Abstract
Autocrine proliferation repressor protein A (AprA) is a protein secreted by Dictyostelium discoideum cells. Although there is very little sequence similarity between AprA and any human protein, AprA has a predicted structural similarity to the human protein dipeptidyl peptidase IV (DPPIV). AprA is a chemorepellent for Dictyostelium cells, and DPPIV is a chemorepellent for neutrophils. This led us to investigate if AprA and DPPIV have additional functional similarities. We find that like AprA, DPPIV is a chemorepellent for, and inhibits the proliferation of, D. discoideum cells, and that AprA binds some DPPIV binding partners such as fibronectin. Conversely, rAprA has DPPIV‐like protease activity. These results indicate a functional similarity between two eukaryotic chemorepellent proteins with very little sequence similarity, and emphasize the usefulness of using a predicted protein structure to search a protein structure database, in addition to searching for proteins with similar sequences.
Keywords: AprA, DPPIV, structure similarity, functional similarity, protease, chemorepellent
Introduction
AprA is a secreted Dictyostelium discoideum protein that inhibits the proliferation of D. discoideum cells.1, 2 AprA functions in conjunction with another secreted protein called CfaD, which also inhibits the proliferation of D. discoideum cells.3 Loss of the AprA or CfaD proteins results in cells that proliferate faster and reach a higher density than wild‐type cells.2, 3 The addition of recombinant AprA (rAprA) or CfaD (rCfaD) to wild‐type cells causes a significant reduction in proliferation.3, 4 In addition to its ability to inhibit D. discoideum cell proliferation, AprA causes chemorepulsion of D. discoideum cells.1 Cells at the edge of wild‐type colonies move away from the dense colony center while cells at the edge of an aprA− colony form a tight edge with little movement outward.5 Both wild type and aprA− cells move away from a source of rAprA.1
DPPIV is a human glycoprotein that can be found in both a transmembrane as well as an extracellular soluble form.6 The trans membrane form is expressed on some lymphocytes and epithelial cells and has a number of binding partners including adenosine deaminase, fibronectin, and collagen.7, 8, 9 The soluble form is found in most bodily fluids.7, 9 Both the membrane and soluble forms have serine protease activity, and cleave proteins or peptides with a proline or alanine at the second position of the N‐terminus.6 One of the major functions of DPPIV is the cleavage of glucagon‐like peptide‐1 (GLP‐1), which normally increases the secretion of insulin to promote glucose uptake.10 DPPIV cleavage of GLP‐1 inhibits this process, and inhibitors of DPPIV are used as treatment for type 2 diabetes.10
Protein structure prediction can reveal protein function that was not obvious from sequence analysis. Structure prediction can be extremely accurate.11, 12, 13, 14, 15, 16 Structure based classification can help identify related proteins that have low sequence similarities.17, 18 For instance, lectins, which bind carbohydrates, contain a characteristic structural fold.18 Although the fold is present in many different protein families with proteins that bind carbohydrates, there is little sequence similarity within the fold.18 These studies are evidence that structural and functional properties may be comparable even when sequence similarities are lacking.
The AprA protein has no significant amino acid sequence similarity to human proteins. We previously found that the predicted structure of AprA has similarity to the crystal structure of DPPIV.19 AprA causes chemorepulsion of D. discoideum cells, and we observed that DPPIV causes chemorepulsion of human and mouse neutrophils.1, 19 In this report, we examine whether other functional similarities exist between AprA and DPPIV, and show that AprA has a DPPIV‐like peptidase activity, and that DPPIV can both inhibit the proliferation and induce chemorepulsion of D. discoideum cells. Together, this indicates conserved properties of two eukaryotic chemorepulsion signals with very different amino acid sequences albeit with possibly similar structures.
Results
Like DPPIV, rAprA has protease activity
DPPIV is a serine peptidase, with a characteristic serine protease catalytic triad in the crystal structure,20, 21 and DPPIV cleaves peptides or proteins with a proline or alanine in the second position at the N‐terminus.22, 23 The predicted structure of AprA has a similar serine peptidase catalytic triad (Fig. 1).19 To test whether AprA may have a DPPIV‐like protease activity, conditioned media collected from log‐phase wild type or aprA− cells were assayed for DPPIV activity. Wild type conditioned media had significantly more DPPIV‐like protease activity, with a K m of 720 ± 150 µM and a V max of 20 ± 1 µM/h (mean ± SEM, n = 3), than conditioned media from aprA− cells [Fig. 2(A)]. The subtracted curve (the aprA− values subtracted from the WT values) had a K m of 590 ± 480 µM and a V max of 10 ± 2 µM/h (n = 3). This suggests that AprA may have DPPIV‐like activity, or that AprA may regulate something that has DPPIV‐like peptidase activity.
Figure 1.
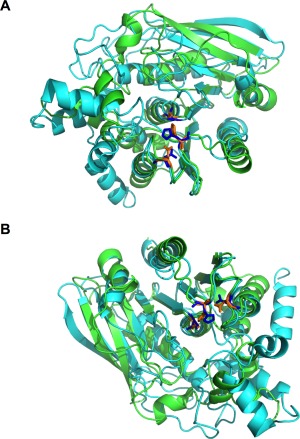
AprA and DPPIV have similar structures. (A,B) The predicted structure of AprA, shown in cyan, is superimposed with the α/β hydrolase domain of DPPIV (Protein Data Bank ID 1J2E), shown in green. The catalytic triad of DPPIV (Asp708, His740, and Ser630) is orange while the predicted catalytic triad of AprA (Asp288, His319, and Ser195) is blue. The predicted structure of AprA did not overlap with the β‐propeller domain of DPPIV. Therefore, the β‐propeller domain was removed for simplicity of the image.
Figure 2.
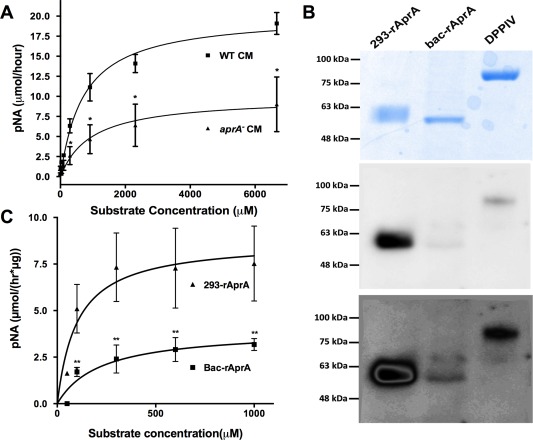
AprA has DPPIV‐like enzymatic activity. (A) Wild‐type or aprA− conditioned media were incubated with various concentrations of H‐Gly‐Pro‐pNA p‐tosylate and the formation of the pNA digestion product was measured. (B) 293‐rAprA, Bac‐rAprA, and rDPPIV were electrophoresed on SDS‐polyacrylamide gels. The upper panel shows Coomassie blue staining of a gel; the middle and lower panels show a corresponding Western blot stained with biotinylated Lens culinaris agglutinin. (C) 293‐rAprA and Bac‐rAprA were incubated with various concentrations of H‐Gly‐Pro‐pNA p‐tosylate as in panel A. Values in A and C are mean ± SEM, n = 3 (n = 7 for Bac‐rAprA in C). Asterisk indicates P < 0.05, **P < 0.01 (t test).
To directly test whether AprA has DPPIV‐like activity, we examined the DPPIV‐like protease activity of recombinant AprA. As glycosylation of DPPIV is required for its peptidase activity,24 we expressed AprA in, and purified recombinant AprA from, HEK 293 cells (293‐rAprA) and E. coli (bac‐rAprA; this is the form of rAprA that we previously observed to both inhibit proliferation and induce chemorepulsion of Dictyostelium cells1, 2). After electrophoresis on SDS‐PAGE gels, 293‐rAprA showed a larger apparent molecular mass than bac‐rAprA [Fig. 2(B), top]. Western blots stained with Lens culinaris agglutinin, which detects sequences containing α‐linked mannose or α‐linked d‐glucose residues,25 indicated that 293‐rAprA and DPPIV are glycosylated, while bac‐rAprA showed much less glycosylation [Fig. 2(B), middle and bottom]. The smeared bac‐rAprA band in the middle panel is two bands, which was revealed by a high contrast view [Fig. 2(B), bottom]. Since there are some glycosylated proteins in E. coli,26, 27, 28 this may be due either to some glycosylation of the bac‐rAprA, or nonspecific staining by the lectin. The apparently lower level of lectin staining of rDPPIV compared to 293‐rAprA may be due to differences in glycosylation content or composition. Both 293‐rAprA and bac‐rAprA had DPPIV‐like peptidase activity [Fig. 2(C)]. The 293‐AprA showed a K m of 100 ± 65 µM and a V max of 9 ± 1 µM/(h × µg) (n = 3), while bac‐rAprA, showed a K m of 220 ± 120 µM and a V max of 4 ± 1 µM/(h × µg) (n = 7). Although the K ms were not significantly different (and were not significantly different from the ‘WT‐aprA−’, activity described above), the bac‐rAprA V max was significantly lower than that of 293‐rAprA (P < 0.05, t test). Together, these data suggest that AprA has DPPIV‐like enzymatic activity, and that glycosylation of AprA potentiates this activity.
Like DPPIV, rAprA can bind fibronectin
DPPIV binds to fibronectin and collagen.7, 9 To determine if AprA also has a similar binding activity, fibronectin, collagen, or 10% serum were coated on plates and the ability of rAprA to bind them was assessed. Bac‐rAprA was able to bind plasma fibronectin [Fig. 3(A)]. The bac‐rAprA also appeared to weakly bind collagen. Although the anti‐AprA antibodies used in this assay recognize both bac‐rAprA and 293‐rAprA on Western blots (data not shown), in parallel assays, 293‐rAprA showed no significant binding to fibronectin or collagen [Fig. 3(B)]. These data suggest that rAprA has some DPPIV‐like binding activity, and that AprA glycosylation is not required for this binding.
Figure 3.
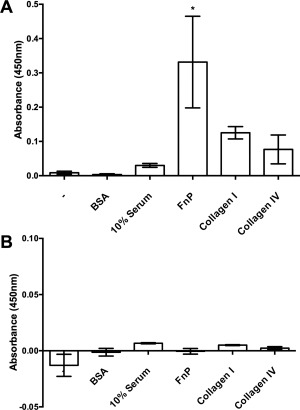
rAprA binds some of the binding partners of DPPIV. Plates were coated with nothing (−), bovine serum albumin (BSA), serum, plasma fibronectin (FnP), collagen I, or collagen IV. After incubation with (A) Bac‐rAprA or (B) 293‐rAprA, and then washing, affinity‐purified anti‐AprA antibodies were used to detect bound rAprA. Values are mean ± SEM, n = 3. * indicates P < 0.05 compared to the (−) control (one‐way ANOVA, Dunnett's test).
rAprA is unable to chemorepulse human neutrophils
DPPIV causes chemorepulsion of human neutrophils.19 To determine if AprA also causes chemorepulsion of human neutrophils, bac‐rAprA or 293‐rAprA were assayed for their ability to cause chemorepulsion. Neither bac‐rAprA nor 293‐rAprA could cause biased movement of human neutrophils [Fig. 4(A)]. Neutrophils are the only cell type that we observed DPPIV to have a chemorepellent effect on,19 and since neutrophils do not proliferate in culture, we were unable to determine if AprA affects neutrophil proliferation. 293‐rAprA could not induce chemorepulsion of D. discoideum cells [Fig. 4(B)], suggesting that glycosylation is not involved in the chemorepulsion activity of AprA.
Figure 4.
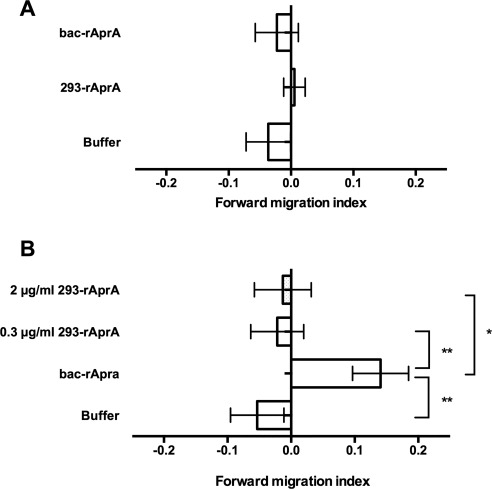
AprA does not induce chemorepulsion of neutrophils. (A) 1.6 µg/mL of rAprA was added to one side of an Insall chamber, and the movement of neutrophils towards or away from the AprA was measured. (B) 0.3 µg/mL of bac‐rAprA or the indicated concentration of 293‐rAprA was added to one side of an Insall chamber, and the movement of D. discoideum cells was measured. Positive values indicate that cells move away from rAprA. Values are mean ± SEM, n = 3. * indicates P < 0.05, **P < 0.01 (t test).
Like AprA, DPPIV can inhibit the proliferation of D. discoideum cells
Bac‐rAprA inhibits the proliferation of D. discoideum cells.4 However, we were unable to detect any significant ability of 293‐rAprA to inhibit (or increase) D. discoideum proliferation. To determine if recombinant DPPIV (rDPPIV) affects the proliferation of D. discoideum cells, rDPPIV was added to wild‐type cells. Like bac‐rAprA and rCfaD, 300 ng/mL rDPPIV inhibited the proliferation of D. discoideum cells [Fig. 5(A)], and this inhibition was observed at rDPPIV concentrations ranging from 1 to 1000 ng/mL [Fig. 5(B)]. To determine if rDPPIV can inhibit proliferation in the absence of endogenous AprA or CfaD, rDPPIV was added to aprA− or cfaD− cells. rDPPIV was able to inhibit the proliferation of both of these strains [Fig. 5(C)]. Together, these data indicate that for unknown reasons, 293‐rAprA is unable to affect D. discoideum proliferation, that rDPPIV can inhibit D. discoideum proliferation, and that this inhibition by rDPPIV does not require AprA or CfaD.
Figure 5.
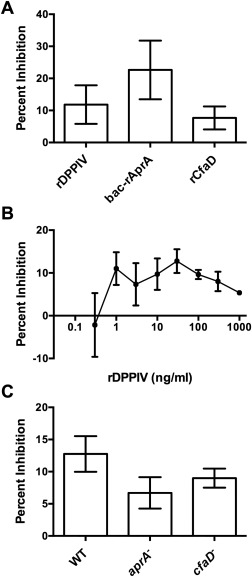
rDPPIV inhibits the proliferation of D. discoideum cells. Buffer, rDPPIV, rAprA, or rCfaD were added to wild‐type D. discoideum cells and the cell density was measured 18 h later. The inhibition of proliferation was then measured in comparison to the proliferation of the buffer control cells. All 3 proteins significantly inhibited proliferation (P < 0.05, t tests). (B) The indicated concentrations of rDPPIV were added to D. discoideum cells and the proliferation inhibition was assayed as in A. rDPPIV concentrations from 1 to 1000 ng/mL significantly inhibited proliferation (P < 0.05, t tests). (C) 300 ng/mL rDPPIV was added to wild‐type, aprA−, and cfaD− cells and proliferation inhibition was measures as in A. rDPPIV significantly inhibited the proliferation of all three cell lines (P < 0.05, t test). Values are mean ± SEM, n = 3.
Like AprA, rDPPIV can induce chemorepulsion of D. discoideum cells
In addition to its ability to inhibit proliferation, AprA causes chemorepulsion of Dictyostelium cells.1 Like bac‐rAprA, rDPPIV also induced chemorepulsion of D. discoideum cells (Fig. 6). These data indicate further functional similarities between AprA and DPPIV.
Figure 6.
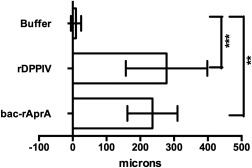
rDPPIV induces chemorepulsion of D. discoideum cells. 2000 ng/mL of rDPPIV or bac‐rAprA was added to one side of an Insall chamber. The movement of D. discoideum cells was observed by video‐microscopy and displacement on the x axis was plotted in microns. Positive values indicate that cells moved away from rDPPIV or rAprA. Values are mean ± SEM, n = 3. ** indicates P < 0.01, *** P < 0.001 (t test).
Discussion
The Dictyostelium discoideum protein AprA and the human protein DPPIV are predicted to share structural similarity, but lack any significant sequence similarity. Here we show that AprA and DPPIV also have functional similarity. AprA appears to be responsible for some but not all of the DPPIV‐like enzymatic activity in D. discoideum conditioned media. The residual activity in aprA− CM may be due to nonspecific proteases, since a variety of proteases are secreted by D. discoideum cells.29, 30 Both bac‐rAprA and 293‐rAprA have DPPIV‐like enzymatic activity, although the 293‐rAprA V max was higher than that of bac‐rAprA, indicating that glycosylation potentiates the enzymatic activity of rAprA. This is partially consistent with observations that glycosylation of DPPIV is required for its enzymatic activity.24 Recombinant DPPIV has a K m of 170 µM and a V max of 8 µM/(h × µg), using the same substrate we used,31 indicating that the K m and V max of 293‐rAprA and DPPIV are quite similar. In addition to having a DPPIV‐like protease activity, like DPPIV bac‐rAprA binds fibronectin and weakly to collagen. Conversely, like AprA, DPPIV both inhibits D. discoideum cell proliferation and induces chemorepulsion of D. discoideum cells. Together, these observations indicate that AprA has some functional properties similar to those of DPPIV, and vice versa.
Despite having DPPIV‐like protease activity, neither bac‐rAprA nor 293‐rAprA could cause chemorepulsion of human neutrophils. We previously observed that DPPIV protease inhibitors can block the chemorepellent activity of DPPIV on neutrophils.19 Together, these observations suggest that either the neutrophil chemorepellent domain site of DPPIV is near its protease active site, but is not part of it, that the chemorepellent active site is not near the protease active site, and is disturbed by the protease inhibitor as an allosteric alteration, or that there is a difference in the recognition sites of the AprA and DPPIV proteases that is not revealed by the H‐Gly‐Pro‐pNA p‐tosylate substrate. Alternatively, DPPIV protease activity could be necessary, but not sufficient, for neutrophil chemorepulsion, with additional protein functions required that are not conserved with AprA.
Bac‐rAprA inhibits the proliferation of, and induces chemorepulsion of, D. discoideum. Despite having a higher V max than bac‐rAprA, the 293‐rAprA was unable to do either of these things to D. discoideum. These observations indicate that the effects of AprA on D. discoideum cells does not involve its DPPIV‐like enzymatic activity, or that as with AprA and DPPIV, there is a difference in the recognition sites of the bac‐rAprA and 293‐rAprA proteases that is caused by the difference in glycosylation, and that this difference is not revealed by the H‐Gly‐Pro‐pNA p‐tosylate substrate. Alternatively, the glycosylation of 293‐rAprA may prevent binding of 293‐rAprA to other proteins, 293‐rAprA‐induced chemorepulsion, and inhibition of proliferation. D. discoideum produces both N‐ and O‐linked glycosylations.32 As such, we expected that a glycosylated rAprA (293‐rAprA) may function more like DPPIV since DPPIV activity is thought to be dependent on its glycosylation.24
Throughout its developmental life cycle, D. discoideum is dependent on cell–cell adhesion proteins for fruiting body formation and cell fate determination.33 Indeed, D. discoideum contains proteins with EGF/Laminin repeat domains and domains for cell adhesion/recognition, such as fibronectin and growth factor receptor domains.33 An additional difference between the two forms of rAprA is their ability to bind fibronectin. The 293‐rAprA, which has higher enzymatic activity, shows no detectable binding. This indicates that the binding of AprA to fibronectin is not dependent on its enzymatic activity. Taken together, our results indicate that AprA and DPPIV have remarkable similarities in their functional properties despite having little sequence similarity, and suggest that the similarities in functional activities do not depend on their similar protease activities. This then suggests the possibility that there is some domain of DPPIV that resembles the receptor–binding site of AprA, and that there may be additional protein regions present on DPPIV but not on AprA that are required to activate the DPPIV receptor.
Materials and Methods
Cell culture
Cells were cultured following Brock et al.34 in HL5 medium (Formedium, Norwich, England) using wild‐type Ax2 cells, aprA− strain DB60T3‐82 and cfaD− strain DB27C‐1.3 Proliferation inhibition and chemorepulsion assays were done as previously described.35
Recombinant DPPIV and AprA
Recombinant DPPIV was purchased from Enzo Life Sciences (Farmingdale, NY). Recombinant AprA was expressed in, and purified from, E. coli as previously described.3 To express recombinant AprA in HEK 293 cells, the DNA encoding the secretion signal sequence of human Serum Amyloid P (SAP) was amplified by PCR using hSAP‐pcDNA3.1− 36 as a template and the primers 5′ ATAGGCGCGCCATGAACAAGCCGCTGC‐3′ and 5′ ATTAAGCTTAGCAAAGGCTTCCAGG‐3′. This was digested with AscI and HindIII and ligated into the corresponding sites of pCMV‐AC‐His (Origene, Rockville, MD) to generate pCMV6‐SAPSec‐His. PCR was then done using a vegetative Ax2 cDNA library and the primers 5′‐CCCAAGCTTACTCCATTGGATGATTATGTC‐3′ and 5′‐CCGCTCGAGTAAAGTTGCAGTTGAACTAGCACTATCACC‐3′ to generate DNA containing the AprA coding region without the AprA secretion signal. After digestion with HindIII and XhoI, the PCR product was ligated into the corresponding sites of pCMV6‐SAPSec‐His to generate pCMV6‐SAP‐AprA‐His. After sequencing to verify the integrity of the insert, the recombinant plasmid was transfected into HEK 293 cells, and recombinant AprA was purified as previously described.37, 38
Western blots and AprA binding
Proteins were electrophoresed on 4–20% tris–glycine gels (Lonza, Rockland, ME) following the manufacturer's instructions. For Western blots, biotinylated Lens culinaris agglutinin (Vector Laboratories, Burlingame, CA) was used following the manufacturer's directions. For rAprA binding, 10% fetal calf serum (VWR, Houston, TX) or 100 µg/mL of bovine collagen I (Santa Cruz Biotechnology, Dallas, TX), human collagen IV (Sigma‐Aldrich, St. Louis, MO) or human plasma fibronectin (Corning, Corning, NY) in PBS buffer were added to wells of a 96‐well tissue culture plate (Corning) and incubated overnight at 4°C. Wells were washed with PBST (3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, 0.05% Tween 20, pH 7.4) three times and blocked with 4% BSA in PBM (20 mM KH2PO4, 10 µM CaCl2, 1 mM MgCl2, pH 6.1 with KOH) for 1 h. Wells were incubated with 10 µg/mL rAprA (+ wells) or 4% BSA (− wells) in PBST for 1 h. Wells were washed with PBST six times and then incubated with 1.14 µg/mL affinity‐purified rabbit anti‐AprA antibodies2 for 1 h, then washed six times with PBST and incubated with 1:5000 HRP‐conjugated donkey antirabbit antibody (Biolegend, San Diego, CA) in PBST for 30 min. Bound antibody was then reacted with 100 µL TMB (Biolegend, San Diego, CA). The reaction was stopped by adding 100 µL of 1N HCl, and reaction products were detected with a SynergyMx plate reader (Biotek, Winooski, VT) at 450 nm. Subtracted values (‘+’ to ‘−’) were calculated.
DPPIV activity assay
For enzymatic activity assays, conditioned media from log phase D. discoideum cells were clarified by centrifugation at 3000×g for 4 min. DPPIV‐like enzymatic activity of conditioned media, buffer (20 mM sodium phosphate, pH 7.4) or rAprA in buffer were measured using pNA substrate (H‐Gly‐Pro‐pNA·p‐tosylate) (Enzo Life Sciences, Farmingdale, NY) for 1 h at room temperature and the reaction product was detected at 410 nm with an Ultrospec 2100 Pro spectrophotometer (for conditioned media) (Amersham Biosciences, Piscataway, NJ) or a SynergyMx plate reader (for recombinant AprA).
Statistics
Statistical analysis was performed using GraphPad Prism 4 software (GraphPad, San Diego, CA). Statistical significance between two groups was determined by t tests or Mann–Whitney tests, or between multiple groups using 1‐way ANOVA with Dunnett's test. Significance was defined as P < 0.05.
Acknowledgments
We thank Dr. Robert Insall for the kind gift of the Insall chambers.
Disclosure: The authors declare no conflicts of interest.
Author statement: A Dictyostelium discoideum protein called AprA and a human protein called DPPIV have essentially no sequence similarity but have a predicted structural similarity. We show here that the two proteins have a remarkable set of functional similarities, including a peptidase activity. This study strongly supports the use of predicted structure comparison to elucidate protein function.
References
- 1. Phillips JE, Gomer RH (2012) A secreted protein is an endogenous chemorepellant in Dictyostelium discoideum . Proc Natl Acad Sci USA 109:10990–10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brock DA, Gomer RH (2005) A secreted factor represses cell proliferation in Dictyostelium. Development 132:4553–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakthavatsalam D, Brock DA, Nikravan NN, Houston KD, Hatton RD, Gomer RH (2008) The secreted Dictyostelium protein CfaD is a chalone. J Cell Sci 121:2473–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choe JM, Bakthavatsalam D, Phillips JE, Gomer RH (2009) Dictyostelium cells bind a secreted autocrine factor that represses cell proliferation. BMC Biochem 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phillips JE, Gomer RH (2010) The ROCO kinase QkgA is necessary for proliferation inhibition by autocrine signals in Dictyostelium discoideum . Eukaryot Cell 9:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walborg EF, Tsuchida S, Weeden DS, Thomas MW, Barrick A, Mcentire KD, Allison JP, Hixson DC (1985) Identification of dipeptidyl peptidase‐Iv as a protein shared by the plasma‐membrane of hepatocytes and liver biomatrix. Exp Cell Res 158:509–518. [DOI] [PubMed] [Google Scholar]
- 7. Cordero OJ, Salgado FJ, Nogueira M (2009) On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immun 58:1725–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu DM, Slaitini L, Gysbers V, Riekhoff AG, Kahne T, Knott HM, De Meester I, Abbott CA, McCaughan GW, Gorrell MD (2011) Soluble CD26/dipeptidyl peptidase IV enhances human lymphocyte proliferation in vitro independent of dipeptidyl peptidase enzyme activity and adenosine deaminase binding. Scand J Immunol 73:102–111. [DOI] [PubMed] [Google Scholar]
- 9. Ohnuma K, Hosono O, Dang NH, Morimoto C (2011) Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem 53:51–84. [DOI] [PubMed] [Google Scholar]
- 10. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ (2011) Secretion of glucagon‐like peptide‐1 (GLP‐1) in type 2 diabetes: what is up, what is down? Diabetologia 54:10–18. [DOI] [PubMed] [Google Scholar]
- 11. Roy A, Kucukural A, Zhang Y (2010) I‐TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy A, Yang J, Zhang Y (2012) COFACTOR: an accurate comparative algorithm for structure‐based protein function annotation. Nucleic Acids Res 40:W471–W477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y (2008) I‐TASSER server for protein 3D structure prediction. BMC Bioinform 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whisstock JC, Lesk AM (2003) Prediction of protein function from protein sequence and structure. Q Rev Biophys 36:307–340. [DOI] [PubMed] [Google Scholar]
- 15. Lee D, Redfern O, Orengo C (2007) Predicting protein function from sequence and structure. Nat Rev Mol Cell Biol 8:995–1005. [DOI] [PubMed] [Google Scholar]
- 16. Kim SH, Shin DH, Choi IG, Schulze‐Gahmen U, Chen S, Kim R (2003) Structure‐based functional inference in structural genomics. J Struct Funct Genomics 4:129–135. [DOI] [PubMed] [Google Scholar]
- 17. Holm L, Sander C (1996) Mapping the protein universe. Science 273:595–603. [DOI] [PubMed] [Google Scholar]
- 18. Chandra NR, Prabu MM, Suguna K, Vijayan M (2001) Structural similarity and functional diversity in proteins containing the legume lectin fold. Protein Eng 14:857–866. [DOI] [PubMed] [Google Scholar]
- 19. Herlihy SE, Pilling D, Maharjan AS, Gomer RH (2013) Dipeptidyl peptidase IV is a human and murine neutrophil chemorepellent. J Immunol 190:6468–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misumi Y, Hayashi Y, Arakawa F, Ikehara Y (1992) Molecular‐cloning and sequence‐analysis of human dipeptidyl peptidase‐Iv, a serine proteinase on the cell‐surface. Biochim Biophys Acta 1131:333–336. [DOI] [PubMed] [Google Scholar]
- 21. Thoma R, Loffler B, Stihle M, Huber W, Ruf A, Hennig M (2003) Structural basis of proline‐specific exopeptidase activity as observed in human dipeptidyl peptidase‐IV. Structure 11:947–959. [DOI] [PubMed] [Google Scholar]
- 22. Proost P, Menten P, Struyf S, Schutyser E, De Meester I, Van Damme J (2000) Cleavage by CD26/dipeptidyl peptidase IV converts the chemokine LD78beta into a most efficient monocyte attractant and CCR1 agonist. Blood 96:1674–1680. [PubMed] [Google Scholar]
- 23. Matteucci E, Giampietro O (2009) Dipeptidyl peptidase‐4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem 16:2943–2951. [DOI] [PubMed] [Google Scholar]
- 24. Fan H, Meng W, Kilian C, Grams S, Reutter W (1997) Domain‐specific N‐glycosylation of the membrane glycoprotein dipeptidylpeptidase IV (CD26) influences its subcellular trafficking, biological stability, enzyme activity and protein folding. Eur J Biochem 246:243–251. [DOI] [PubMed] [Google Scholar]
- 25. Casset F, Hamelryck T, Loris R, Brisson JR, Tellier C, Daothi MH, Wyns L, Poortmans F, Perez S, Imberty A (1995) NMR, molecular modeling, and crystallographic studies of lentil lectin–sucrose interaction. J Biol Chem 270:25619–25628. [DOI] [PubMed] [Google Scholar]
- 26. Benz I, Schmidt MA (2001) Glycosylation with heptose residues mediated by the AAH gene product is essential for adherence of the AIDA‐I adhesin. Mol Microbiol 40:1403–1413. [DOI] [PubMed] [Google Scholar]
- 27. Lindenthal C, Elsinghorst EA (1999) Identification of a glycoprotein produced by enterotoxigenic Escherichia coli . Infect Immun 67:4084–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sherlock O, Dobrindt U, Jensen JB, Munk Vejborg R, Klemm P (2006) Glycosylation of the self‐recognizing Escherichia coli Ag43 autotransporter protein. J Bacteriol 188:1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. North MJ, Franek KJ, Cotter DA (1990) Differential secretion of dictyostelium–discoideum proteinases. J Gen Microbiol 136:827–833. [Google Scholar]
- 30. North MJ, Scott KI, Lockwood BC (1988) Multiple cysteine proteinase forms during the life‐cycle of dictyostelium–discoideum revealed by electrophoretic analysis. Biochem J 254:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aertgeerts K, Ye S, Shi L, Prasad SG, Witmer D, Chi E, Sang BC, Wijnands RA, Webb DR, Swanson RV (2004) N‐linked glycosylation of dipeptidyl peptidase IV (CD26): effects on enzyme activity, homodimer formation, and adenosine deaminase binding. Protein Sci 13:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arya R, Bhattacharya A, Saini KS (2008) Dictyostelium discoideum—a promising expression system for the production of eukaryotic proteins. FASEB J 22:4055–4066. [DOI] [PubMed] [Google Scholar]
- 33. Eichinger L, Pachebat JA, Glockner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B, Kummerfeld S, Madera M, Konfortov BA, Rivero F, Bankier AT, Lehmann R, Hamlin N, Davies R, Gaudet P, Fey P, Pilcher K, Chen G, Saunders D, Sodergren E, Davis P, Kerhornou A, Nie X, Hall N, Anjard C, Hemphill L, Bason N, Farbrother P, Desany B, Just E, Morio T, Rost R, Churcher C, Cooper J, Haydock S, van Driessche N, Cronin A, Goodhead I, Muzny D, Mourier T, Pain A, Lu M, Harper D, Lindsay R, Hauser H, James K, Quiles M, Babu MM, Saito T, Buchrieser C, Wardroper A, Felder M, Thangavelu M, Johnson D, Knights A, Loulseged H, Mungall K, Oliver K, Price C, Quail MA, Urushihara H, Hernandez J, Rabbinowitsch E, Steffen D, Sanders M, Ma J, Kohara Y, Sharp S, Simmonds M, Spiegler S, Tivey A, Sugano S, White B, Walker D, Woodward J, Winckler T, Tanaka Y, Shaulsky G, Schleicher M, Weinstock G, Rosenthal A, Cox EC, Chisholm RL, Gibbs R, Loomis WF, Platzer M, Kay RR, Williams J, Dear PH, Noegel AA, Barrell B, Kuspa A (2005) The genome of the social amoeba Dictyostelium discoideum . Nature 435:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brock DA, Gomer RH (1999) A cell‐counting factor regulating structure size in Dictyostelium. Genes Dev 13:1960–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Herlihy SE, Tang Y, Gomer RH (2013) A Dictyostelium secreted factor requires a PTEN‐like phosphatase to slow proliferation and induce chemorepulsion. PloS One 8:e59365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crawford JR, Pilling D, Gomer RH (2012) FcgammaRI mediates serum amyloid P inhibition of fibrocyte differentiation. J Leukocyte Biol 92:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brock DA, Hatton RD, Giurgiutiu D‐V, Scott B, Ammann R, Gomer RH (2002) The different components of a multisubunit cell number‐counting factor have both unique and overlapping functions. Development 129:3657–3668. [DOI] [PubMed] [Google Scholar]
- 38. Cox N, Pilling D, Gomer RH (2015) DC‐SIGN activation mediates the differential effects of SAP and CRP on the innate immune system and inhibits fibrosis in mice. Proc Natl Acad Sci USA 112:8385–8390. [DOI] [PMC free article] [PubMed] [Google Scholar]


