Abstract
Emerging findings disclose unexpected components of G protein-coupled receptor (GPCR) signaling and cell biology. Select GPCRs exhibit classical signaling that is restricted to cell membranes and newly described persistent signaling that depends on internalization of the GPCR bound to β-arrestins. Termination of non-canonical endosomal signaling requires intraluminal acidification and sophisticated protein trafficking machineries. Recent studies reveal the structural determinants of the trafficking chaperones. This review summarizes advances in GPCR signaling and trafficking with a focus on the parathyroid hormone receptor as prototype, and the actin-SNX27-retromer tubule complex, an endosomal sorting hub responsible for recycling and preservation of cell surface receptors. The findings are integrated into a model of PTHR trafficking with implications for signal transduction, bone growth, and mineral-ion metabolism.
Keywords: GPCR, PTHR, endosome, SNX27, retromer, ASRT complex
The Classical View
The long-accepted paradigm for G protein-coupled receptor (GPCR) activation involves a cyclical model, whereby ligand binding to its cognate receptor promotes transient activation, downstream signaling, followed by inactivation and receptor recycling, setting the stage for another round of stimulation. This process understandably guards cells against excessive stimulation, while resensitization protects cells against prolonged hormone resistance [1]. Receptor inactivation proceeds at the cell surface but is commonly followed by endocytosis [2]. Such internalized receptors can be targeted for degradation or recycled to the cell membrane for another round of stimulation. It was long assumed that internalized GPCRs were inactive [3, 4]. This assumption was predicated both on conceptual grounds and technical limitations. Theoretical arguments against signaling by internalized GPCRs hinged on the fact that receptor phosphorylation and β-arrestin recruitment uncouple the receptor from its associated G proteins, thus terminating signaling. Further, biochemical measurements of GPCR signaling lacked the spatial or temporal resolution to delineate more nuanced aspects of compartmentalized signaling. Such constraints have been overcome by the application of Fluorescence Resonance Energy transfer (FRET)-based sensors that permit real-time observations of signaling and trafficking events in live cells [5–7], and enhanced Bioluminescence Resonance Energy Transfer BRET procedures that permit measurement of GPCR activation and trafficking [8–12].
Employing such contemporary approaches, recent studies now reveal that some internalized GPCRs not only remain active but also exhibit sustained signaling. The functional consequences in some instances display biased responses, where the cell surface signal elicits one set of actions while the signal from internalized receptors exhibits an alternate effect. Emerging evidence demonstrates that signaling by some GPCRs including peptide hormone receptors PTHR [13], thyroid-stimulating receptor (TSHR) [14], glucagon-like peptide 1 receptor (GLP1R) [15], the pituitary adenylate cyclase activating polypeptide (PACAP) type 1 receptor [16], the vasopressin V2R receptor [17], corticotropin-releasing hormone receptor 1 (CRHR1) [18] and others (Table 1) is not restricted to cell membranes but exhibits ligand-biased persistent G protein signaling that depends on internalization of the GPCR bound to β-arrestins. Such β-arrestin-dependent sustained Gs interaction and cAMP signaling has been expanded to monoamine receptors including the β2 adrenergic receptor (β2AR) [7], and dopamine D1R receptor [19]. Persistent signaling of internalized receptor tyrosine kinases epidermal growth factor receptor (EGFR) has also been reported [20–22]. Such prolonged signaling is referred to as non-canonical to distinguish it from the transient activation of receptors residing at the cell surface. Termination of non-canonical endosomal PTHR signaling requires intraluminal acidification and sophisticated protein trafficking machineries, described herein. That said, chemical biological features of diverse ligands engaging a common GPCR can direct biased signaling responses. In the case of the PTHR, for example, PTH and PTHrP both bind the receptor but only PTH elicits transient and sustained signaling, while PTHrP displays only brief receptor activation [23, 24]. Furthermore, semi-synthetic long-acting PTH triggers both short and persistent signaling and physiological responses [25]. A recently described homozygous human PTH mutation, where Arg25 is replaced by Cys [26], cAMP signaling is less prolonged. The chemical basis for these varied responses remains unknown but could be related to pharmacokinetic and elementary chemical differences. Such behavior may extend to other peptide hormone receptors, such as the V2R, where distinct differences in the duration of agonist stimulation have been reported [27].
Table 1.
GPCRs Exhibiting Sustained Signaling
| Receptor | Family | Ligand | G Protein | PDZ Motif | PDZ Adapter | Refs |
|---|---|---|---|---|---|---|
| PTHR | B | parathyroid hormone | Gs, Gq, Gi | -ETVM | NHERF1/2 | [13, 42, 43, 53] |
| S1PR1 | A | sphingosine 1-phosphate | Gs, Gi/Go | -NSSS | RhoGEF (LARG) | [98] |
| β2AR | A | isoproterenol | Gs | -DSLL | NHERF1/2 | [7] |
| TRHR | A | thyrotropin releasing hormone | Gs, Gq/G11 | -QTVL | nNOS | [14] |
| GLP1R | B | glucagon like-peptide | Gs | -ASCS | PDZD2 | [15] |
| PACAPR1 | B | pituitary adenylate cyclase-activating polypeptide | Gs | -NLAT | PICK1 | [16] |
| V2R | A | vasopressin | Gs | -DTSS | unknown | [17] |
| CRHR1 | B | corticotropin-releasing hormone | Gs | -STAV | NHERF3 (PDZK1), MAGI2 | [18] |
| D1R D2R D3R |
A | dopamine | Gs | -QHPT | NHERF1 Spinophilin PICK1 |
[19] |
Abbreviations: PTHR, parathyroid hormone receptor; NHERF1/2/3, Na-H exchange regulator 1, 2 or 3; S1P1R, sphingosine 1 receptor; RhoGEF, Rho guanine nucleotide exchange factor; LARG, Leukemia-associated RhoGEF; β2AR, beta-2 adrenergic receptor; TRHR, thyrotropin-releasing hormone receptor; nNOS, neuronal nitric oxide synthase; GLP1R, glucagon-like peptide-1 receptor; PDZD2, PDZ-domain containing protein-2; PACAPR1, pituitary adenylate cyclase-activating polypeptide type I receptor; PICK1, protein interacting with C kinase-1; V2R, vasopressin V2 receptor; CRHR1, corticotropin-releasing factor receptor 1; PDZK1, PDZ domain-containing protein-1; MAGI2, membrane-associated guanylate kinase, WW and PDZ domain-containing protein 2; D1R, D2R, D3R, dopamine receptor-1, -2, or -3.
To β-arrestin or Not to β-arrestin
In the traditional model, GPCR occupancy initiates conformational changes that stimulate G protein binding that is followed by receptor phosphorylation by G protein-coupled receptor kinases (GRKs) and second messenger-dependent kinases, PKA and PKC (Figure 1). These events, in turn, recruit β-arrestin to the active phosphorylated receptor, thereby uncoupling the GPCR from its associated heterotrimeric G proteins and facilitating association of the receptor with the clathrin and the endocytic machinery. Although this is outlined as the common series of events, important deviations from this scheme are well characterized. Some GPCRs, for instance, are internalized in a manner demonstrably independent of β-arrestin [28–31].
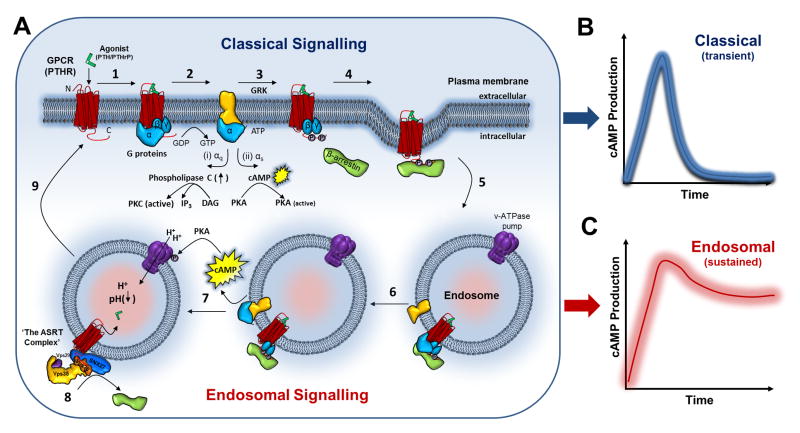
Figure 1. Classical (Brief) vs. Endosomal (Extended) GPCR Signaling.
(A) (1) Binding of agonist ligand (PTH/PTHrP) to PTHR initiates signaling through the recruitment of heterotrimeric G proteins (αβγ). (2) Guanine nucleotide exchange activates the G alpha subunit (α) which transduces parallel downstream signaling cascades, either via (i) PKC (αq) or (ii) PKA (αs) leading to transient signaling at the cell surface (B). (3) Like many, GPCRs, PTHR is then phosphorylated by GRK leading to recruitment of and engagement with β-arrestins. (4) β-arrestin-binding initiates receptor internalization into clathrin-coated pits by interaction with endocytic machinery. (5) PTHR G-protein signaling continues upon entry into early endosomes. (6) Endosomal signaling induces prolonged cAMP responses from ligand-receptor complexes (C). (7) Termination of non-canonical signaling requires PKA driven V-ATPase-mediated intraluminal acidification and recruitment of the ASRT complex. (8) These events coincide with the release of β-arrestin and the ligand from the receptor. (9) The ASRT complex subsequently facilitates post-endocytic recycling of the receptor back to the plasma membrane thereby resensitizing the cell surface.
Several recent findings reveal a far more dynamic and nuanced range of arrestin conformations and interactions than previously appreciated. These discoveries stem from the use of conformationally sensitive fluorescent probes and application of allosteric nanobodies. Receptor-specific patterns of rapid β-arrestin2 conformational changes occur upon GPCR binding [32]. Unexpectedly, β-arrestins remain active following receptor dissociation, suggesting that β-arrestins exert receptor-independent actions before returning to their quiescent conformational state. Related studies revealed that GPCRs impose distinct conformational signatures on β-arrestin [33]. The signatures define spatial and steric elements that could be discerned by introducing the Fluorescein Arsenical Hairpin binder (FlAsH) motif at different sites within β-arrestin2. Further, the magnitude of the fluorescent conformational changes correlated with patterns of receptor trafficking and ERK activation [33]. The stepwise activation of β-arrestin confirms the generally held view that initial contact of β-arrestin with the phosphorylated GPCR guides the receptor to the polar core, with subsequent conformational reorganization that enhances the stability of binding. Moreover, persistence of the active state of β-arrestin after disengaging the bound GPCR would allow for signal amplification by another round of binding of an available receptor [32]. A modified version of the β2AR, where its C-terminus was exchanged for that of the V2R C-terminal tail, maintains the pharmacological properties of the β2AR but displays the prolonged Gs-mediated cAMP signaling previously demonstrated for V2R vasopressin receptor [17, 34]. Remarkably, this β2V2R simultaneously interacts with both β-arrestin and Gs in a so-called megaplex. Single-particle, negative stain electron microscopy revealed that the G protein binds to the receptor transmembrane core. The functional implications of the megaplex architecture permits the β-arrestin bound, internalized GPCR to promote GDP-GTP exchange, thereby activating Gs. This finding explains the mechanism whereby β-arrestin facilitates GPCR internalization without interfering with G protein coupling to the receptor. These findings open the possibility for designing molecules that could selectively stabilize conformationally active or inactive multimolecular receptor states.
ERK1/2 signaling may proceed through GPCR activation pathways as well as independent of G proteins [35]. Nonetheless, a curious paradox surrounds the seemingly contradictory GPCR requirements between in vitro and in vivo ERK1/2 signaling in some instances. Thus, β1-adrenergic signaling in vivo transactivates ERK1/2 through β-arrestin and EGFR to promote cardiac myocytes growth and survival. This action (presumably) entails β1AR internalization. The requirement for receptor endocytosis is questioned, due to the modest extent of β1AR in vitro phosphorylation and sequestration [36, 37]. This inconsistency was recently addressed by von Zastrow and colleagues [38], who demonstrated that β-arrestin can be remotely activated without antecedent accumulation in clathrin-coated pits.
In addition to the four major structural families into which GPCRs are divided [39], the receptors are also grouped into two classes based on the strength of the interaction with β-arrestins and the brief or sustained nature of GPCR internalization. Thus, Class A receptors such as β2AR, μ-opioid, and amine GPCRs have weak interactions and display transient internalization, whereas Class B receptors, including peptide hormone receptors such as V2R and PTHR, exhibit stronger and more stable binding to β-arrestins and undergo sustained endocytosis [40, 41]. For reasons that presently remain unclear, β-arrestins may exert opposite effects on membrane-delimited GPCRs, but nonetheless be required for their persistent signaling upon internalization. In the case of the prototype β2AR, for instance, β-arrestins inhibit or diminish cell surface signaling, whereas they enhance signaling of the PTHR. The PTHR, however, deviates from the classical pattern, wherein it forms a high-affinity complex with PTH in the absence of G proteins [42]. Nonetheless, in both cases, and with other GPCRs exhibiting sustained cAMP signaling, β-arrestin internalizes and traffics to the endosome together with the GPCR, though not in the case of the β2AR. The regulation and termination of endosomal signaling is discussed later on. Family B GPCRs like the PTHR exhibit bifurcating signaling pathways, one involving adenylyl cyclase and using cAMP as a second messenger, the other including phospholipase C and using calcium and inositol phosphates as second messengers. Interestingly, sustained PTHR signaling is only of the cAMP pathway and not calcium pathway [43].
Boston Two-Step
A distinguishing feature of Family B GPCRs is the large N-terminal extracellular domain defined by 3 conserved disulfide bonds. This class of receptors transduces signals from a variety of peptide hormones including secretin, PTH, calcitonin, corticotropin releasing factor, glucagon, and others [44]. Binding is thought to involve an initial fast-binding step of the C-terminus of the hormone to the extracellular receptor domain, followed by a conformational change and a slower but higher affinity engagement with the N-terminus of the receptor and activation [45]. A recent report challenges this two-step mechanism and shows that activation of the glucagon receptor (GCGR) and the glucagon-like peptide 1 receptor (GLP1R) require the extracellular receptor domain, whereas for other tested Family B GPCRs activation depends on mass action [46]. The requirement for tethering of PTHR, Corticotropin-releasing hormone receptor 1 (CRF1R), and Proteinase-activated receptor 1 (PAR1) suggests a binding mechanism consistent with the two-step activation process, whereas binding to the extracellular domain of GCGR and GLP1R directly affects the interaction between extracellular and transmembrane receptor domains that facilitate the conformational changes and receptor activation. The propensity of Family B GPCRs to exhibit sustained signaling when occupied by full agonists likely derives from their ability to form stable complexes with β-arrestins, hence promoting the assembly of the newly defined GPCR–β-arrestin–G protein complex [34].
If GPCR phosphorylation and internalization terminate classical, membrane-delimited GPCR signaling and action, what then regulates and terminates signaling from the endosomal compartment?
What’s in it for Me?
Both transient and sustained GPCR signaling employ cAMP as a second messenger. In this age of self-obsession, we need to ask why then are two types of signaling needed and what do they achieve? The answer to this question comes from observations that sustained signaling is involved in transcriptional activation. In the case of PTHR, brief, membrane-associated activation likely causes rapid mobilization of calcium from the bone surface and, perhaps, of calcium absorption by kidney tubules. Acute effects to diminish renal phosphate excretion are also probable [47, 48]. Persistent PTH signaling seems to be a requisite for skeletal growth [49] and, we surmise for induction of Cyp27B1 and stimulation of 1,25-dihydroxyvitamin D synthesis. The anabolic action of PTH on bone, at least in mice, displays further regulatory input insofar as it requires tonic β2AR activity. Ablation of β2AR suppressed expression of PTH-target genes involved in bone turnover. Further evidence that sustained PTHR signaling is required for normal skeletal development comes from studies showing the interfering with receptors internalized to endosomes in osteoblasts reduces bone mineralization and causes major skeletal deficits [50], as discussed below.
In the case of β2AR sustained cAMP signaling, phosphoenolpyruvate carboxykinase 1, encoded by PCK1, is a particularly strongly isoproterenol-induced gene [51]. Blockage of endocytosis markedly reduced transcriptional activation of a subset of β2AR-sensitive genes. These results establish that although some genes are induced by cell surface receptor activation others require GPCR internalization.
Termination of Endosomal GPCR Signaling
Recently, major progress has been made towards understanding the mechanisms responsible for termination of endosomal GPCR signaling [52]. In the case of PTHR, two seminal studies uncovered distinct but overlapping mechanisms that correspond with termination of PTHR-mediated cAMP signaling at the endosome [43, 53]. First, by following fluorescently tagged PTHR in endosomes, Feinstein et al. observed that termination of cAMP production from the receptor coincides with (1) displacement of β-arrestin from PTH-PTHR complexes and; (2) parallel recruitment of retromer, a component of the multiprotein endosomal sorting Actin-Sorting Nexin 27-Retromer Tubule (ASRT) complex [43]. Shortly thereafter, this mechanism was revised prompted by the observation that treatment of cells with Bafilomycin A1, an inhibitor of vacuolar ATPase (v-ATPAse) proton pumps, prolongs PTH-PTHR complex association as well as the duration of cAMP signaling [53]. According to the updated model, termination of cAMP production from PTHR requires sequential activation of a negative feedback loop involving intracellular cAMP, PKA and v-ATPases that collectively drive intraluminal acidification that, in turn, promotes dissociation of PTH-PTHR complexes as it progresses along the endocytic pathway. It is as yet unclear whether these mechanisms work cooperatively or independently; or universally apply to other GPCRs. What is clear is that both of these events correspond with the dissociation of PTH-PTHR complexes and of cAMP-production, thereby releasing the ligand from the receptor for ensuing hand-off to post-endocytic sorting machinery (Figure 1).
The implication from these comments is that sustained signaling involves obligate receptor internalization to endosomes. However, in at least one example, the bile acid GPCR, TGR5, is neither internalized nor interacts with β-arrestins but nonetheless exhibits sustained cAMP signaling [54]. Instead, TGR5 signals from plasma membrane lipid rafts that facilitate transactivation of EGFR and ERK1/2 stimulation. These findings imply that cAMP originating from different sources [18] and the spatiotemporal corralling of cAMP signaling [55] also condition the duration of receptor activity.
Out and About
As recounted below, internalized GPCRs embark on a long and tortuous itinerary. What is the endocytic pathway and the intracellular machinery that sorts internalized receptors within and between cytoplasmic organelles and redirects some back to the plasma membrane? Upon sequestration in clathrin-coated pits GPCRs are trafficked with or without β-arrestin to endosomes. Family A receptors, which includes receptors for visual pigments and a wide variety of small molecules, neurotransmitters (e.g. β2AR), generally exhibit weak interaction with β-arrestins and recycle promptly to the plasma membrane. An obvious exception here is the Family-A V2R peptide receptor that exhibits prolonged endosomal signaling [17]. The ligands for Family B receptors are polypeptide hormones. These receptors, such as the PTHR, display strong interactions with β-arrestins and are internalized together to endosomes. Endosomes or, more correctly, the endosomal network, is a highly elaborated and dynamic tubulovesicular network that controls, and through which, protein sorting and processing proceeds. Endosomal compartments mature from early-to-late endosomes that are characterized by the recruitment and presence of the small GTPase Rab5 for early endosomes, Rab7 by late endosomes, and Rab4 or 11 for recycling endosomes [56] (Figure 2). Maturation of endosomes and trafficking of the GPCR through the network involves acidification of the endosome lumen. These processes are critically at play for sustained PTHR signaling and its subsequent recycling.
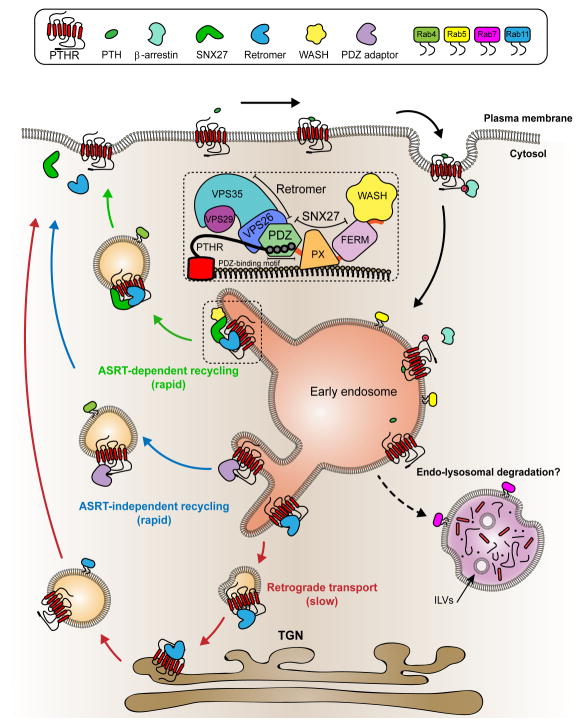
Figure 2. Alternative Modes of GPCR Trafficking Along the Endocytic Network.
Upon ligand-induced activation GPCRs are internalized into early endosomes for sorting. At the early endosome, GPCRs containing C-terminal PDZ binding motifs are recognized by compatible PDZ-adaptor proteins such as SNX27, which works in conjunction with retromer and the WASH complex (Inset). Following sorting, receptors are packaged into recycling tubules which emanate and bud from the membranes of early endosomes. These recycling intermediaries transport GPCRs back to the plasma membrane leading to resensitization of the cell surface. Alternatively, GPCRs marked for degradation are sequestered into intraluminal vesicles (ILVs) of multivesicular endosomes that fuse with lysosomes resulting in receptor proteolysis and down-regulation at the cell surface. Membrane retrieval of endocytosed PTHR occurs via three divergent trafficking itineraries: (i) ASRT-mediated endosome-to-plasma membrane recycling (green); (ii) ASRT-independent endosome-to-PM recycling (blue) or (iii) retrograde endosome-to-TGN-PM recycling (red). This GPCR signaling-trafficking cycle is superimposed by the recruitment and functional transition of small Rab GTPases: early endosome (Rab5), late-endosome/lysosome (Rab7) and recycling endosomes (Rab4 and Rab11).
Post-Endocytic Sorting of GPCRs: The PTHR Paradigm
Spatiotemporal sorting of GPCRs into their correct post-endocytic destinations is critical to the duration and magnitude of endosomal signaling as well as the preservation of receptor homeostasis [2]. In general, following signal termination in early endosomes, the inactive receptor is faced with one of two finite destinations: (1) the lysosomal pathway for degradation, leading to down-regulation of the receptor at the plasma membrane or (2) to renewal (‘recycling’) pathways either via the trans-Golgi-Network (TGN) or directly to the plasma membrane, leading to repopulation, and in turn, resensitization of the cell surface receptor. Emerging evidence shows that the fidelity of this process depends on the ability of receptors to be recognized by molecular adaptor chaperones, which link them to ‘cargo sorting’ complexes assembled on the surface of endosomes.
The ASRT Complex
Among the endosomal sorting machineries that participate in the recruitment and recycling of GPCRs, and many other transmembrane proteins (including GASP and ESCRT [2]), the ASRT complex has emerged as a master regulator [57, 58]. The ASRT complex is itself comprised of sorting nexin 27 (SNX27) and retromer, which together with the WASH complex, organize the assembly of tubules on early endosomes, into which cargo proteins are then packaged, and transported back to the plasma membrane via recycling intermediaries [59]. The retromer sub-complex consists of the core vacuolar protein sorting (Vps) proteins Vps26-Vps29-Vps35 that interact with two Bin-Amphiphysin-Rvs (BAR) domain-containing sorting nexins (SNXs). Originally identified as mediating retrograde endosome-to-Golgi retrieval of acid hydrolases via the cation independent-mannose 6-phosphate receptor (CI-MPR) pathway, retromer more recently has been shown to function in endosome-to-plasma membrane recycling of a multitude of membrane proteins in conjunction with SNX27, with β2AR the prototype GPCR [59]. Like other members of the SNX protein family (up to 34 are expressed in humans), SNX27 possesses a lipid-binding phox-homology (PX)-domain that anchors it to the surface of endosomes [60]. It also houses a 4.1/erzin/moesin/radixin (FERM)-like domain that binds cargos containing F-X-N-P-X-Y sequences [61], as well as a PSD95/Dlg/ZO1 (PDZ) domain, a feature that distinguishes it from other SNX proteins. The PDZ domain allows SNX27 to recognize and engage with cargo harboring compatible PDZ binding ligands with the consensus sequence [D/E]-[S/T]-x-Φ (Φ = any hydrophobic residue) that lie encoded within the cytosolic tails of many membrane proteins, including most GPCRs (see also Text Box 1). At the same time, the PDZ domain serves as a docking site for Vps26 which, in turn, stabilizes and enhances SNX27-PDZ cargo interactions [62]. In this way, SNX27 can be viewed a ‘cargo selector’ for the ASRT trafficking complex, simultaneously linking cargoes to retromer by virtue of its PDZ domain.
Box 1. Structural basis for SNX27-cargo interaction.
Recent high-resolution crystal structures afford considerable insights into the structural determinants of SNX27-cargo interactions [62, 76–79]. More recently, the crystal structure of an extended PTHR PDZ-ligand (EEWETVM) in complex with the SNX27-PDZ domain has also been resolved at near atomic resolution (0.95Å) [50]. Several salient structural features including two acidic residues (Glu−5 and Glu−3) that lie upstream of the canonical C-terminal PTHR PDZ binding triplet (TVM) were uncovered and shown to form strong contacts with Arg58 within the PDZ-domain. Similar acidic acid sequences were previously shown to reside upstream of the canonical PDZ ligand of other SNX27-PDZ interacting cargo such as Kir3.3 [77]. Moreover, the significance of these acidic contacts has been unveiled in a recent study [80]. By comparing the SNX27 binding affinities of the native PTHR PDZ-ligand with a series of alanine mutants spanning the extended PDZ ligand by ITC, Clairfeuille et al revealed that these acidic residues not only afford great strength to SNX27 binding but also impart selectivity and specificity to SNX27-PDZ cargo interaction(s). Remarkably, while not observed in all SNX27-PDZ cargos, those lacking these sequence determinants were, instead, found to contain conserved sites of phosphorylation that substitute for acid residues, and enhance SNX27 interactions as confirmed by additional biochemical studies along with the contribution of several other high resolution SNX27-ligand complexes. This seminal contribution has enabled the formulation of a ‘molecular code’ for SNX27-ligand binding that, through bioinformatics, has unveiled more than four hundred ligands predicted to bind to the SNX27-PDZ domain alone, including signaling receptors, membrane transporters and many others [80].
ASRT-mediated Endosome-to-Plasma Membrane Recycling
Like β2AR and other GPCRs, the PTHR contains a PDZ ligand within its far C-terminus. This PDZ ligand assumes a Type I consensus sequence (E-T-V-M) that is compatible with the canonical recognition signature of the SNX27-PDZ domain. Parallel studies recently confirmed this engagement, thereby providing a direct molecular link to the ASRT complex [50, 63]. First, a PDZ-dependent interaction was demonstrated between PTHR and SNX27 by combining coimmunoprecipitation studies in HEK293 cells with isothermal titration calorimetry (ITC), the later titrating a series of native and mutant PTHR-PDZ-ligand peptides against the SNX27-PDZ domain. This association was allosterically enhanced upon association with Vps26, which binds simultaneously to the SNX27-PDZ domain, thereby wiring SNX27 and PTHR to the ASRT complex. These findings were corroborated independently with complementary biochemical assays that showed this interaction to be enhanced upon brief agonist-stimulation (PTH, 5 min). Both studies examined the spatiotemporal relationship between PTHR, SNX27 and retromer by directly visualizing the trafficking and/or recycling of fluorescently and pH-sensitive tagged PTHR variants overexpressed in fixed and live HEK293 cells. As observed for other GPCRs (e.g. β2AR), knockdown of SNX27 or retromer expression by short-interfering hairpin RNAs led to reduced PTHR recycling and/or cell surface availability. Paradoxically, despite receptor down-regulation, loss of either ASRT component coincided with elevated endosomal cAMP-signaling in HEK293 cells [43] and osteoblastic cells derived from the bones of SNX27 knockout [50], and haplodeficient Vps35 mice [64]. Together, these studies infer that the ASRT complex functions as a ‘molecular brake’ to restrict PTHR-signaling at the endosomes. Additional considerations, however, including potential alterations in auxiliary proteins that intersect with the ASRT complex and impinge on receptor entry into endosomal tubules, need also be considered. The protein phosphatase 1 regulatory subunit 14C (PPP1R14C), an inhibitory subunit of PP1 phosphatase, which binds directly to Vps35, for example, was recently shown to promote PTHR-endosomal, but not cell surface, signaling in osteoblastic cells [64]. How PPP1R14C interacts with Vps35 and modulates endosomal PTHR signaling remains poorly understood but may relate to delayed entry into ASRT endosomal tubules, resulting in prolonged endosomal residence time, enhanced intracellular PTHR signaling and eventual misdirection into degradative lysosomes as observed in this study [65]. In fact, this mechanism has been recently shown to underscore the ability of arrestin domain-containing protein 3 (ARRDC3), a member of the β-arrestin family of visual/β-arrestins and Vps26-related proteins [66] to prolong on the endosomal occupancy and associated cAMP-signaling of β2AR [67]. Although future work will be required to appreciate the full complement of proteins that may intersect with the ASRT-signaling-trafficking nexus, it appears that in addition to its canonical function in directing entry of receptors into recycling tubules, the ASRT complex, together with accessory proteins, is capable of integrating endosomal GPCR-signal termination with post-endocytic sorting. Additional proteins may participate in stabilizing GPCRs within the recycling apparatus or facilitating their movement through the ASRT complex. The Golgi-associated protein interacting specifically with Tc10 (also called PIST, GOPC, CAL, or FIG), for instance, stabilizes the β1AR with the TGN, leading to activation of the MAPK pathway [68]. This may be a more general theme of formation, dissolution, and hand-off of GPCRs harboring PDZ ligands as they traverse the endocytic machinery.
Alternative Post-endocytic Pathways of PTHR recycling
The discovery of the aforementioned direct endosome-to-plasma membrane-recycling route is contrary to the classical paradigm of PTHR trafficking, which has long assumed that the receptor transits via the Golgi compartment en route to the plasma membrane. A key question that arises therefore is how many endosomal trafficking itineraries is the receptor capable of following back to the plasma membrane? While we have only just begun to appreciate the spectrum and diversity of endocytic pathways traversed by GPCRs destined for the cell surface, at least two alternative trafficking routes can be envisaged for the PTHR based on recent observations (Figure 2).
SNX27-independent endosome-to-plasma membrane recycling
The first alternative PTHR sorting route is a direct endosome-to-plasma membrane PTHR recycling pathway that is independent of SNX27. This hypothesis is based on the observation that a mutant PTHR PDZ ligand (ETVA), although incapable of binding SNX27, recycles at the same rate as the native receptor in HEK293 cells [63]. Through a series of coimmunoprecipitation and recombinant protein overlay assays, the mutated PTHR was shown, instead, to bind retromer directly via assembly of a ternary complex that involves sequential interaction between PTHR and Vps26, which, in turn, then binds SNX27. Although less common, this scenario would bypass the general requirement for SNX27-PDZ cargo pre-engagement. The fact that the rate of recycling of this PTHR mutant was not appreciably altered when either SNX27 or retromer was lacking implies, however, that this pathway operates through distinct mechanism(s). One possibility is that SNX27 is substituted for by another PDZ-adaptor protein. The N-ethylmaleimide-sensitive factor (NSF), for example, an ATPase which usually functions to drive the disassembly of SNARE fusion complexes [69], has been previously implicated in the endosome-plasma membrane recycling of β2AR [70]. Moreover, NSF contains a PDZ-domain assuming the recognition sequence of [D/E]-[S/T]-[L/V]-x that would accommodate indiscriminant binding to either native (ETVM) or mutant (ETVA) PTHR-PDZ ligands, a position later substantiated by coimmunoprecipitation experiments in the same study [63].
Endosome-to-Golgi-plasma Membrane Recycling
The second alternative transport pathway involves PTHR recycling via the Golgi intermediate. This pathway was previously identified and shown to occur in a phospholipase-D dependent manner in response to prolonged PTH-stimulation (i.e. 30 min) [71], consistent with the slow recycling times reported for this Family B GPCR (~ 1–2 hours for complete recycling) [72] as compared to the more rapid Family A receptors (e.g. β2AR). By mapping the subcellular distribution of exogenously expressed PTHR in osteoblast-like cells (MC3T3), recent work by Xiong et al. not only confirmed the existence of this indirect recycling route, but also suggested that retromer (Vps35) governs this pathway [64]. Of note however, in this study, the PTHR was monitored as a C-terminal fusion protein (PTHR-mCherry), which would occlude its PDZ-recognition motif and thereby its interaction with SNX27. Nevertheless, PTHR Golgi-to-plasma membrane translocation was shown to coincide with the recruitment of retromer/Vps35, which bound PTHR directly in coimmunoprecipitation assays. A structural basis for this engagement was not determined. Further support for a retromer-directed role for this pathway is furnished by the observation that PTHR translocation to the Golgi is reduced when Vps35 expression is suppressed (knockdown by miRNA-Vps35), instead being redistributed into late-endosomes/lysosomes. Together, with the recent studies of Chan et al that observed similar ‘leakage’ of PTH-PTHR complexes into lysosomes when either SNX27 or retromer was lacking [50], these findings support the view that a major function of ASRT complex is to rescue endocytosed receptors away from the lysosomal degradative pathway and redirect their recycling back to the cell surface [6].
It all comes out in the WASH
Over the last few years it has come to light that post-endocytic recycling via the ASRT complex also relies on auxiliary protein complexes that couple it to the underlying cytoskeleton, as well as to facilitate protein-lipid interactions. Recycling by the ASRT complex, for example, depends on the filamentous actin (F-actin) network in conjunction with the Wiskott-Aldrich syndrome protein and SCAR homologue (WASH) complex, which activates Arp2/3-mediated actin polymerization on endosomes [73]. Accordingly, disruption of the actin network by latrunculin A (an inhibitor of actin polymerization) reduces PTHR recycling rates to levels similar to those observed following SNX27 or retromer knockdown [63]. Moreover, FAM21, the largest WASH component, restricts SNX27 to endosomal recycling tubules by modulating local Golgi phosphatidylinositol 4-phosphate [PI(4)P] concentrations, thereby preventing misdirection of SNX27 cargoes to the Golgi apparatus [74]. Curiously, the same phosphoinositide has more recently been shown serve as a control mechanism for cholesterol distribution via the retromer [75]. Exactly how these studies might be extrapolated to endosomal GPCR signaling and trafficking awaits future investigations, yet these examples highlight additional layers of regulation imparted by protein-protein and protein-lipid interactions on internalized cargoes as they navigate their way through the endomembrane system. These interactions are additionally modulated by phosphorylation and, likely, other post-translational modifications.
Concluding Remarks and Future Perspectives
GPCRs transduce a broad spectrum of extracellular signals to intracellular actions. Signaling was thought to be brief, to allow for rapid responses of limited duration to preserve responsivity. New live-cell imaging technologies have revolutionized this view to reveal that some receptors engage in persistent signaling, and that such sustained signaling exerts biological actions that differ from those elicited by transient receptor activation. The perpetuation of GPCR signaling and its termination proceeds in endosomes and involves a host of adaptive protein machinery, exquisite protein sequences and conformational alterations.
A majority of the GPCRs displaying hybrid canonical and non-canonical signaling harbor carboxy-terminal PDZ recognition sequences. Intriguingly, SNX27 possesses a prominent and required PDZ domain that is needed for accelerated GPCR recycling. These findings suggest that the involved GPCRs are handed off, from one PDZ protein to another. Further studies will be needed to determine if this is the case and, if so, how the conformational or adaptive mechanisms are regulated to expedite this activity.
The ASRT complex and endosomal proteins associated with GPCR recycling assemble multimolecular complexes that are formed and then rapidly dissembled. There are likely additional protein components recruited for this process and identifying them will permit a more complete picture of these processes to be developed. As these various elements are identified, with the distinct possibility that unique components are involved with particular receptors or in different cells, the opportunity to develop novel molecules that selectively enhance or interfere with discrete steps in the signaling-trafficking cycle that could be used for experimental studies and, potentially, for clinical therapeutics may arise. Selective targeting of the endosomal GPCR–G protein–ASRT complex may afford more specific treatments with fewer untoward effects than global targeting of cell surface GPCRs.
Although these advances represent important progress in understanding novel aspects of GPCR signaling, significant gaps remain to be bridged and key questions (see outstanding questions box) remain to be resolved. Issues that will likely come to light include in vivo physiological situations and pharmacological interventions, where signaling from internal compartments importantly can help distinguish examples of prolonged signaling at the cell surface from extended signaling arising from endosomes; the spatiotemporal logic of producing a rapidly diffusing messengers (cAMP) at two locations; and the co-trafficking of downstream G proteins, cyclases, in addition to arrestins, and the structural and conformational dynamics by which the protein trafficking hand-offs occur.
OUTSTANDING QUESTIONS BOX.
How many post-endocytic trafficking pathways participate in the sorting and recycling of GPCRs, and what are the nature and identities of the molecular chaperones that operate when SNX27 or the ASRT complex is lacking?
What are the key structural determinants governing GPCR selectivity affinity, and post-endocytic fate? What do sequences upstream of the canonical PDZ binding ligand contribute to the spatiotemporal pattern of trafficking? How does phosphorylation modify the nature of these interactions and GPCR trafficking destinations and behavior?
What is the post-endocytic fate of PTHR upon reaching the end of its functional life cycle?
Can post-endocytic sorting machineries be pharmacologically targeted to improve current GPCR-linked therapeutic strategies? For instance, PTH(1–34) is the only anabolic agent on the market currently approved for the treatment of post-menopausal osteoporosis, but it is expensive and requires daily subcutaneous injection. Therefore exploiting the ability of the ASRT complex to prolong endosomal PTHR signaling may negate the need for daily administration of PTH-based therapy.
Box 2. Final Destination: Post-endocytic fate and degradation of PTHR.
Despite several recent advances in our understanding of the molecular regulation of endosomal PTHR signaling and trafficking, considerably less is known about the fate of the receptor upon reaching the end of its functional life cycle. By analogy with other GPCRs [81], it is generally assumed that the internalized receptor, destined for degradation, is packaged into membrane invaginations that bud inward to form intraluminal vesicles (ILVs). Once formed, these multivesicular endosomes (MVEs) fuse with lysosomes (the cell’s primary degradative center), initiating receptor proteolysis and down-regulation at the cell surface. Several degradative pathways for GPCRs, and other membrane proteins, have been identified, with the ubiquitination and the endosomal sorting complex required for transport (ESCRT) complex pathway, best characterized. However, several GPCRs (e.g. protease-activated receptor 1) are also degraded through ubiquitin-independent mechanisms, instead utilizing adaptor and scaffolding proteins (i.e. GASP1 and ALIX) that mediate sorting into ILVs and link the receptor to ESCRT complexes (I–IV). While direct evidence for PTHR sequestration into ILVs is lacking, a mechanism controlling the final destination and fate of ligand-activated PTHR has been shown to operate through the ubiquitin-proteasome pathway [82]. Remarkably, unlike many other transmembrane proteins destined for degradation along the proteasomal pathway, the addition of ubiquitination to PTHR does not irreversibly mark the receptor for degradation. Rather the ultimate fate of the receptor is dictated by an intricate balance between ubiquitinating and deubiquitinating enzymes, which target PTHR either towards (1) the proteasome for long term down-regulation or (2) rescue the receptor by directing it towards renewal pathways, such as that operated by the ASRT complex. In this way the cell is able to rapidly adapt and fine-tune its ligand responsiveness by dually controlling cell surface PTHR abundance and the fate of the receptor. Such an adaption is advantageous, as it provides an important safeguard against pathological conditions associated with PTH resistance, as observed patients with chronic renal failure.
Box 3. The ASRT complex in Human and Mouse Disease.
Owing to a diverse suite of cargos that depend on the ASRT complex for recycling and preservation [6], it is unsurprising that genetic alteration of a single component corresponds with prominent and pleiotropic pathologies, both in humans and in mice. Insofar, all genetic mutations identified in SNX27 or retromer in humans have been linked to severe neuropathologies including Alzheimer’s disease [83], Parkinson’s disease [84, 85], Down syndrome [86] and infantile epilepsy [87] for recent reviews see [88, 89]. Accordingly, lowered expression of SNX27 or retromer reduces the surface cell levels of several cargo known to influence synaptic neurotransmission (N-methyl-D-aspartate receptors; NMDARs [90] and AMPARs[91], neuronal signaling and excitably (β2AR [90], GIRK2a [92] mGLuR5 [93]), glial cell differentiation (GPCR 17; GPR17 [94]) and, more recently, limiting the distribution of amyloid precursor protein (APP), a precursor linked to the onset and progression of Alzheimer’s disease [95]. Recent assessment of several mouse models has further extended the physiological contribution(s) of the ASRT complex to peripheral systems [96], such as the skeleton [50, 97]. By examining the bones of mice lacking SNX27, Chan et al demonstrated that they were not only smaller compared to their control littermates, but also displayed a sharp loss in bone mass (i.e. severe congenital osteoporosis). Mechanistically, this defect was attributed, in part, to disturbances in the differentiation and function of osteoblasts, which exhibited alterations in PTHR signaling, although there are likely to be additional cargoes involved, such as those that synergize with PTHR anabolic action (e.g. β2AR [49]). In an earlier study, mice possessing at least one functional VPS35 allele were shown to manifest a similar, albeit less serve, low bone mass phenotype [97]. On this occasion, the reduction in bone mass corresponded with dysregulation in bone-resorbing osteoclasts, which were hyper-activated, owing to reduced retromer-mediated recycling of the receptor RANK, a master regulation of osteoclast formation that resides on the surface of osteoclast progenitors (i.e. monocyte/macrophages). More recently, a follow up study by the same group, using specific Vps35 knockout mice in the osteoblast-lineage (osteocalcin-Cre), revealed a mild low bone mass phenotype associated with altered PTH-driven PTHR-signaling in osteoblasts [64]. Consequently, treatment with PTH(1–34) promoted exaggerated PTH-induced anabolic responses during bone remodeling (turnover) which was paralleled by a decrease in the catabolic activity of osteoclasts, resulting in a net elevation in trabecular (primary) bone mass. Together these recent studies extend the role(s) for the ASRT complex to the development and maintenance of the skeleton and highlight the value of using model organisms to exploit the full range of disorders that may be attributed to disturbances in this enigmatic sorting ensemble.
TRENDS BOX.
Some GPCRs exhibit transient as well as sustained signalling following sequestration in endosomes.
GPCRs displaying persistent signalling and accelerated recycling harbor a carboxy-terminal recognition motif that facilitates handoffs between PDZ proteins.
Emerging evidence suggests sustained signalling exerts distinct functional actions.
Post-endocytic sorting machineries assembled on the surface of endosomes link GPCR signalling and trafficking.
GPCRs are capable of following more than one endosomal trafficking itinerary back to the cell surface.
Acknowledgments
The authors acknowledge the assistance of Audrey Chan in preparing illustrations. This work was supported by grants from the National Health and Medical Research Council of Australia (NHMRC) (APP1078280) to NJP and the National Institutes (NIH) (DK105811) to PAF.
GLOSSARY BOX
- Actin-SNX27-Retromer-Tubule (ASRT) complex
A multiprotein trafficking complex comprised of SNX27 and retromer that assembles tubules on endosomes, into which cargo proteins are sorted, and then recycled back to the plasma membrane. It works in conjunction with actin via the WASH complex.
- BRET
in Bioluminescence Resonance Energy Transfer, the donor fluorophore of the FRET pair is replaced with a luciferase, which in the presence of a luciferin substrate excites the acceptor fluorophore as in FRET.
- Endosomal Sorting Complexes Required for Transport (ESCRT) complexes
evolutionary conserved complexes that facilitate the biogenesis of MVEs containing GPCRs, and other membrane proteins, through a process involving sequential recruitment of ESCRT sub-complexes (ESCRT-0/I,-II, -III and –IV) which mediate formation of ILVs, encapsulation of ubiquitinated cargo, deubiquitination and, finally scission of the ILVs.
- Fluorescein Arsenical Hairpin binder (FlAsH)
is a nonfluorescent molecule but becomes highly fluorescent upon binding to a specific sequence consisting of a biarsenical-tetracysteine tag with the form CCXXCC.
- Fluorescence Resonance Energy transfer (FRET)
is an optical technique that measures changes in intra-molecular conformations or protein-protein interactions. It is based on the transfer of conserved energy between two fluorophores with overlapping excitation and emission spectra.
- G protein-coupled receptor (GPCR)
The largest class of 7 transmembrane-spanning receptors that transduce signals ranging from light to growth and hormone action. GPCRs are classified in 4 major families, designated A–D. Family B incorporates peptide hormone receptors including the parathyroid hormone receptor (PTHR).
- Nanobodies
single domain antibody fragments containing the structural and functional properties of heavy-chain dimers antibodies that naturally occur in llamas, dromedaries, and camels (Camelidae) and are devoid of light chains.
- PDZ binding ligand
A short linear motif that resides within the far C-terminus of many transmembrane proteins including GPCRs. It interacts with PDZ-domain-containing adaptor proteins containing compatible consensus sequences.
- Postsynaptic density protein/Drosophila disc large tumor suppressor/Zonula occludens-1 protein (PSD95/Dlg/ZO1 or PDZ) domain
A scaffolding module found in many cytoplasmic adaptor proteins. It serves as a platform for the recruitment and assembly of protein-protein complexes.
- Retromer
An ancient evolutionarily conserved pentameric protein complex comprised of the core vacuolar protein sorting (Vps) proteins Vps26-Vps29-Vps35 that interact with two Bin-Amphiphysin-Rvs (BAR) domain containing SNXs. It forms the major sub-complex of the ASRT trafficking complex.
- Sorting Nexin 27 (SNX27)
A unique PDZ-domain-containing member of the SNX protein family that acts as a ‘cargo selector’ for the ASRT complex. It also possesses PX- and FERM-like- domain(s) that links it to endosomal membranes and associated cargos, respectively.
- Ubiquitination
a posttranslational modification whereby ubiquitin is covalently added to protein substrates by the sequential actions of three ubiquitin enzymes: E1 (ubiquitin activation), E2 (ubiquitin carrying), E3 (ubiquitin ligase). The ubiquitinated substrates are targeted for proteosomal degradation.
- WASH complex
A multimeric protein complex composed of WASH1, strumpellin, SWIP, FAM21 and CCDC53 that activates the actin-related protein 2/3 (Arp2/3) complex which nucleates filamentous actin required to drive the elongation of ASRT-positive tubules from endosomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohan ML, et al. G-protein coupled receptor resensitization-appreciating the balancing act of receptor function. Curr Mol Pharmacol. 2012;5:350–361. [PMC free article] [PubMed] [Google Scholar]
- 2.Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–16. doi: 10.1016/j.ceb.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bünemann M, Hosey MM. G-protein coupled receptor kinases as modulators of G-protein signalling. J Physiol (Lond) 1999;517:5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 5.Vilardaga JP, et al. GPCR and G proteins: drug efficacy and activation in live cells. Mol Endocrinol. 2009;23:590–9. doi: 10.1210/me.2008-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calebiro D, et al. Imaging of persistent cAMP signaling by internalized G protein-coupled receptors. J Mol Endocrinol. 2010;45:1–8. doi: 10.1677/JME-10-0014. [DOI] [PubMed] [Google Scholar]
- 7.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Namkung Y, et al. Monitoring G protein-coupled receptor and β-arrestin trafficking in live cells using enhanced bystander BRET. Nat Commun. 2016;7:12178. doi: 10.1038/ncomms12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiulpakov A, et al. Mutations of vasopressin receptor 2 including novel L312S have differential effects on trafficking. Mol Endocrinol. 2016;30:889–904. doi: 10.1210/me.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoddart LA, et al. Application of BRET to monitor ligand binding to GPCRs. Nature methods. 2015;12:661–3. doi: 10.1038/nmeth.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan TH, et al. Sensitive and high resolution localization and tracking of membrane proteins in live cells with BRET. Traffic. 2012;13:1450–6. doi: 10.1111/j.1600-0854.2012.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szalai B, et al. Improved methodical approach for quantitative BRET analysis of G Protein Coupled Receptor dimerization. PLoS One. 2014;9:e109503. doi: 10.1371/journal.pone.0109503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrandon S, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calebiro D, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuna RS, et al. Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2013;305:E161–170. doi: 10.1152/ajpendo.00551.2012. [DOI] [PubMed] [Google Scholar]
- 16.Merriam LA, et al. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci. 2013;33:4614–22. doi: 10.1523/JNEUROSCI.4999-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinstein TN, et al. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem. 2013;288:27849–60. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inda C, et al. Different cAMP sources are critically involved in G protein-coupled receptor CRHR1 signaling. J Cell Biol. 2016;214:181–195. doi: 10.1083/jcb.201512075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotowski SJ, et al. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–90. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigismund S, et al. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–19. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Brankatschk B, et al. Regulation of the EGF transcriptional response by endocytic sorting. Sci Signal. 2012;5:ra21. doi: 10.1126/scisignal.2002351. [DOI] [PubMed] [Google Scholar]
- 22.Villasenor R, et al. Regulation of EGFR signal transduction by analogue-to-digital conversion in endosomes. eLife. 2015;4 doi: 10.7554/eLife.06156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean T, et al. Altered selectivity of parathyroid Hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazaki M, et al. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci USA. 2008;105:16525–30. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu M, et al. Pharmacodynamic actions of a long-acting PTH analog (LA-PTH) in thyroparathyroidectomized (tptx) rats and normal monkeys. J Bone Miner Res. 2016;31:1405–12. doi: 10.1002/jbmr.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, et al. A homozygous [Cys25]PTH(1–84) mutation that Impairs PTH/PTHrP receptor activation defines a novel form of hypoparathyroidism. J Bone Miner Res. 2015;30:1803–13. doi: 10.1002/jbmr.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favory R, et al. Investigational vasopressin receptor modulators in the pipeline. Expert Opin Investig Drugs. 2009;18:1119–1131. doi: 10.1517/13543780903066764. [DOI] [PubMed] [Google Scholar]
- 28.Paing MM, et al. β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, et al. Agonist-induced internalization of leukotriene B4 receptor 1 requires G-protein-coupled receptor kinase 2 but not arrestins. Mol Pharmacol. 2004;66:377–386. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 30.Van Koppen CJ, Jakobs KH. Arrestin-independent internalization of G protein-coupled receptors. Mol Pharmacol. 2004;66:365–367. doi: 10.1124/mol.104.003822. [DOI] [PubMed] [Google Scholar]
- 31.Giebing G, et al. Arrestin-independent internalization and recycling of the urotensin receptor contribute to long-lasting urotensin II-mediated vasoconstriction. Circ Res. 2005;97:707–15. doi: 10.1161/01.RES.0000184670.58688.9F. [DOI] [PubMed] [Google Scholar]
- 32.Nuber S, et al. β-Arrestin biosensors reveal a rapid, receptor-dependent activation/deactivation cycle. Nature. 2016;531:661–4. doi: 10.1038/nature17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MH, et al. The conformational signature of β-arrestin2 predicts its trafficking and signalling functions. Nature. 2016;531:665–8. doi: 10.1038/nature17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomsen AR, et al. GPCR-G Protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166:907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strungs EG, Luttrell LM. Arrestin-dependent activation of ERK and Src family kinases. Handb Exp Pharmacol. 2014;219:225–257. doi: 10.1007/978-3-642-41199-1_12. [DOI] [PubMed] [Google Scholar]
- 36.Freedman NJ, et al. Phosphorylation and desensitization of the human β1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J Biol Chem. 1995;270:17953–61. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- 37.Shiina T, et al. Interaction with β-arrestin determines the difference in internalization behavor between β1- and β2-adrenergic receptors. J Biol Chem. 2000;275:29082–29090. doi: 10.1074/jbc.M909757199. [DOI] [PubMed] [Google Scholar]
- 38.Eichel K, et al. β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nat Cell Biol. 2016;18:303–310. doi: 10.1038/ncb3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander SP, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. Br J Pharmacol. 2015;172:5744–5869. doi: 10.1111/bph.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oakley RH, et al. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 41.Oakley RH, et al. Differential affinities of visual arrestin, βarrestin1, and βarrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 42.Wehbi VL, et al. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc Natl Acad Sci USA. 2013;110:1530–1535. doi: 10.1073/pnas.1205756110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feinstein TN, et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollenstein K, et al. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014;35:12–22. doi: 10.1016/j.tips.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro M, et al. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc Natl Acad Sci USA. 2005;102:16084–16089. doi: 10.1073/pnas.0503942102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao LH, et al. Differential requirement of the extracellular domain in activation of class B G protein-coupled receptors. J Biol Chem. 2016;291:15119–15130. doi: 10.1074/jbc.M116.726620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okazaki M, et al. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci USA. 2008;105:16525–30. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagai S, et al. Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem. 2011;286:1618–1626. doi: 10.1074/jbc.M110.198416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanyu R, et al. Anabolic action of parathyroid hormone regulated by the β2-adrenergic receptor. Proc. Natl. Acad. Sci. USA. 2012;109:7433–7438. doi: 10.1073/pnas.1109036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan ASM, et al. Sorting Nexin 27 couples PTHR trafficking to retromer for signal regulation in osteoblasts during bone growth. Mol Cell Biol. 2016;27:1367–1382. doi: 10.1091/mbc.E15-12-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol. 2014;10:1061–1065. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irannejad R, et al. Effects of endocytosis on receptor-mediated signaling. Curr Opin Cell Biol. 2015;35:137–43. doi: 10.1016/j.ceb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gidon A, et al. Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nat Chem Biol. 2014;10:707–9. doi: 10.1038/nchembio.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jensen DD, et al. The bile acid receptor TGR5 does not interact with beta-arrestins or traffic to endosomes but transmits sustained signals from plasma membrane rafts. J Biol Chem. 2013;288:22942–60. doi: 10.1074/jbc.M113.455774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caldieri G, Sigismund S. Spatial resolution of cAMP signaling by soluble adenylyl cyclase. J Cell Biol. 2016;214:125–127. doi: 10.1083/jcb.201606123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harbor perspectives in biology. 2014;6:a022616. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberg F, et al. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol. 2013;15:461–71. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeffer SR. A nexus for receptor recycling. Nat Cell Biol. 2013;15:446–8. doi: 10.1038/ncb2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Temkin P, et al. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13:715–21. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teasdale RD, Collins BM. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem J. 2012;441:39–59. doi: 10.1042/BJ20111226. [DOI] [PubMed] [Google Scholar]
- 61.Ghai R, et al. Phosphoinositide binding by the SNX27 FERM domain regulates localisation at the immune synapse of activated T-cells. J Cell Sci. 2014;128:553–565. doi: 10.1242/jcs.158204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallon M, et al. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc Natl Acad Sci USA. 2014;111:E3604–13. doi: 10.1073/pnas.1410552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGarvey JC, et al. Actin-sorting nexin 27 (SNX27)-retromer complex mediates rapid parathyroid hormone receptor recycling. J Biol Chem. 2016;291:10986–1002. doi: 10.1074/jbc.M115.697045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong L, et al. Retromer in osteoblasts interacts with protein phosphatase 1 regulator subunit 14C, terminates parathyroid hormone’s signaling, and promotes its catabolic response. EBioMedicine. 2016;9:45–60. doi: 10.1016/j.ebiom.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiong L, et al. Retromer in osteoblasts interacts with protein phosphatase 1 regulator subunit 14C, terminates parathyroid hormone’s signaling, and promotes its catabolic response. EBioMedicine. 2016;9:45–60. doi: 10.1016/j.ebiom.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang DS, et al. Role of beta-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol. 2014;27:63–71. doi: 10.1016/j.ceb.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian X, et al. The α-Arrestin ARRDC3 regulates the endosomal residence time and intracellular signaling of the β2-adrenergic receptor. J Biol Chem. 2016;291:14510–14525. doi: 10.1074/jbc.M116.716589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koliwer J, et al. The Golgi-associated PDZ domain protein PIST/GOPC stabilizes the β1-adrenergic receptor in intracellular compartments after internalization. J Biol Chem. 2015;290:6120–9. doi: 10.1074/jbc.M114.605725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryu JK, et al. Review: Progresses in understanding N-ethylmaleimide sensitive factor (NSF) mediated disassembly of SNARE complexes. Biopolymers. 2016;105:518–31. doi: 10.1002/bip.22854. [DOI] [PubMed] [Google Scholar]
- 70.Cong M, et al. Binding of the beta2 adrenergic receptor to N-ethylmaleimide-sensitive factor regulates receptor recycling. J Biol Chem. 2001;276:45145–52. doi: 10.1074/jbc.M106087200. [DOI] [PubMed] [Google Scholar]
- 71.Garrido JL, et al. Role of phospholipase D in parathyroid hormone type 1 receptor signaling and trafficking. Mol Endocrinol. 2009;23:2048–59. doi: 10.1210/me.2008-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conway BR, et al. Quantitative analysis of agonist-dependent parathyroid hormone receptor trafficking in whole cells using a functional green fluorescent protein conjugate. Journal of cellular physiology. 2001;189:341–55. doi: 10.1002/jcp.10028. [DOI] [PubMed] [Google Scholar]
- 73.Seaman MN, et al. Retromer-mediated endosomal protein sorting: all WASHed up! Trends in cell biology. 2013;23:522–8. doi: 10.1016/j.tcb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S, et al. FAM21 directs SNX27-retromer cargoes to the plasma membrane by preventing transport to the Golgi apparatus. Nat Commun. 2016;7:10939. doi: 10.1038/ncomms10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marquer C, et al. Arf6 controls retromer traffic and intracellular cholesterol distribution via a phosphoinositide-based mechanism. Nat Commun. 2016;7:11919. doi: 10.1038/ncomms11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balana B, et al. Ras-association domain of sorting Nexin 27 is critical for regulating expression of GIRK potassium channels. PloS one. 2013;8:e59800. doi: 10.1371/journal.pone.0059800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balana B, et al. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc Natl Acad Sci U S A. 2011;108:5831–6. doi: 10.1073/pnas.1018645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghai R, et al. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci U S A. 2013;110:E643–E652. doi: 10.1073/pnas.1216229110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghai R, et al. Phox homology band 4.1/ezrin/radixin/moesin-like proteins function as molecular scaffolds that interact with cargo receptors and Ras GTPases. Proc Natl Acad Sci U S A. 2011;108:7763–8. doi: 10.1073/pnas.1017110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clairfeuille T, et al. A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex. Nat Struct Mol Biol. 2016;23:921–932. doi: 10.1038/nsmb.3290. [DOI] [PubMed] [Google Scholar]
- 81.Bowman SL, Puthenveedu MA. Postendocytic sorting of adrenergic and opioid receptors: new mechanisms and functions. Prog Mol Biol Transl Sci. 2015;132:189–206. doi: 10.1016/bs.pmbts.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alonso V, et al. Ubiquitination-deubiquitination balance dictates ligand-stimulated PTHR sorting. J Bone Miner Res. 2011;26:2923–34. doi: 10.1002/jbmr.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Follett J, et al. The Vps35 D620N mutation linked to parkinson’s disease disrupts the cargo sorting function of retromer. Traffic. 2014;15:230–44. doi: 10.1111/tra.12136. [DOI] [PubMed] [Google Scholar]
- 84.Follett J, et al. Parkinson’s disease linked Vps35 R524W mutation impairs the endosomal association of retromer and induces α-synuclein aggregation. J Biol Chem. 2016;291:18283–18298. doi: 10.1074/jbc.M115.703157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McMillan KJ, et al. Atypical parkinsonism-associated retromer mutant alters endosomal sorting of specific cargo proteins. J Cell Biol. 2016;214:389–99. doi: 10.1083/jcb.201604057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, et al. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down’s syndrome. Nat Med. 2013;19:473–80. doi: 10.1038/nm.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Damseh N, et al. A defect in the retromer accessory protein, SNX27, manifests by infantile myoclonic epilepsy and neurodegeneration. Neurogenetics. 2015;16:215–221. doi: 10.1007/s10048-015-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Follett J, et al. Retromer’s role in endosomal trafficking and impaired function in neurodegenerative diseases. Current protein & peptide science. 2016 doi: 10.2174/1389203717666160311121246. [DOI] [PubMed] [Google Scholar]
- 89.Small SA, Petsko GA. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nature reviews Neuroscience. 2015;16:126–32. doi: 10.1038/nrn3896. [DOI] [PubMed] [Google Scholar]
- 90.Choy RW, et al. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron. 2014;82:55–62. doi: 10.1016/j.neuron.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loo LS, et al. A role for sorting nexin 27 in AMPA receptor trafficking. Nat Commun. 2014;5:3176. doi: 10.1038/ncomms4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munoz MB, Slesinger PA. Sorting nexin 27 regulation of G protein-gated inwardly rectifying K+ channels attenuates in vivo cocaine response. Neuron. 2014;82:659–69. doi: 10.1016/j.neuron.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin TB, et al. VPS26A-SNX27 interaction-dependent mGluR5 recycling in dorsal horn neurons mediates neuropathic pain in rats. J Neurosci. 2015;35:14943–55. doi: 10.1523/JNEUROSCI.2587-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meraviglia V, et al. SNX27, a protein involved in down syndrome, regulates GPR17 trafficking and oligodendrocyte differentiation. Glia. 2016;64:1437–60. doi: 10.1002/glia.23015. [DOI] [PubMed] [Google Scholar]
- 95.Huang TY, et al. SNX27 and SORLA interact to reduce amyloidogenic subcellular distribution and processing of amyloid precursor protein. J Neurosci. 2016;36:7996–8011. doi: 10.1523/JNEUROSCI.0206-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai L, et al. Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-D-aspartate receptor 2C (NR2C) Mol Cell Biol. 2011;31:1734–47. doi: 10.1128/MCB.01044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xia WF, et al. Vps35 loss promotes hyperresorptive osteoclastogenesis and osteoporosis via sustained RANKL signaling. J Cell Biol. 2013;200:821–37. doi: 10.1083/jcb.201207154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mullershausen F, et al. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–34. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]