details below).
Figure 1.
Experimental set-up. (a) A hand-reared fledgling (on the left) that was imprinted on a ‘mother’ (on the right), following her on the foraging grid during one of the training sessions. (b) A schematic illustration of one of the artificial leaves comprising the task. Note the X-shaped slit located in the middle of the leaf (right above the well), through which the pecking demonstration was done, and the folding of the leaf tip that made the pushing behaviour easier. (Online version in colour.)
(b). Training and test procedure
Fledglings were assigned to one of two experimental groups while controlling for body mass and nest of origin in a randomized block design. The groups differed in the action demonstrated by the ‘mother’—pushing versus pecking, forming a two-action experiment. We did not run a control group without a ‘mother’, because (i) fledglings of this age would be unlikely to approach the grid without a mother and (ii) this control was not necessary given the goals of our study (see Results and Discussion).
In both groups, during each demonstration, the ‘mother’ pecked at the seeds a few times (applying either the pushing or the pecking demonstration, depending on experimental group), pulled back, waited a few seconds—providing the fledgling with the opportunity to perform the action by itself, and then repeated the demonstrated action again (unless it was clear to the experimenter that all seeds in the well had already been consumed). In both groups, the ‘mother’ also pecked once at the leaf edge at the beginning of each demonstration, to reduce the possible effect of local enhancement (thus actions were different but differences in action locations were minimized). Short videos depicting the different demonstrations are provided in the electronic supplementary material.
The training procedure of both groups comprised 10 training sessions: four on days 1 and 2, and two additional sessions in the morning of day 3. One hour of food deprivation preceded each pair of training sessions. Each training session (the presentation of a new foraging grid) lasted 3 min, or until the seeds in all six foraging wells had been consumed (whichever occurred first). During the session, the ‘mother’ moved between the leaves, and occasionally returned to the same leaf if some of the seeds that it covered had not been consumed, thus providing additional learning opportunities. At the end of the training phase, the fledglings of both groups were tested in three successive sessions, during which the foraging grid was presented in the absence of the ‘mother’.
(c) Behavioural and data analyses
All training and test sessions were videorecorded to allow a step-by-step analysis of individual experience and behavioural preferences. Each interaction that the fledglings conducted with a leaf was classified according to its social context (joining the ‘mother’ versus performing independent actions), success in solving the task (successful versus unproductive actions) and action type.
Success in solving the task was defined according to the fledgling's ability to reach the interior of the well (which presumably allows them to consume the seeds located in it). Successful actions were classified as either: (i) successful pecking, in which the fledglings pecked through the slit, or as (ii) successful pushing, in which the fledglings pushed the beak and head below the leaf's surface and pecked inside the well in this position. Unproductive actions were divided into four distinct categories: (i) leaf peck attempt—pecks that were directed towards the leaf's surface, but not the slit itself. (ii) Beak insertion—partial insertion of the beak underneath the leaf surface without reaching the interior of the well. (iii) Grabbing leaf's side—grabbing the edge of the leaf's side (along its wider part) with the beak, as well as lifting or pulling it. (iv) Grabbing leaf's tip—grabbing the edge of the leaf's tip, lifting, pushing or pulling while holding it with the beak, or some combination of these possibilities.
For each action performance, two data measurements were taken: action initiation (marking only the beginning of the action performed), and number of beak movements invested during action performance. While the statistical analysis of the two measures reveals similar patterns, the analysis presented in this manuscript is based on the number of beak movements invested, which serves as a more conservative measure, as it is less affected by constant switching between action types. The analysis of joining events during the training phase, however, was based on the number of action initiations, as we were specifically interested in the effect of the number of times the fledglings joined the mother's demonstration (hence, the initiation of joining), and not of the number of beak movements invested following the onset of such joining.
Of the 24 fledglings that participated in the experiment, only 18 fledglings were sufficiently active during the test phase and thus provided data for our final analysis: 10 from the ‘pushing demonstration group’ (five in each cohort), and eight from the ‘pecking demonstration group’ (six and two in the first and second cohorts, respectively).
Statistical analysis was conducted in JMP version 10 and R v. 3.2.4. Differences between experimental treatments were tested using linear models with treatment group and cohort as factors, and number or proportion of actions as dependent variables (after square-root transformation for count data and arcsine-square-root transformation for proportions). The same data were also analysed using permutation-based factorial ANOVA models with 10 000 unrestricted permutations (which is independent of assumptions regarding the distribution of our data). The results of this analysis did not differ from those of our linear models, and are provided in the electronic supplementary material (electronic supplementary material, table S3). Explanations of further statistical analysis reported in the electronic supplementary material are provided therein.
3. Results
(a). Successful performance of demonstrated behaviours
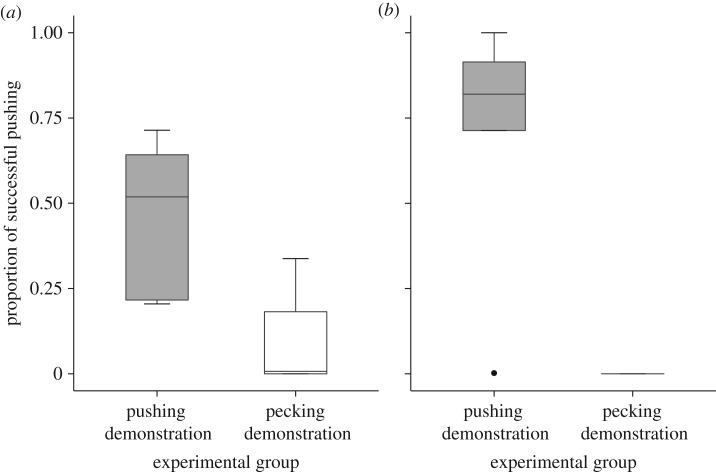
Analysis of the sparrows' performance during the test phase, revealed a significant effect of the two-action demonstration. When tested after the training period, and without the ‘mother’, individuals of the ‘pushing demonstration’ group used a much higher proportion of pushing behaviour to solve the task than individuals of the ‘pecking demonstration’ (figure 2, table 1 model A; electronic supplementary material, figure S1a). This difference between experimental groups in the performance of successful actions was mainly derived from the effect of the pushing demonstration: birds of both experimental groups did not differ in the amount of pecking they performed, but did differ in the amount of pushing (electronic supplementary material, figure S1a and table S2 models SF–SG). Similar differences in performance of demonstrated solutions are frequently observed in social learning experiments, and are often attributed to task difficulty and relevance to natural foraging contexts [5–8]. Nevertheless, the significant difference between groups in the proportion of pushing actions suggests that exposure to demonstrator's behaviour increased the probability that the observer would produce the same behaviour, and stands in line with conventional analysis of two-action experiments [12].
Figure 2.
The proportion of successful pushing performed during the test phase (out of the total number of successful actions performed by a fledgling) in the ‘pushing demonstration’ group (grey) and ‘pecking demonstration’ group (white). (a) Fledglings raised in the first cohort (pushing demonstration group: n = 5, pecking demonstration group: n = 6). (b) Fledglings raised in the second cohort (pushing demonstration group: n = 5, pecking demonstration group: n = 2). Data are represented as median ± quantiles. Note that as there were only two types of successful actions performed, the proportion of pushing is a relative measure, reciprocal to the proportion of pecking.
Table 1.
Analysis of action performance in the test phase. Linear models exploring the effect of experimental group and cohort on the fledglings' performance of different action types during the test phase. Model A refers to successful action performance, whereas models B–C refer to the performance of unproductive attempts. All models included intercept (not shown) and were based on N = 18 birds. All analyses performed on transformed data.
| model | response | effects | d.f. | likelihood ratio χ2 | p |
|---|---|---|---|---|---|
| A | pushing proportion (out of successful action performances) | group | 1 | 13.478 | 0.0002 |
| cohort | 1 | 0.029 | 0.866 | ||
| interaction | 1 | 1.819 | 0.177 | ||
| B | unproductive attempts (total) | group | 1 | 9.856 | 0.002 |
| cohort | 1 | 12.909 | 0.0003 | ||
| interaction | 1 | 1.599 | 0.206 | ||
| C | grabbing leaf tip | group | 1 | 12.635 | 0.0004 |
| cohort | 1 | 11.897 | 0.0006 | ||
| interaction | 1 | 4.835 | 0.028 |
(b). Performance of unproductive actions during the test phase
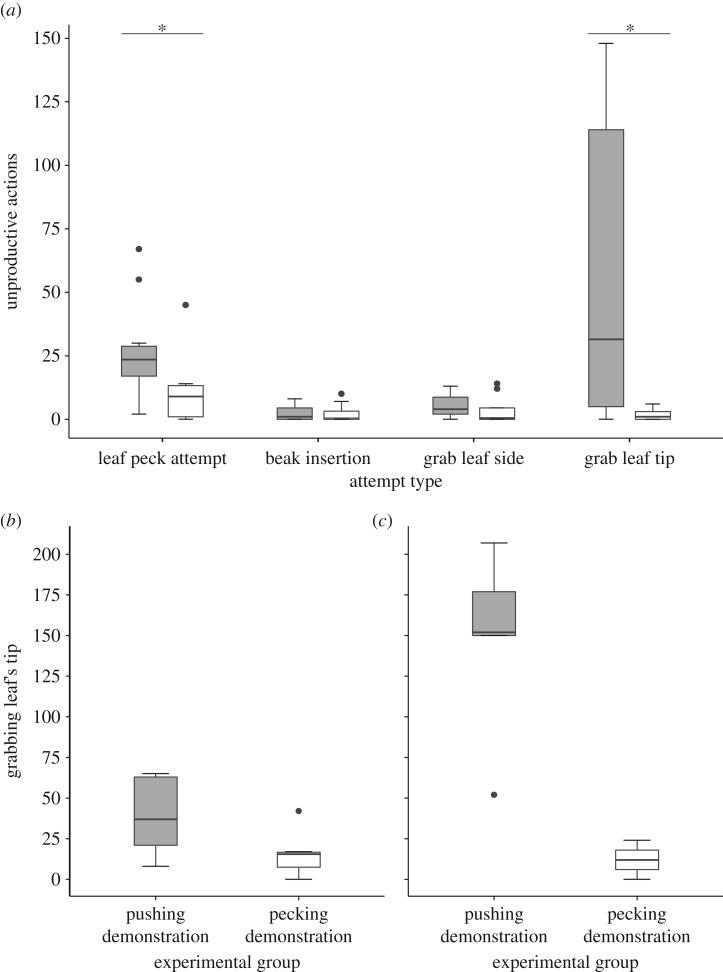
Our analysis of the fledglings' behaviour during the test phase revealed that alongside their performance of successful actions, the fledglings also produced a range of unproductive actions that involved interacting with the leaves, but without successfully gaining access to the location of the seeds. Importantly, birds in the pushing demonstration group were much more likely to produce such unproductive actions (figure 3a, table 1 model B), the most common of which was ‘grabbing-leaf-tip’, a behaviour expressed by grabbing the tip of the leaf with the beak, and then holding and pressing it, lifting, pulling and pushing it in different directions, or a combination of these possibilities. This type of behaviour was seldom displayed by the birds exposed to the pecking demonstration, and was by far the most common unproductive behaviour produced by the pushing demonstration group (figure 3; a short video presenting an example of this behaviour is available in the electronic supplementary material). This difference between treatment groups was highly significant (table 1 model C), and is somewhat surprising because ‘grabbing-leaf-tip’ was never demonstrated by the ‘mother’ and could not be demonstrated even by mistake (a stuffed bird cannot perform this behaviour with its immobile beak). Nonetheless, producing this behaviour makes sense if, after observing the pushing demonstration, the sparrows tried to use actions from their natural behavioural repertoire in order to produce the observed behaviour or its consequences. Grabbing and manipulating small objects with the beak is quite natural for young fledglings. In fact, it is quite probable that if the artificial leaves had not been attached to the surface with thumbtacks (figure 1), this behaviour could have been effective in exposing the seeds.
Figure 3.
Unproductive actions performed by fledglings of the ‘pushing demonstration’ group (grey) and ‘pecking demonstration’ group (white) in the test phase. (a) The distribution of different types of unproductive actions. (b) The amount of grabbing of the leaf tip performed by fledglings of the first cohort. (c) The amount of grabbing performed by fledglings of the second cohort. Sample sizes for the pushing and pecking groups of the first and second cohorts were 5 versus 6 and 5 versus 2, respectively. Data are represented as median ± quantiles.
Unproductive actions were also more common when solving the task was more difficult, which is highly consistent with a trial-and-error process. Among birds of the pushing-mother group, both unproductive actions in general and ‘grabbing-leaf-tip’ in particular were more common among birds of the second cohort, for which we had made the task a little more difficult (figure 3b,c, table 1 models B–C). This was done by slightly flattening the leaves after we suspected that their uplifted tip in the first cohort had allowed the birds to perceive the hidden seeds. Another relevant observation is that unproductive pecking was performed more frequently by birds of the pushing-mother group (figure 3a), the group for which it was more difficult to learn the demonstrated act (see section (a) above). Thus, facing a more difficult task or trying to copy the more difficult demonstration (i.e. pushing), increased the number of unproductive attempts.
(c). Actions performed during the training phase
We also looked at the behaviour of the fledglings during the training period when they followed the ‘mother’. Although fledglings of both groups followed the ‘mother’ equally frequently (no difference in the number of joining events, electronic supplementary material, table S1 model SA), those of the pecking group showed a sharper increase in performance of independent actions during training, and most of these actions were in the form of successful pecking (electronic supplementary material, figure S2). In contrast, fledglings of the pushing demonstration learned to rely on the ‘mother’ and scrounge on her food finding by running swiftly to the leaf and pushing their heads underneath it when the ‘mother’ started the pushing demonstration. Most of them, especially those of the second cohort, did not perform many independent actions during training (electronic supplementary material, figure S1b). Recall that during the test phase, when the same fledglings faced the task without a demonstrator, they did attempt to imitate the demonstrated action or to produce its consequences, based on whatever they remembered from the training phase. While almost all of them were eventually successful (figure 2; electronic supplementary material, figure S1), a trial-and-error process is clearly indicated by their unproductive actions during the test (figure 3; electronic supplementary material, figure S1a).
(d). The effect of accidental demonstration errors
In the pecking-mother group, the mother's pecks through the slit would occasionally cause the leaf to be lifted in a way that allowed the fledgling to insert its head and reach the seeds—similarly to the effect of the pushing demonstration. Such events were relatively rare and accidental (2.1 ± 1.88 events per individual as opposed to 63.3 ± 21.71 joining events per individual in the pushing demonstration). Nevertheless, the fact that they produced leaf movements that were similar to the movements obtained during pushing demonstration, but through a different action pattern (as the ‘mother’ pecked through the slit rather than pushed and touched the leaf edge), allowed us to examine whether the fledglings paid attention only to the movements of the leaf or also to the actions of the ‘mother’. Analysis of these data revealed that for birds of the pecking group, the number of accidental leaf-lifting events during training was not correlated with the tendency to use pushing or to produce the ‘grab-leaf-tip’ copying errors during the test (pushing: rs = 0.366, p = 0.373; ‘grab-leaf-tip’: rs = 0.210, p = 0.617; N = 8 in both cases), but was significantly correlated with the number of times they attempted to insert the beak under the leaf without reaching the well's interior (rs = 0.88, p = 0.004, N = 8). Thus, leaf-lifting events led to beak insertions rather than to grabbing or pushing behaviours, which means that leaf movement alone cannot explain how the birds of the pushing demonstration learned to push. Rather, they probably did so by paying attention to the actions of the ‘mother’, or at least to the location of the contact area between her beak and the leaf.
4. Discussion
In this study, we have shown that young sparrows following a mother model learned to solve a foraging task using the actions demonstrated by the ‘mother’ (either pushing the leaf or pecking it): when tested independently, fledglings in our two experimental groups differed significantly in their tendency to successfully perform the different actions. Thus, similar to the findings of other two-action experiments (e.g. [5–8,23,24]), our results are consistent with the possibility that the fledglings were imitating the mother's actions. However, our results further suggest that success-level analysis of two-action experiments may fail to identify trial-and-error processes that can be involved when animals learn to perform an act from seeing it done by others [18]. Alongside their performance of successful actions, the sparrows in our experiment also conducted many unproductive actions that were not demonstrated to them, but were nevertheless directed towards the leaves. Both the amount and distribution of these actions varied between our experimental groups, suggesting that they, too, were the result of differences in demonstration. Overall, these findings suggest that the sparrows engaged in a trial-and-error learning process, in which they gradually fitted their own behavioural repertoire to demonstrated task solutions.
It is important to note that learning to imitate through a trial-and-error process [18] is not necessarily inconsistent with the common approach that emphasizes the distinction between different social learning mechanisms, such as local enhancement, stimulus enhancement, emulation and imitation [13]. The two approaches can be combined. First, adding information on trial-and-error copying may help to identify which of these mechanisms is at work (or whether some of them work together). For example, without the evidence of inaccurate copying of the pushing demonstration in our study, the main effect of our two-action experiment (figure 2) could have been explained by instantaneous imitation and/or simpler mechanisms. However, adding the evidence of inaccurate copying makes instantaneous imitation highly unlikely. Similarly, the above-mentioned leaf-lifting events during the pecking demonstration had no apparent effect on the main results (i.e. on how the birds eventually solved the task), but the fact that such events led to different trial-and-error actions than those induced by the pushing demonstration, suggests that the sparrows did not merely emulate the movement of the leaf but also paid attention to some aspects of the mother's behaviour. Note that we cannot determine whether these aspects were the actual actions of the ‘mother’, or merely the location of the contact area between her beak and the leaf, or a combination of both. As a result, in this study, we cannot determine whether the process of learning to ‘imitate’ through trial-and-error learning was more consistent with traditional imitation or with trial-and-error refinement of local enhancement (see also reference [6]).
Second, the idea that animals try to match their behaviour to a template in memory that had been previously learned from observation [18] may be relevant to all forms of social learning, not only to imitation. Accordingly, different social learning mechanisms may be viewed as part of a continuum, and differ mainly in what has been learned from observation and represented by such templates. For example, if the template merely represents the location where other individuals found food, then matching the produced behaviour to the template may be viewed as local enhancement. If the template represents how other individuals handle an object, but without further details, then matching would be consistent with stimulus enhancement. If the template represents specific movements of an object, but not the movements of the demonstrator, then trying to produce behaviours that move the object in a way that matches the memorized template may be viewed as emulation. Finally, as suggested by Galef [18], and in line with recent views [4], the process may be viewed as imitation when the template directly represents the behaviour of the demonstrator, and repeated matching of behaviour to the template (i.e. of action to perception) may lead to the formation of mirror neurons [4].
This unified approach to social learning mechanisms makes it easy to see why, in practice, it may be difficult to distinguish between different mechanisms, especially when the template contains a combination of the above-mentioned components [25]. This unified approach also offers a causal link between social attention (data acquisition and input mechanisms [26,27]) and social learning: the extent to which humans and other animals attend to social stimuli and the resulting quality of the template that they can construct in memory, establishes the limits for what they are able to learn through social learning.
5. Conclusion
By shifting the focus from successful to unsuccessful behaviours in a two-action social learning experiment, we were able to provide evidence of a trial-and-error process that may explain how animals learn to perform an act from seeing it done by others. Our results are consistent with recent views that stress the role of individual learning and social attention in the development of imitation and other social learning skills [22,25–32], and may contribute to a unified process-level analysis of social learning mechanisms.
Supplementary Material
Acknowledgements
We thank Oren Kolodny for fruitful discussions, Shira Landman, Sigal Avraham and Gal Shalev for laboratory and technical assistance, Eli Geffen and Alex Slavenko for their help with statistical analysis and Will Hoppitt, Oren Kolodny and Yosef Prat for comments on the manuscript.
Ethics
This study was carried out under animal care permit no. L-12-028 of Tel-Aviv University.
Authors' contributions
N.T. and A.L. designed the experiment and wrote the manuscript. N.T. conducted the experiment and analysed the data.
Competing interests
We have no competing interests.
Funding
Funding for this experiment was provided by the Israel Science Foundation grant no. 1312/11.
References
- 1.Hoppitt W, Laland K. 2013. Social learning: an introduction to mechanisms, methods and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Shettleworth SJ. 2010. Cognition, evolution, and behavior, 2nd edn, i–xiii, 1–700 pp. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Byrne RW. 2002. Imitation of novel complex actions: what does the evidence from animals mean? Adv. Stud. Behav. 31, 77–105. ( 10.1016/S0065-3454(02)80006-7) [DOI] [Google Scholar]
- 4.Cook R, Bird G, Catmur C, Press C, Heyes C. 2014. Mirror neurons: from origin to function. Behav. Brain Sci. 37, 177–192. ( 10.1017/S0140525X13000903) [DOI] [PubMed] [Google Scholar]
- 5.Akins CK, Zentall TR. 1996. Imitative learning in male Japanese quail (Coturnix japonica) using the two-action method. J. Comp. Psychol. 110, 316–320. ( 10.1037/0735-7036.110.3.316) [DOI] [PubMed] [Google Scholar]
- 6.Aplin LM, Sheldon BC, Morand-Ferron J. 2013. Milk bottles revisited: social learning and individual variation in the blue tit, Cyanistes caeruleus. Anim. Behav. 85, 3–10. ( 10.1016/j.anbehav.2013.03.009) [DOI] [Google Scholar]
- 7.Fawcett TW, Skinner AMJ, Goldsmith AR. 2002. A test of imitative learning in starlings using a two-action method with an enhanced ghost control. Anim. Behav. 64, 547–556. ( 10.1006/anbe.2002.3092) [DOI] [Google Scholar]
- 8.Voelkl B, Huber L. 2000. True imitation in marmosets. Anim. Behav. 60, 195–202. ( 10.1006/anbe.2000.1457) [DOI] [PubMed] [Google Scholar]
- 9.Zentall TR, Sutton JE, Sherburne LM. 1996. True imitative learning in pigeons. Psychol. Sci. 7, 343–346. ( 10.1111/j.1467-9280.1996.tb00386.x) [DOI] [Google Scholar]
- 10.van de Waal E, Claidiere N, Whiten A. 2015. Wild vervet monkeys copy alternative methods for opening an artificial fruit. Anim. Cogn. 18, 617–627. ( 10.1007/s10071-014-0830-4) [DOI] [PubMed] [Google Scholar]
- 11.Zentall TR. 2001. Imitation in animals: evidence, function, and mechanisms. Cybernet. Syst. 32, 53–96. ( 10.1080/019697201300001812) [DOI] [Google Scholar]
- 12.Heyes CM, Ray ED. 2000. What is the significance of imitation in animals? Adv. Stud. Behav. 29, 215–245. ( 10.1016/S0065-3454(08)60106-0) [DOI] [Google Scholar]
- 13.Hoppitt W, Laland KN. 2008. Social processes influencing learning in animals: a review of the evidence. Adv. Stud. Behav. 38, 105–165. ( 10.1016/s0065-3454(08)00003-x) [DOI] [Google Scholar]
- 14.Byrne RW. 1999. Imitation without intentionality. Using string parsing to copy the organization of behaviour. Anim. Cogn. 2, 63–72. ( 10.1007/s100710050025) [DOI] [Google Scholar]
- 15.Heyes CM. 1994. Social-learning in animals: categories and mechanisms. Biol. Rev. Camb. Phil. Soc. 69, 207–231. ( 10.1111/j.1469-185X.1994.tb01506.x) [DOI] [PubMed] [Google Scholar]
- 16.Custance D, Whiten A, Fredman T. 1999. Social learning of an artificial fruit task in capuchin monkeys (Cebus apella). J. Comp. Psychol. 113, 13–23. ( 10.1037/0735-7036.113.1.13) [DOI] [Google Scholar]
- 17.Zentall TR. 2004. Action imitation in birds. Learn. Behav. 32, 15–23. ( 10.3758/BF03196003) [DOI] [PubMed] [Google Scholar]
- 18.Galef BG. 2015. Laboratory studies of imitation/field studies of tradition: towards a synthesis in animal social learning. Behav. Process. 112, 114–119. ( 10.1016/j.beproc.2014.07.008) [DOI] [PubMed] [Google Scholar]
- 19.Oostenbroek J, Suddendorf T, Nielsen M, Redshaw J, Kennedy-Costantini S, Davis J, Clark S, Slaughter V. 2016. Comprehensive longitudinal study challenges the existence of neonatal imitation in humans. Curr Biol. 26, 1334–1338. ( 10.1016/j.cub.2016.03.047) [DOI] [PubMed] [Google Scholar]
- 20.Katsnelson E, Motro U, Feldman MW, Lotem A. 2008. Early experience affects producer–scrounger foraging tendencies in the house sparrow. Anim. Behav. 75, 1465–1472. ( 10.1016/j.anbehav.2007.09.020) [DOI] [Google Scholar]
- 21.Katsnelson E, Motro U, Feldman MW, Lotem A. 2011. Individual-learning ability predicts social-foraging strategy in house sparrows. Proc. R. Soc. B 278, 582–589. ( 10.1098/rspb.2010.1151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truskanov N, Lotem A. 2015. The importance of active search for effective social learning: an experimental test in young passerines. Anim. Behav. 108, 165–173. ( 10.1016/j.anbehav.2015.07.031) [DOI] [Google Scholar]
- 23.Klein ED, Zentall TR. 2003. Imitation and affordance learning by pigeons (Columba livia). J. Comp. Psychol. 117, 414–419. ( 10.1037/0735-7036.117.4.414) [DOI] [PubMed] [Google Scholar]
- 24.Dawson BV, Foss BM. 1965. Observational learning in budgerigars. Anim. Behav. 13, 470 ( 10.1016/0003-3472(65)90108-9) [DOI] [PubMed] [Google Scholar]
- 25.Alem S, Perry CJ, Zhu X, Loukola OJ, Ingraham T, Søvik E, Chittka L. 2016. Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol. 14, e1002564 ( 10.1371/journal.pbio.1002564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotem A, Halpern JY. 2012. Coevolution of learning and data-acquisition mechanisms: a model for cognitive evolution. Phil. Trans. R. Soc. B 367, 2686–2694. ( 10.1098/rstb.2012.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyes C. 2012. What's social about social learning? J. Comp. Psychol. 126, 193–202. ( 10.1037/a0025180) [DOI] [PubMed] [Google Scholar]
- 28.Dawson EH, Avargues-Weber A, Chittka L, Leadbeater E. 2013. Learning by observation emerges from simple associations in an insect model. Curr. Biol. 23, 727–730. ( 10.1016/j.cub.2013.03.035) [DOI] [PubMed] [Google Scholar]
- 29.Galef BG. 2013. Imitation and local enhancement: detrimental effects of consensus definitions on analyses of social learning in animals. Behav. Process. 100, 123–130. ( 10.1016/j.beproc.2013.07.026) [DOI] [PubMed] [Google Scholar]
- 30.Leadbeater E. 2015. What evolves in the evolution of social learning? J. Zool. 295, 4–11. ( 10.1111/jzo.12197) [DOI] [Google Scholar]
- 31.Fragaszy DM, Visalberghi E. 2001. Recognizing a swan: socially-biased learning. Psychologia 44, 82–98. [Google Scholar]
- 32.Galef BG, Giraldeau LA. 2001. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15. ( 10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.