Abstract
Introduction
Patients with primary immunodeficiency (PID) often report fatigue, yet this symptom has not been studied in PID. Fatigue affects 6–7.5% of healthy adults. The goal of this study is to estimate the prevalence of fatigue in patients with PID and investigate its associated factors.
Methods
We analyzed 2537 PID patients registered in USIDNET to determine responses to the field “fatigue” in the core registry form. Demographics, immune phenotypes, and comorbid conditions were compared between fatigued and non-fatigued patients to identify relevant associations and potential drivers. A focused analysis was performed for patients with predominantly antibody deficiency disorders (PADs).
Results
Fatigue was reported in 25.9%(95% CI 23.7–28.3) of PAD patients, compared to 6.4% (95% CI 4.9–8.2) of non-PAD. Patients with common variable immunodeficiency (CVID) had the highest prevalence of fatigue (p < 0.001) among all PID diagnoses. Other factors that were associated with a higher rate of fatigue among PAD patients included female sex, higher BMI, depression, bronchiectasis, and autoimmunity. Additionally, fatigued PAD patients had lower absolute lymphocyte, CD3, CD4, and CD8 counts compared to non-fatigued patients.
Conclusion
Our findings suggest that fatigue is overrepresented in PAD patients. Prospective studies to estimate prevalence, risk factors, and fatigue etiology in PID are warranted, so therapeutic interventions can be considered.
Keywords: Fatigue, Primary immune deficiency disorder, USIDnet
Introduction
Primary immunodeficiency disorders (PIDs) are rare inherited diseases of the immune system that lead to frequent infections and increased risk of autoimmune disorders and malignancy [1]. Based on the affected immune mechanisms, PIDs are divided into a number of categories as defined by the International Union of Immunological Societies including immunodeficiencies affecting cellular and humoral immunity, combined immunodeficiency with associated or syndromic features (generally associated with T cell immunodeficiency), predominantly antibody deficiencies, diseases of immune dysregulation, congenital defects of phagocyte number, function, or both, defects in intrinsic and innate immunity, autoinflammatory disorders, complement deficiencies, and phenocopies of primary immunodeficiencies [2]. Among these categories, the predominantly antibody deficiency group accounts for approximately half of all patients with a PID diagnosis [1].
In 2013, there were an estimated 6 million people living with PID worldwide [3], with PID affecting 29.1–50.5 per 100,000 [4]. It is likely that prevalence of PID will continue to increase as the extended recognition of these diagnoses grows, significantly affecting both adults and children at the population level [3]. With this increased prevalence, there have been major improvements in the clinical care of PID patients, mainly due to improved awareness, earlier diagnosis, and appropriate immunoglobulin G (IgG) replacement therapies that led to increased life expectancy of individuals with primary antibody deficiency (PAD) [5–7]. In addition to the improvements in clinical care, morbidity, and mortality, there has been an increased focus on the quality of life (QoL) in PID patients as a way to improve perceived clinical and psychosocial dimensions of the diseases. Unfortunately, despite improvements in diagnosis and life expectancy, PID patients have impaired QoL [8].
Self-reported fatigue is the most important factor influencing overall QoL [9] and is a major prognostic factor for survival, independent from other risk factors in diseases such as chronic myelogenous leukemia patients [10] and terminally ill cancer patients [11]. Additionally, fatigue was associated with worse prognosis in patients with heart failure [12], had negative impact on health status, function, and mortality in an elderly population [13], and predicted mortality in chronic obstructive lung disease [14]. In another study, low levels of fatigue independently predicted longer recurrence-free and overall survival from breast cancer, when controlling for biological factors [15]. Taken together, these data suggest that fatigue could be a powerful indicator of overall survival for many different diseases.
Currently, there are no published studies that specifically address the prevalence of fatigue in PID patients. The purpose of this study is to estimate the prevalence of fatigue among PID patients using data from the US Immunodeficiency Network registry and investigate these data for characteristics that may be associated with fatigue.
Methods
Data Source
Data for this retrospective study were collected in October 2015 and December 2015 from the US Immunodeficiency Network (USIDnet), a research consortium established to advance scientific research on PIDs. This registry actively collects validated data on all PID diagnoses, as entered by approved physicians, researchers, and other staff at enrolling institutions [16]. The study was given Institutional Review Board (IRB) approvals from participating institutions, and all patients provided consent to include their data in the registry. The USIDnet uses patient registry form, which contained on page 6 a question about presence of fatigue, as one of the constitutional symptoms. The analyses were based on the answers to this question and others from the core registry form.
For each of the PID diagnosis, USIDnet has specific diagnostic criteria. For CVID, USIDnet used the following criteria: marked decrease (at least 2 SD below the mean for age) in two out of three of the major isotypes (IgG and IgA or IgM), IgG <500 mg/dl for adults, age above 2 years at the onset of immunodeficiency, absent isohemagglutinins and/or poor response to vaccines, and defined causes of hypogammaglobulinemia have been excluded.
Inclusion/Exclusion Criteria
Two queries were made to the USIDnet registry: a general prevalence analysis (October 2015) and a PAD-focused analysis (December 2015). All PID patients who were reported to have fatigue in the core registry form were included for the general prevalence analysis. Data from patients with PID, who were reported not to have fatigue in the USIDnet, were used as the control group. There was a large difference in prevalence of fatigue between PAD and non-PAD patients in our general prevalence analysis; however, USIDnet policies minimize the clinical data released to answer discrete research questions. Thus, we focused on patients with PAD and submitted a second query to the USIDnet to compare the demographics, immune phenotypes, comorbidities, and associated conditions of fatigued vs. non-fatigued PAD patients. At the time of the PAD-focused query, additional patients had been added to the USIDnet dataset. Thus, we included the additional patients in the PAD-focused analysis, but not in the general prevalence analysis. We excluded eight individuals that had a body mass index (BMI) over 100 from the body mass-related analyses as they were considered to be extreme outliers. Patients with transient hypogammaglobulinemia and patients with hypogammaglobulinemia secondary to malignancy were not included in the PAD diagnosis.
Finally, when assessing the prevalence of fatigue in PAD patients based on IgG replacement status, we have excluded patients who were reported not to be on IgG replacement, or when the data about IgG replacement therapy was not available.
Statistical Analysis
The prevalence of fatigue and 95% exact confidence interval were reported for all patients by disease and age. Summary statistics are presented for demographics, lymphocyte phenotype, non-infectious complications, and immunoglobulin therapy as mean ± standard deviation, median with 25th and 75th percentiles, and frequency reported as a percentage. T-test, chi-square, Fisher’s exact, and Wilcoxon rank sum tests were used for comparisons between fatigued and non-fatigued patients.
Results
Specific Diagnoses of PID Patients in the USIDnet Registry
First, we conducted a general prevalence analysis investigating the prevalence of fatigue among patients diagnosed with PIDs. At the time of this analysis, there were 2537 patients registered in the USIDnet, which included a diverse list of all the diagnoses registered (Fig. 1) 2366 of those patients had clinical visits indicating fatigue status. The most common PID diagnosis was common variable immunodeficiency (CVID) with 987 (41.7%) patients, followed by DiGeorge syndrome (DGS) with 393 patients (16.6%). Other diagnoses included miscellaneous antibody deficiency (MAD) (N = 202 (8.5%)), agammaglobulinemia (AGAMMA) (N = 200 (8.5%), chronic granulomatous disease (CGD) (N = 116 (4.9%)), and severe combined immunodeficiency disorder (SCIDs) (N = 168 (7.1%)). USIDnet combines several rare diagnoses within the core registry, as they did not have a “sub-registry.” We therefore refer to these non-sub-registry diagnoses as “CORE” and include autoimmune lymphoproliferative syndrome (ALPS), autoimmune polyendocrinopathy with candidiasis and ectodermal dystrophy (APECED), ataxia telangiectasia, autoinflammatory syndrome, CD16 deficiency, chronic mucocutaneous candidiasis, Comel-Netherton syndrome, dyskeratosus congenita, GATA2 deficiency, hyper IgE syndrome, IFN-γ receptor-1 deficiency, IL-10 deficiency, immune dysregulation with autoimmunity due to uncertain or unlisted cause, immunodeficiency of unknown cause, immune dysregulation, polyendocrinopathy, enteropathy X-linked (IPEX) IRAK-4 deficiency, hyper IgD syndrome, neutropenia due to uncertain or unlisted cause, other defects in innate immunity, STAT1 deficiency, STAT1 gain-of-function mutations, and UNC13D/Munc 13-4 deficiency. The total number of patients with CORE disorders was 159.
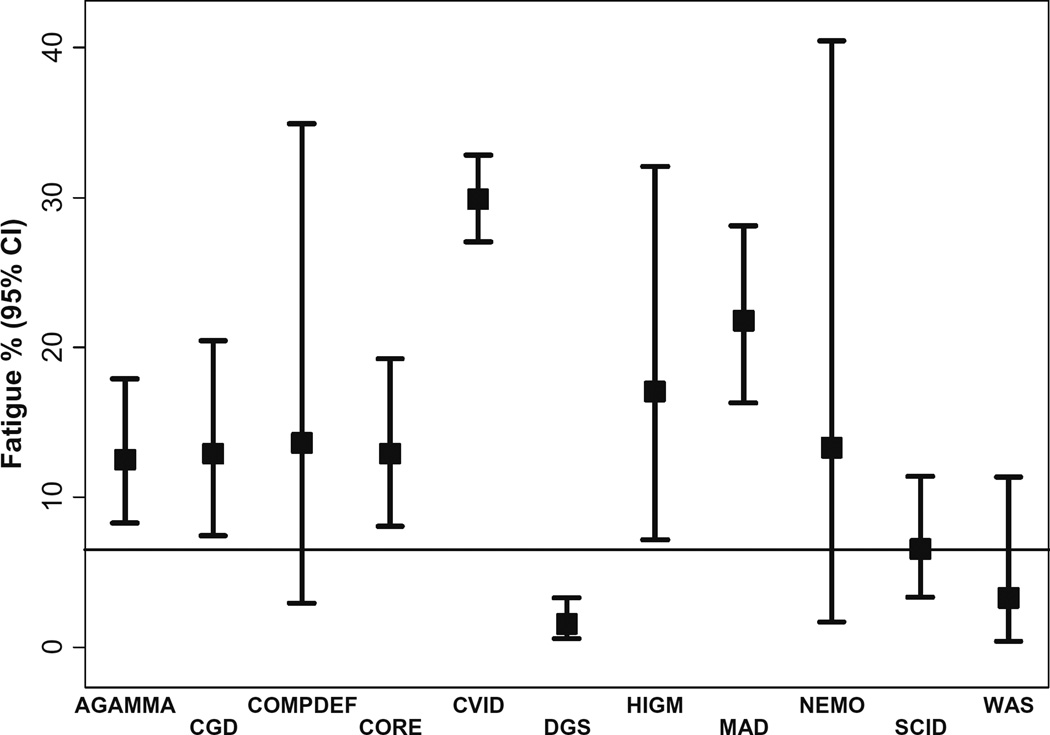
Fig. 1.
Prevalance of fatigue overall and by disease. Horizontal black line represents the prevalence of fatigue in the general population. Abbreviations: AGAMMA agammaglobulinemia, CGD chronic granulomatous disease, COMPDEF complement deficiency; CORE diagnosis included autoimmune lymphoproliferative syndrome (ALPS), autoimmune polyendocrinopathy with candidiasis and ectodermal dystrophy (APECED), ataxia telangiectasia, autoinflammatory syndrome, CD16 deficiency, chronic mucocutaneous candidiasis, Comel-Netherton syndrome, dyskeratosus congenita, GATA2 deficiency, hyper IgE syndrome, IFN-γ receptor-1 deficiency, IL-10 deficiency, immune dysregulation with autoimmunity due to uncertain or unlisted cause, immunodeficiency of unknown cause, immune dysregulation, polyendocrinopathy, enteropathy X-linked (IPEX) IRAK-4 deficiency, hyper IgD syndrome, neutropenia due to uncertain or unlisted cause, other defects in innate immunity, STAT1 deficiency, STAT1 gain-of-function mutations, and UNC13D/Munc13–4 deficiency; CVID common variable immunodeficiency, DGS DiGeorge syndrome, HIGM hyper-IgM syndrome, LAD leukocyte adhesion deficiency, MAD miscellaneous antibody deficiency, NEMO nuclear factor-kappa B essential modulator, SCID severe combined immunodeficiency, WAS Wiskott Aldrich syndrome
Prevalence of Fatigue by Disease Category
We examined the data from all 2537 PID patients for the prevalence of fatigue. Of those patients, 2366 had clinical visits indicating their fatigue status. Four hundred thirty patients out of the 2366 registered PIDs with available fatigue data were reported to have fatigue (18%) while the reported prevalence of fatigue in general population varies from 6 to 7.5% [17, 18]. CVID had the highest prevalence of fatigue among patients PID with 295/987 (29.9%). MAD had the second highest prevalence of fatigue with 44/202 patients reported to have fatigue (21.8%). Conversely, DiGeorge syndrome had very low prevalence (1.5%), and there were no recorded cases of fatigue among patients with leukocyte adhesion deficiency (LAD). Figure 1 summarizes the fatigue prevalence for each PID diagnosis, including the CORE subcategory defined above which contained 20 patients with fatigue (12.9%).
Prevalence of Fatigue by Age
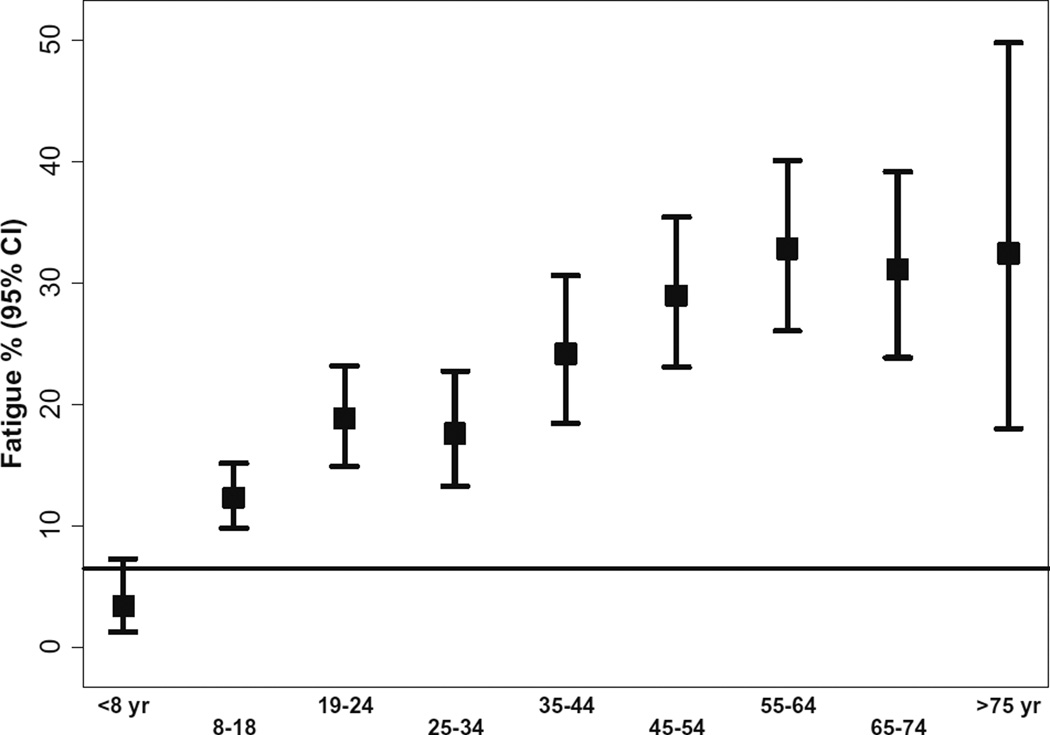
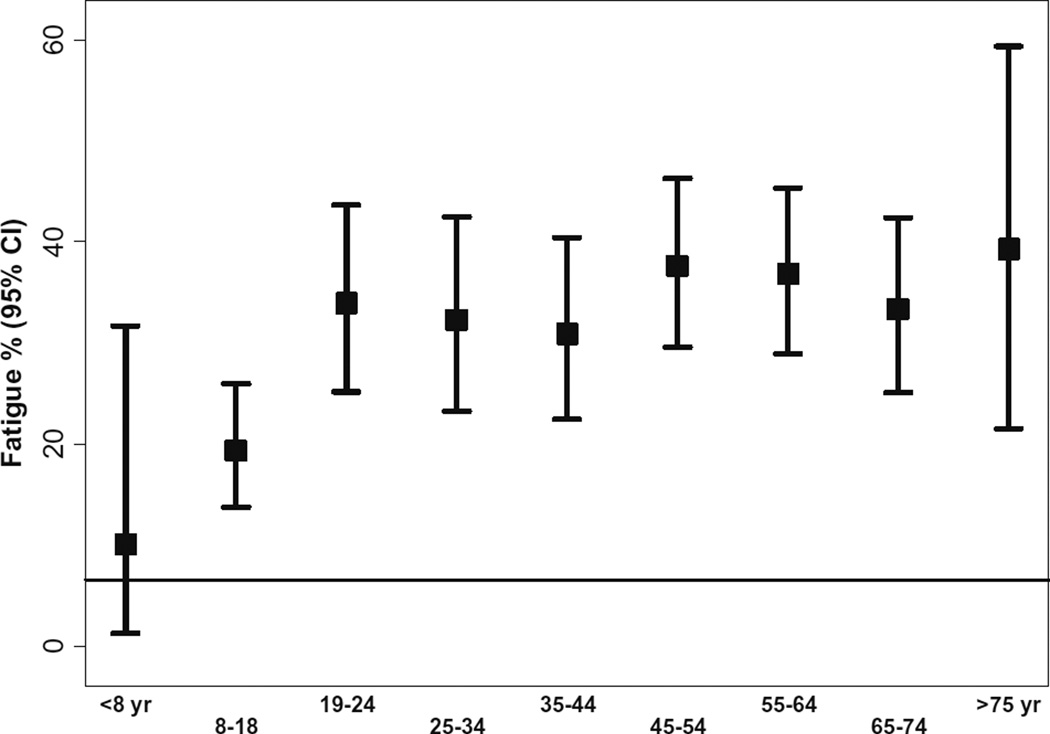
Patients were categorized into the following age groups: ≤8, 9–18, 19–24, 25–34, 35–44, 45–54, 55–64, 65–75, and >75 years). In the general fatigue analysis, PID patients above the age of 8 years had the higher prevalence of fatigue compared to the general population. We observed a trend toward a higher prevalence in patients 44 years old and above (p < 0.001: Cochran-Armitage trend test) (Figs. 2 and 3 summarizes the fatigue prevalence for PID patients by age)
Fig. 2.
Fatigue prevalence by age; all PID diagnosis in USIDnet combined. Horizontal black line represents the prevalence of fatigue in the general population. Except for children below the age of 8, prevalance of fatigue is increased is all other age groups
Fig. 3.
Fatigue proportion in patients with common variable immunodeficiency by age. Horizontal black line represents the prevalence of fatigue in the general population. In CVID, the prevalance of fatigue is increased in patients above the age of 8 years
Demographics of Fatigued PID Patients
Next, we focused our analysis on PID patients who were reported to have fatigue (430 patients). Table 1 includes the demographics of all PID patients in the USIDnet reported to have fatigue. Of the 430 patients with fatigue, 200 (47%) were males and the average (±SD) age was 39.1 years (±20.4). Over two thirds of fatigued patients (69%) had CVID. Race and ethnicity information were available for 343 of the fatigued patients. Of these, 94% of fatigued patients were Caucasian/white, 4% were African American, 0.9% were Asian, and 0.3% American Indian/Native American. Finally, BMI data were available for 346 fatigued patients, and the mean BMI was 24.4 (±7.2). These data suggest that PID patients with fatigue are primarily white with a CVID diagnosis.
Table 1.
Demographics of all PID patients in the USIDnet registry reported to have experienced fatigue
| Demographic | N(%) |
|---|---|
| Total PID patients with fatigue | 430 |
| Disease | |
| AGAMMA | 25 (6) |
| CGD | 15 (3) |
| COMPDEF | 3(1) |
| CORE | 20 (5) |
| CVID | 295(69) |
| DGS | 6(1) |
| HIGM | 7(2) |
| LAD | 0(0) |
| MAD | 44 (10) |
| NEMO | 2(1) |
| SCID | 11(3) |
| WAS | 2(1) |
| Male | 200(47) |
| Race/ethnicity(N=343) | |
| African American/Black | 15 (4) |
| American Indian/Native American | 1(0.3) |
| Asian or Pacific Islander | 3(0.9) |
| Caucasian/White | 324(94) |
| Initial BMI, mean (SD) N = 346 | 24.4 (7.2) |
| Age, in years mean (SD) | 39.1 (20.4) |
Immune Phenotypes of Fatigued PID Patients
We examined the immune phenotype for all PID patients with fatigue as there are substantive data fields within the USIDNET Registry to allow for the input of laboratory findings. The number of patients for whom laboratory data were entered varied for each immune characteristic, but all data were included when available. As expected, the average lymphocyte counts varied based on the underlying immune defect. However, overall, the median absolute lymphocyte count (ALC) CD3, CD4, CD19, and CD56CD16 NK cells were generally within normal ranges for the average age of the fatigued PID patients. Table 2 summarizes the immune phenotypes of PID patients with fatigue.
Table 2.
Summary of immune phenotypes of all PID patients with fatigue
| Characteristic | Median (25th, 75th) |
|---|---|
| Absolute lymphocyte count (N= 179) | 1800(1200, 2390) |
| CD3% (N= 210) | 75(68, 82.5) |
| CD3(µl) (N= 215) | 1249(769, 1700) |
| CD4% (N= 229) | 45(36.2, 54.4) |
| CD4(µl) (N= 206) | 704 (462, 1072) |
| CD8% (N= 197) | 25(18, 32.2) |
| CD8(µl) (N= 195) | 440(265, 621) |
| CD19%(N= 191) | 13 (6.3, 19) |
| CD19 (µl) (N= 186) | 227.5 (55, 404) |
| CD20%(N= 56) | 11.1 (3.4, 19.1) |
| CD20 (µl) (N= 49) | 246(46, 404) |
| CD56CD16 NK cells (%)(N= 158) | 8(5, 13) |
| CD56CD16 NK cells (µl) (N= 152) | 121(69.5, 224.5) |
| CD4CD45RA naïve T(%) (N= 46) | 27.4 (9.6, 37) |
| CD4CD45RA naïve T(µl), (N= 36) | 320.5 (131.5, 655) |
| CD4CD45RO memory T(%) (N= 44) | 39.45 (23.05, 78.1) |
| CD4CD45RO memory T(µl), (N= 33) | 290(181, 380) |
Non-Infectious Comorbidities and Complications of Fatigued PID Patients
Next, we analyzed the frequency of select comorbidities and complications reported for PID patients with fatigue. Table 3 summarizes the non-infectious complication in all fatigued patients. The most common non-infectious comorbidities and complications among all fatigued patients were depression, reported in 23% of fatigued PID patients (compared to 4.2–12.7 in the general population [19], followed by bronchiectasis (16%) and arthritis (16%).
Table 3.
Summary of non-infectious comorbidities and complications observed in PID patients with fatigue (N = 430)
| Complication | N(%) |
|---|---|
| Hemolytic anemia | 10 (2%) |
| Idiopathic thrombocytopenia (ITP) | 6(1%) |
| Thrombocytopenia | 64 (15%) |
| Thrombotic thrombocytopenia | 8(2%) |
| Leukopenia | 16 (4%) |
| Splenomegaly | 60 (14%) |
| Cancer (any type) | 47 (11%) |
| Granuloma | 37 (9%) |
| Colitis | 32 (7%) |
| Lymphoma (any type) | 10 (2%) |
| Melanoma | 3(1%) |
| Adrenal Insufficiency | 9(2%) |
| IBD, not otherwise specified | 20 (5%) |
| Interstitial lung disease | 23 (5%) |
| Pulmonary fibrosis | 5(1%) |
| Bronchiectasis | 68 (16%) |
| Arthritis (rheumatoid-juvenile) | 69 (16%) |
| Bronchitis olbitrans | 4(1%) |
| Bronchiolitis—follicular | 1(0.2%) |
| Internal lung parenchyma | 1(0.2%) |
| Hashimoto’s thyroiditis | 8(2%) |
| Pulmonary lymphoproliferative | 1(0.2%) |
| Bronchiolitis obliterans organizing pneumonia (BOOP) | 6(1%) |
| Chronic inflammatory demyelinating polyneuropathy | 1(0.2%) |
| Vasculitis | 4(1%) |
| Hepatomegaly | 23 (5%) |
| Lymphadenopathy | 65 (15%) |
| Raynaud’s | 8 (2%) |
| Sjogren’s disease | 8(2%) |
| Eosinophilic esophagitis, Celiac disease | 18 (4%) |
| Depression | 101(23%) |
Prevalence of Fatigue in Patients with Primary Antibody Deficiency Disorders
Within the set of PIDs included in this analysis, several disorders may be classified as primary antibody disorders (PADs), including CVID, MAD, HIGM, and AGAMMA.
The prevalence of fatigue among PADs was 25.9% (95% CI 23.67–28.29), while among individuals with non-PADs, the proportion of fatigue is significantly lower 6.3% (95% CI 4.91, 8918.15). The prevalence of fatigue in non-PAD patients resembles that in the general population [17, 18].
Demographics of Fatigued PAD Patients
Due to this large difference in prevalence of fatigue between PAD and non-PAD patients, we decided to focus further analyses on PAD patients. To advance a PAD-focused analysis, we submitted a second query to the USIDnet requesting further information about patients with PAD reported to have fatigue. We compared the demographics, immune phenotypes, and comorbid conditions of fatigued vs. non-fatigued. The characteristics of fatigued patients with PAD were notable in light of our prior analyses of PAD overall (Table 4). In this second analysis, we found that fatigued patients with PADs were more likely to be white; 57% of fatigued PAD patients were females compared to 43% of non-fatigued patients (P < 0.001). Additionally, PAD patients with fatigue had a significantly larger average BMI (25 vs. 22.7 kg/m2, P < 0.001). We also found that the odds of fatigue increases by 4% for every unit increase in BMI (OR = 1.04; 95% CI = 1.02, 1.07). Finally, PAD patients on IVIG had similar prevalence of fatigue compared to those on SCIG (36.6 vs. 35.6%, p = 0.77)
Table 4.
Comparison of patient characteristics by fatigue status among patients with primary antibody deficiency disorders—second query
| No fatigue | Fatigue | p Valuea | |
|---|---|---|---|
| Total patients with PAD, N (%) | 855(69%) | 385(31%) | |
| Specific disease, N(%) | <0.001 | ||
| AGAMMA | 173(20%) | 25 (6%) | |
| CVID | 564(66%) | 307(80%) | |
| HIGM | 27 (3%) | 7(2%) | |
| MAD | 91 (11%) | 46 (12%) | |
| Female, N (%) | 369(43%) | 220(57%) | <0.001 |
| Caucasian, N (%) | 602/652 (92%) | 290/302 (96%) | 0.034 |
| Initial BMI, mean (SD) N = 568, 305 | 22.7 (6.8) | 25.0(7.4) | <0.001 |
Significant p values are provided
PAD primary antibody deficiency, AGAMMA agammaglobulinemia, CVID common variable immunodeficiency, HIGM hyper-IgM syndrome, MAD miscellaneous antibody deficiencies, BMI body mass index
From these data, we conclude that unlike in PIDs as a whole, fatigue in PAD patients appears to be more common in females and overweight patients.
Lymphocyte Phenotype Fatigue Patients with PAD
Next, we analyzed the lymphocytic phenotypes in the PAD-focused subset of fatigued patients with specific comparison to the non-fatigued PAD patients (Table 5). Overall, fatigued PAD patients had a lower median ALC than did non-fatigued PAD patients (P = 0.0015). Fatigued PAD patients also had lower CD3 (P = 0.0014), CD4 (P = 0.015), and CD8 (P = 0.001) counts than non-fatigued PAD patients. Fatigued PAD patients had statistically significant higher memory T cell counts (P = 0.003). Thus, these data suggest that lymphocytic phenotype differences exist between fatigued and non-fatigued patients with PADs.
Table 5.
Comparison of lymphocyte phenotype based on of fatigue status among patients with primary antibody deficiency disorders—second query
| Total patients with anti body deficiency | No fatigue 855(69%) Median (25th, 75th) |
Fatigue 385(31%) Median (25th, 75th) |
p Value |
|---|---|---|---|
| Absolute lymphocyte count (N=253, N= 158) | 2100 (1360, 3185) | 1775 (1200, 2291) | 0.0015 |
| CD3% (N=375, N= 188) | 76 (68, 85) | 76 (70, 83) | 0.8667 |
| CD3(µl) (N=348, N= 186) | 1404 (928, 2134) | 1239 (762, 1692) | 0.0014 |
| CD4% (N=377, N= 198) | 44 (36, 53) | 46.9 (38, 57) | 0.0499 |
| CD4(µl) (N=341, N= 179) | 802(506, 1295) | 701(462, 1050) | 0.0158 |
| CD8% (N=347, N= 173) | 25.9 (19, 33) | 25 (19.3, 33) | 0.7142 |
| CD8(µl) (N=328, N= 168) | 501 (312, 854) | 416 (249, 610) | 0.0010 |
| CD19%(N=335, N= 171) | 13 (6, 20) | 12.2 (6, 18.9) | 0.7747 |
| CD19 (µl) (N=318, N= 162) | 221(75, 482) | 209.5(52, 344) | 0.1561 |
| CD20%, (N= 116, N= 48) | 9.85 (3, 17.5) | 10.5 (2.85, 16.7) | 0.9755 |
| CD20 (µl) (N=100, N= 45) | 237(83.5, 438) | 223.8(46, 363) | 0.6973 |
| CD56CD16 NK cells %(N=281, N= 141) | 7.8(5, 11.6) | 8(5, 13) | 0.3414 |
| CD56CD16 NK cells (µl) (N=256, N= 134) | 137(82.5, 248.5) | 120.9(66, 216) | 0.0881 |
| CD4CD45RA naïve T %(N=69, N= 41) | 33 (13, 48) | 29.9 (14.8, 36.9) | 0.2244 |
| CD4CD45RA naïve T (µl) (N=51, N= 32) | 437(102, 992) | 359(143.5, 698) | 0.4892 |
| CD4CD45RO memory T %(N=59, N= 38) | 17 (9.1, 42.4) | 39.5 (22, 80.2) | 0.0030 |
| CD4CD45RO memory T(µl) (N=49, N= 29) | 268(163, 408) | 294(194, 384) | 0.6679 |
Significant p values are indicated in bold
Non-Infectious Comorbidities and Complications in Fatigued PAD Patients
Next, we analyzed the occurrence of comorbidities and complications in PAD patients. In the fatigued PAD group, the most common non-infectious complication was depression (24%; see Table 6). This comorbidity was not observed in non-fatigued PAD patients, and the difference between these two groups was statistically significant (P < 0.001). After depression, the second most common comorbidities were arthritis (16 vs. 0% in non-fatigued, P < 0.001) and bronchiectasis (16 vs. 0% in non-fatigued, P < 0.001). Other non-infectious comorbidities and complications that were statistically significantly different between fatigued and non-fatigued PAD patients were leukopenia, granuloma, colitis, adrenal insufficiency, IBD, interstitial lung disease, hepatomegaly, eosinophilic esophagitis, and Celiac disease. Thus, selected comorbidities occur more frequently in fatigued PAD patients, which suggests that they either speak to a particular severity of PAD or may be particular drivers of fatigue within this category of the deficiency.
Table 6.
Comparison of non-infectious complications according to fatigue status among patients with primary antibody deficiency disorders—second query
| No fatigue | Fatigue | p Value | |
|---|---|---|---|
| All antibody-deficient patients (N= 1240) | 855(69%) | 385(31%) | |
| Non-infectious complications | N(%) | N(%) | |
| Hemolytic anemia | 16/380(4%) | 7(2%) | 0.059 |
| Idiopathic thrombocytopenia (ITP) | 25/380(7%) | 3(1%) | <0.001 |
| Thrombocytopenia | 57/380(15%) | 49 (13%) | 0.363 |
| Thrombotic thrombocytopenia | 3/380(1%) | 7(2%) | 0.341 |
| Leukopenia | 4/380(1%) | 13 (3%) | 0.047 |
| Splenomegaly | 43/380(11%) | 51 (13%) | 0.416 |
| Cancer (any type) | 47/380(12%) | 47 (12%) | 0.946 |
| Granuloma | 12/380(3%) | 28 (7%) | 0.014 |
| Colitis | 0 | 25 (6%) | <0.001 |
| Lymphoma (any type) | 13/380(3%) | 11(3%) | 0.684 |
| Melanoma | 0 | 3(1%) | 0.249 |
| Adrenal Insufficiency | 0 | 7(2%) | 0.015 |
| IBD, not otherwise specified | 0 | 18 (5%) | <0.001 |
| Interstitial lung disease | 0 | 22 (6%) | <0.001 |
| Pulmonary fibrosis | 0 | 3(1%) | 0.249 |
| Bronchiectasis | 0 | 60 (16%) | <0.001 |
| Arthritis (juvenile rheumatoid) | 0 | 62 (16%) | <0.001 |
| Bronchitis olbitrans | 0 | 2(0.5%) | 0.499 |
| Bronchiolitis-follicular | 0 | 1(0.3%) | 0.999 |
| Internal lung parenchyma | 0 | 1(0.3%) | 0.999 |
| Hashimoto’s thyroiditis | 1/380(0.3%) | 7(2%) | 0.069 |
| Pulmonary lymphoproliferative | 0 | 1(0.3%) | 0.999 |
| Bronchiolitis obliterans organizing pneumonia (BOOP) | 0 | 5(1%) | 0.062 |
| Chronic inflammatory demyelinating polyneuropathy | 0 | 1(0.3%) | 0.999 |
| Vasculitis | 4/380(1%) | 3(1%) | 0.724 |
| Hepatomegaly | 2/380(1%) | 17 (5%) | 0.001 |
| Lymphadenopathy | 49/380(13%) | 52 (14%) | 0.831 |
| Raynoud’s | 2/380(1%) | 8(2%) | 0.107 |
| Sjogren’s disease | 3/380(1%) | 7(2%) | 0.341 |
| Eosinophilic esophagitis Celiac disease | 0 | 16 (4%) | <0.001 |
| Depression | 0 | 91 (24%) | <0.001 |
Significant p values are indicated in bold
Prevalence of Fatigue in Patients with Common Variable Immunodeficiency
Since we observed that patients with CVID had the highest prevalence of fatigue among all PID patients, we performed focused analysis on CVID patients.
First, we analyzed the prevalence of fatigue among CVID patients based on age. The mean age of fatigued CVID patients was higher than non-fatigued (43.7 vs. 38.8, P = 0.001). Across all age groups, fatigue prevalence was higher than the general population. Figure 4 summarized the prevalence of fatigue in CVID patients by age.
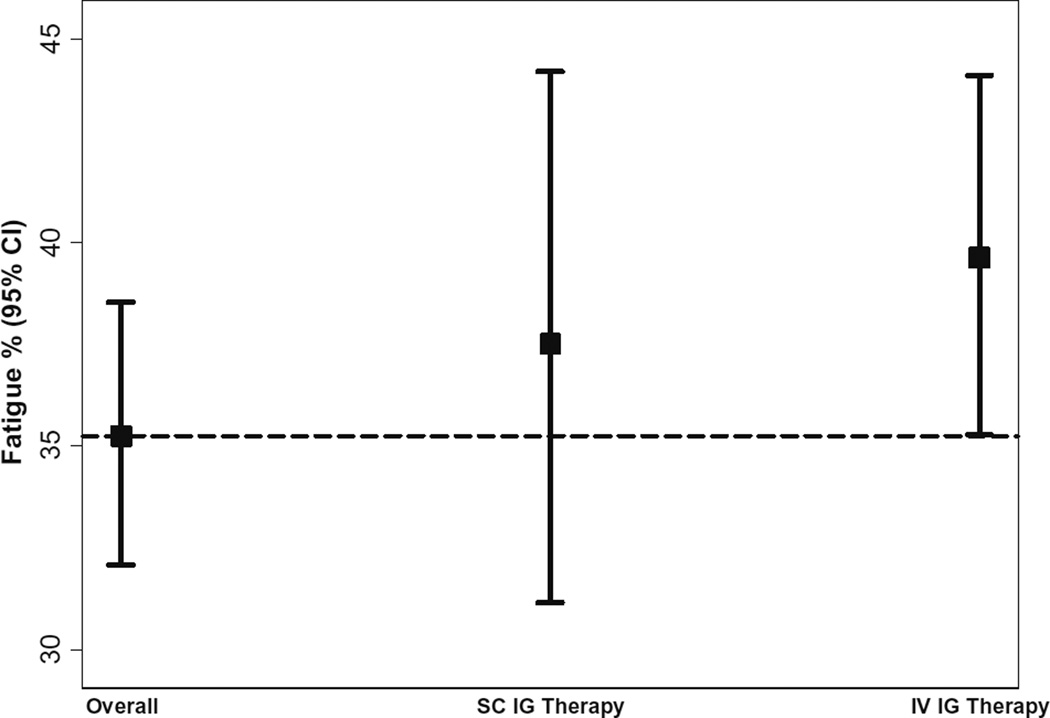
Fig. 4.
Proportion of fatigued CVID patients on SCIG and IVIG. Prevalance of fatigue was not different between CVID patients receiving IVIG versus SCIG. Abbreviations: SCIG subcutaneous immunoglobulin, IVIG intravenous immunoglobulin
Next, we examined the demographics of fatigued and non-fatigued CVID patients. Similar to what we observed in PAD patients, fatigued CVID patients had higher BMI compared to non-fatigued patients (25.4 vs 23.4, P = 0.0004). There was no difference of fatigue prevalence in CVID based on sex or ethnicity. Table 7 summarizes the characteristics of CVID patients based on their fatigue status.
Table 7.
Comparison of patient characteristics by fatigue status among patients with common variable immunodeficiency—second query
| CVID patients | Non-fatigue | Fatigue | p Value |
|---|---|---|---|
| Age (years), mean (SD) N= 540, 307 | 38.8 (21.3) | 43.7 (19.8) | 0.001 |
| Age, N(%) | 0.001 | ||
| <8 | 16 (3%) | 3(1%) | |
| 8–18 | 121(21%) | 35 (11%) | |
| 19–24 | 61 (11%) | 40 (13%) | |
| 25–34 | 57 (10%) | 33 (11%) | |
| 35–44 | 61 (11%) | 34 (11%) | |
| 45–54 | 71 (13%) | 55 (18%) | |
| 55–64 | 74 (13%) | 54 (18%) | |
| 65–74 | 65 (12%) | 42 (14%) | |
| ≥75 | 38 (7%) | 11(4%) | |
| Initial BMI, mean (SD) N = 386, 242 | 23.4 (6.7) | 25.4 (7.5) | 0.0004 |
| Caucasian/white, N(%) | 419/440 (95%) | 240/246 (98%) | 0.155 |
| Female, N(%) | 313/564 (55%) | 1807/307(61%) | 0.132 |
Significant p values are indicated in bold
Finally, we were interested to know if fatigue prevalence differed between CVID patients receiving IVIG versus SCIG. For this analysis, we excluded CVID patients not reported to be on IgG replacement therapy or if the route of administration was not included. We found that prevalence of fatigue was not statistically significantly different between CVID patients receiving IVIG versus SCIG (39.63 vs 37.5%, P = 0.59).
Discussion
Our analysis of fatigue prevalence in PID patients revealed several interesting findings. The prevalence of fatigue among patients with PID in the USIDnet registry was 18%. In comparison, population-based surveys in Britain and the USA report fatigue prevalence at 6.0–7.5% [17, 20]. When we divided the PID population into the PAD vs. non-PAD subset, we found that non-PAD patients had similar fatigue prevalence compared to general population (6.4%), while PAD patients had a higher prevalence of fatigue at 25.9%. Particularly, CVID patients who had the highest prevalence of fatigue (29.9%). Thus, PAD patients have an above-average frequency of fatigue and are distinguished not only from the general population but among the PID population as well.
When we analyzed the prevalence of fatigue based on age, we found that in PID in general, patients above the age of 8 years have a higher prevalence of fatigue compared to the general population, and there was a trend toward increase fatigue by age. We observed the same pattern among CVID patients. In those patients, even children below the age of 8 years old had a higher prevalence of fatigue compared to the general population. This high prevalence was an interesting finding and is consistent with fatigue data available in children with other chronic diseases. Fatigue is a common symptom in children [21]. The National Longitudinal Study of Adolescent Health (Add Heath) indicated that 21% of US children aged 7–12 years [22] and 15–30% of adolescents aged 11–21 years reported frequent fatigue [23]. Fatigue prevalence is particularly high in children with special healthcare needs such as cancer, diabetes, and rheumatologic disorders [24–26]. Therefore, it was not surprising to find that fatigue was observed and reported by providers who cared for children with primary immunodeficiency disorders. Prospective studies on children with PID using validated tools would be critical to measure the prevalence and burden of fatigue in this unique patient population.
Through our analysis, we identified several factors that were associated with increased prevalence of fatigue among PID patients, including having PAD and receiving IgG replacement therapy to treat it. We did observe that 27% of PAD patients in the USIDnet registry were not receiving IgG replacement. IgG replacement is the most accepted treatment for CVID, HIGM, and agammaglobulinemia. Patients with miscellaneous antibody deficiency (MAD) included a variety of diagnosis, including selective antibody deficiency, and here, the indications for IgG replacement are more variable and at times controversial [6]. It could be that more symptomatic PAD patients were placed on IgG replacement, while those who had less clinical difficulties were not. The alternative explanation, which must be raised in light of the observation, is that there is the perception of fatigue associated with the receipt of immunoglobulin therapy.
When we compared PAD patients receiving SCIG to IVIG and excluded PAD patient not receiving IgG replacement, the prevalence of fatigue appeared to be similar between the two groups. Similarly, CVID patients receiving IVIG had similar fatigue prevalence to patients receiving SCIG
Published observations suggested temporal dynamics between fatigue and IgG replacement route. For example, patients with CVID reported statistically significant improvement in fatigue symptoms 10 days post-infusion compared to the day of infusion. [27]. This improvement in fatigue is sometimes followed by an exacerbation in the third to fourth week, just prior to the next infusion. This phenomenon is often referred to as the “wear-off effect” [28]. Many patients who receive IVIG infusions every 21 to 28 days report general malaise, fatigue, arthralgia, myalgia, and increased susceptibility to infections during the last 7 to 10 days of each dosing interval [29]. The first national survey of the Immune Deficiency Foundation (IDF) for Treatment Experiences and Preferences of Patients with Primary Immune Deficiency Diseases revealed that 42% of patients with PID receiving IVIG reported that they could feel the wear-off effect. [30] The point at which the patient feels the wear-off effect was closely related to the frequency of infusion. For example, those who infused every 4 weeks felt the effects wearing off at 21 days post-infusion [30]. In contrast to patients receiving IVIG therapy, those receiving SCIG therapy less frequently report feeling the wear-off effect [31]. This difference is thought to be due to the lower variability in and more consistently physiological level of serum IgG concentration when Ig is administered via the subcutaneous route [32]. A recent study however showed that the probability of fatigue was greatest in the first week of both the 3- and 4-week dosing cycles and was significantly lower in the subsequent weeks of the treatment cycle, suggesting that fatigue may actually be a result of IVIG administration itself [33]. Further, more specific and prospective studies are needed to identify the relationship between fatigue and IgG replacement therapy.
Our analysis also showed that fatigued PAD patients have higher rates of select comorbidities and complications, with depression having the most prevalence at 23%. The 2014 National Survey on Drug Use and Health estimated that 4.3% of US adults had at least one episode of major depression [34]. There are no available data on the criteria used to diagnose depression in those patients. One explanation for this high prevalence of depression could be due to overlap between fatigue and depression diagnosis. This overlap depends significantly on how each of those diseases is defined [35]. While some suggested that fatigue is merely a form of depression, research in chronic fatigue suggests that those two conditions are distinct from each other. For example, central symptoms of depression such as guilt and self-esteem issues are not typically features of chronic fatigue syndrome (CFS) [18]. Additionally, biological differences have been observed between fatigue and depression such as the regulation of the hypothalamic-pituitary-adrenal axis which is downregulated in CFS and upregulated in depression [36]. Studies of twins demonstrated that genetic and environmental risk factors for fatigue are independent from psychiatric symptoms [37] by showing that in twins, 44% of the genetic contribution to fatigue was not shared with the other common psychological forms of distress, and that environmental factors which determined the psychological forms of distress were almost totally independent of the environmental determinant of fatigue [37]. And finally, cohort studies identified that different and specific risk factors exist between fatigue and psychiatric comorbidity (e.g., family history of psychiatric disorder) and fatigue without psychiatric comorbidity (e.g., having excessive energy as a child) [35, 38]. Together these support the notion of separating fatigue and depression and any further study of fatigue in PID should focus directly upon discerning this dichotomy.
We found that fatigued PAD patients had a higher rate of autoimmunity, lung disease, and gastrointestinal disease and lower numbers of certain lymphocytes (though the lymphocyte counts in both groups were within normal ranges). Fatigue has been increasingly recognized as a comorbidity of chronic diseases, such as multiple sclerosis [39], systemic lupus erythematosus [40], chronic liver diseases [41], and rheumatoid arthritis (RA) [42], and in patients infected with HIV [43]. Patients with these chronic conditions consistently identify fatigue as one of the most problematic and challenging aspects of their disease [44].We hypothesize here that the chronic inflammation associated with several of the non-infectious complications in fatigued PID patients is interfering with lymphocyte homeostasis and thus explaining the observed mild immunophenotypical differences.
Prior studies of fatigue prevalence in the general population showed clear sex differences, with a female odds ratio (OR 1.9) [45] and females reporting fatigue 1.2 to 2.3 times more frequently than do males [46]. In our study, a similarly directed sex difference emerged in the PAD-focused analysis, but it was not a significant factor in the general prevalence of fatigue among all PID patients or in the focused CVID analysis. The reasons for this sex difference remain elusive and warrant further study with regard to the interplay of known sex differences [47], drivers of fatigue, and immunity.
Most of the patients in the registry were white. The prevalence of fatigue was not significantly different between different ethnic groups. This could be due to underrepresentation of other ethnic groups in this dataset. Population-based studies showed that a higher risk of chronic fatigue may be specific to certain ethnic groups, such as Native Americans and African Americans in the USA [48], and that Hispanics report higher levels of fatigue compared to Anglos [49, 50].
Along with sex and ethnic differences, obesity has been associated with chronic fatigue [51]. BMI of 25 kg/m2 and above is considered overweight, and BMI >30 kg/m2 is considered obese. A longitudinal study investigated weight trends over time in a community-based sample of individuals with chronic fatigue syndrome (CFS) and healthy controls [52]. Over 10-year follow up, the authors found that the overweight/obese CFS group had significantly more fatigue when compared to overweight controls. In one study, fatigue correlated strongly with being obese (BMI >30 kg/m2; OR = 4.1), and this characteristic was associated with eating less healthy diet (less fruits, vegetables, and cereal in meals), as well as being less physically active [45]. Excessive body fat is associated with increased levels of circulating inflammatory cytokines [53]. For example, in sleep apneic patients with fatigue and sleepiness symptoms, levels of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) were elevated. Interestingly, in sleep apneic patients, the primary determinant for IL-6 levels was the body mass index [54]. Indeed, we observed a significant increase in fatigue among PID patients who were also clinically obese with mean BMI of (25 ± 7.4), and that as BMI increases, the odds of fatigue increases.
Obesity can contribute to fatigue due to increased sleep problems, comorbid medical illnesses, psychiatric disorders, and unexplored metabolic processes, in addition to increased inflammatory status [55, 56]. At a minimum, this finding validates known general associations between obesity and fatigue within a PID population and suggests that measures to control obesity in PID patients should be considered for many reasons including the association with fatigue.
Finally, we need to note that fatigue is a multidimensional concept; it manifests in multiple independent dimensions, including physical, cognitive, motivational, and reduced activities [57]. Various diseases showed that fatigue has a major impact on QoL [8–10, 58, 59], and in a large cross-sectional study of over 11,000 cancer patients, fatigue was identified as a root factor in driving global health and overall QoL, independent of tumor stage [59]. Additionally, fatigue was found to affect perceived health (PH), in a way that higher presence of fatigue in daily activities correlated negatively with perceived health [60]. A recent study identified several factors that were associated with poor perceived health among patients with PID registered in the Immunodeficiency Foundation IDF [61]. Of those factors, female sex, CVID diagnosis, comorbid conditions, and limited physical activity pointed to similar demographics that we found to be associated with increased prevalence of fatigue among PIDs. Although perceived health status is a reasonable indicator of global quality of life, disagreement between patients’ reported health status and their perceptions of their global quality of life is common [62]. This suggest that fatigue is a stronger indicator of QoL than perceived health is. Thus, it is important to identify and isolate fatigue as a component of PH and QoL as it may be treatable and has the potential to improve both.
This study has several limitations that should be acknowledged. First, our analysis was limited to the data available in the USIDnet. Given that this is a healthcare-reported database, fatigue definition probably varied based on each provider’s understanding, and the severity and temporality of the fatigue could not be quantified. As a result, fatigue prevalence could be underestimated, especially when one considers previous research showing that physicians often underestimate their patients’ health symptoms, particularly fatigue [63]. We also do not know if the physicians have utilized specific assessments for fatigue or if the registry simply contains their best assessment or perspective. In our analysis, we found that none of the patients with DiGeorge syndrome were reported to have fatigue and noted a low prevalence of fatigue among patients with SCID. This could be explained by underreporting fatigue in children as discussed above, and in the case of DiGeorge, an additional limitation would be the difficulty in measuring symptoms in those with learning disability without using specific tools. Another thought would be that patients who survived life-threatening diseases such as SCID and complete DiGeorge might be more tolerant to subjective adversities. For example, cancer survivors sometimes report “life-transforming” changes [64], mainly due to positive psychological, social, or spiritual experiences that occurred during their illness. Those lessons learned from suffering/or healing allow them to develop coping methods such as resilience [65] and subjective well-being [66].
As we have discussed above, the use of a validated fatigue instrument is of the essence. This would represent an appropriate next step given the signal discerned through our analyses of registry reported data. Despite the possible limitations, however, the apparent difference in fatigue prevalence between PAD and non-PAD patients suggests that our findings likely reflect a bonafide increase in the prevalence of fatigue among those suffering from PAD. A further limitation is that the immune phenotyping, BMI data, IgG replacement therapy, and non-infectious complication data were not consistently available for all patients analyzed, and thus, our current conclusions may suffer from underreporting bias. Finally, the data about the frequency of infections and IgG trough levels, in correlation with fatigue, were not available; hence, we were unable to correlate IgG trough levels and infection rates with fatigue in our analysis. Prior studies suggest that fatigue increased toward the end of IVIG infusion [28] when IgG levels trend down, and patients had a higher susceptibility to infections. Despite these limitations, our findings provide rationale for a prospective research design and patient-reported symptoms.
In conclusion, our results suggest an increased prevalence in fatigue among PID patients, particularly in the PAD population, specifically in CVID patients. Prospective studies using validated instruments to estimate prevalence, risk factors, and fatigue etiology in PID are warranted, so therapeutic interventions can be conceived and implemented to improve the QoL for patients coping with this challenging disease
Acknowledgments
We grateful acknowledge Ms. Marla Goldsmith USIDNET Registry Manager and Tara Caulder Project Director for their expertise, dedication and support through every step of this project.
Abbreviations
- PID
Primary immune deficiency disorder
- PAD
primary antibody deficiency
- QoL
quality of life
- USIDnet
US Immunodeficiency Network
- CVID
common variable immunodeficiency
- DGS
DiGeorge syndrome
- MAS
miscellaneous antibody deficiency
- AGAMMA
agammaglobulinemia
- CGD
chronic granulomatous disease
- SCID
severe combined immunodeficiency disorder
- ALPS
autoimmune lymphoproliferative syndrome
- APCED
autoimmune polyendocrinopathy with candidiasis and ectodermal dystrophy
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy X-linked
- HIGM
hyperIgM syndrome
- ALC
Absolute lymphocyte count
- IgG
immunoglobulin G
- IVIG
intravenous immunoglobulin G
- SCIG
subcutaneous immunoglobulin G
- IBD
inflammatory bowel disease
- IDF
immune deficiency foundation
- TNF
tumor necrosis factor
- STAT
signal transducer and activator of transcription
- BMI
body mass index
- CFS
chronic fatigue syndrome
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Bonilla FA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136(5):1186–1205. e78. doi: 10.1016/j.jaci.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 2.Bousfiha A, et al. The 2015 IUIS Phenotypic Classification for Primary Immunodeficiencies. J Clin Immunol. 2015;35(8):727–38. doi: 10.1007/s10875-015-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousfiha AA, et al. Primary immunodeficiency diseases worldwide: more common than generally thought. J Clin Immunol. 2013;33(1):1–7. doi: 10.1007/s10875-012-9751-7. [DOI] [PubMed] [Google Scholar]
- 4.Kobrynski L, Powell RW, Bowen S. Prevalence and morbidity of primary immunodeficiency diseases, United States 2001–2007. J Clin Immunol. 2014;34(8):954–961. doi: 10.1007/s10875-014-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109(6):1001–1004. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 6.Orange JS, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(4 Suppl):S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Roifman CM, et al. Benefit of intravenous IgG replacement in hypogammaglobulinemic patients with chronic sinopulmonary disease. Am J Med. 1985;79(2):171–4. doi: 10.1016/0002-9343(85)90006-3. [DOI] [PubMed] [Google Scholar]
- 8.Jiang F, Torgerson TR, Ayars AG. Health-related quality of life in patients with primary immunodeficiency disease. Allergy Asthma Clin Immunol. 2015;11:27. doi: 10.1186/s13223-015-0092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efficace F, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27(7):1511–1519. doi: 10.1038/leu.2013.51. [DOI] [PubMed] [Google Scholar]
- 10.Efficace F, et al. Prognostic value of self-reported fatigue on overall survival in patients with myelodysplastic syndromes: a multicentre, prospective, observational, cohort study. Lancet Oncol. 2015;16(15):1506–1514. doi: 10.1016/S1470-2045(15)00206-5. [DOI] [PubMed] [Google Scholar]
- 11.Gripp S, et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25(22):3313–3320. doi: 10.1200/JCO.2006.10.5411. [DOI] [PubMed] [Google Scholar]
- 12.Fink AM, et al. Fatigue, inflammation, and projected mortality in heart failure. J Card Fail. 2012;18(9):711–716. doi: 10.1016/j.cardfail.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887–895. doi: 10.1093/gerona/glq064. [DOI] [PubMed] [Google Scholar]
- 14.Stridsman C, et al. Fatigue Affects Health Status and Predicts Mortality Among Subjects with COPD: Report from the Population-Based OLIN COPD Study. COPD. 2015;12(2):199–206. doi: 10.3109/15412555.2014.922176. [DOI] [PubMed] [Google Scholar]
- 15.Groenvold M, et al. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105(2):209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 16.USIDnet. from USIDNET.org. Available at: http://www.usidnet.org/
- 17.Walker EA, Katon WJ, Jemelka RP. Psychiatric disorders and medical care utilization among people in the general population who report fatigue. J Gen Intern Med. 1993;8(8):436–440. doi: 10.1007/BF02599621. [DOI] [PubMed] [Google Scholar]
- 18.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160(2):221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 19.Health, N.I.o.M. Major Depression Among Adults. 2010 Available from: http://www.nimh.nih.gov/health/statistics/prevalence/ majordepression-among-adults.shtml.
- 20.Lawrie SM, et al. A population-based incidence study of chronic fatigue. Psychol Med. 1997;27(2):343–353. doi: 10.1017/s0033291796004357. [DOI] [PubMed] [Google Scholar]
- 21.Huang IC, et al. The relationships between fatigue, quality of life, and family impact among children with special health care needs. J Pediatr Psychol. 2013;38(7):722–731. doi: 10.1093/jpepsy/jst016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee H. Relationships between physical symptoms and pubertal development. J Pediatr Health Care. 2005;19(2):95–103. doi: 10.1016/j.pedhc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Rhee H, et al. Prevalence of recurrent physical symptoms in U.S. adolescents. Pediatr Nurs. 2005;31(4):314–319. 350. [PubMed] [Google Scholar]
- 24.Varni JW, et al. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002a;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Burwinkle TM, Szer IS. The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: reliability and validity. J Rheumatol. 2004;31(12):2494–2500. [PubMed] [Google Scholar]
- 26.Varni JW, et al. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory Generic Core Scales and Rheumatology Module. Arthritis Rheum. 2002b;46(3):714–725. doi: 10.1002/art.10095. [DOI] [PubMed] [Google Scholar]
- 27.Kanani A, Schellenberg R, Stark D. A pilot study of quality of life, mood, sleepiness and fatigue in patients with primary immunodeficiency receiving IVIG. Journal of Allergy and Clinical Immunology. 2007;119(1):S251. [Google Scholar]
- 28.Misbah S, et al. Subcutaneous immunoglobulin: opportunities and outlook. Clin Exp Immunol. 2009;158(Suppl 1):51–59. doi: 10.1111/j.1365-2249.2009.04027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am. 2008;28(4):779–802. viii. doi: 10.1016/j.iac.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Foundation, I.D. [Cited 23 March 2015];Treatment Experiences and Preferences of Patients with Primary Immune Deficiency Diseases: First National Survey. 2003 Available from: https://primaryimmune.org/wp-content/uploads/2011/04/Treatment-Experiences-and-Preferences-of-Patients-with-Primary-Immune-Deficiency-Disease-First-National-Survey-2002.pdf.
- 31.Kerr J, et al. Is dosing of therapeutic immunoglobulins optimal? A review of a three-decade long debate in europe. Front Immunol. 2014;5:629. doi: 10.3389/fimmu.2014.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abolhassani H, et al. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta analysis. J Clin Immunol. 2012;32(6):1180–1192. doi: 10.1007/s10875-012-9720-1. [DOI] [PubMed] [Google Scholar]
- 33.Rojavin MA, Hubsch A, Lawo JP. Quantitative Evidence of Wear- Off Effect at the End of the Intravenous IgG(IVIG) Dosing Cycle in Primary Immunodeficiency. J Clin Immunol. 2016;36(3):210–219. doi: 10.1007/s10875-016-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quality., C.f.B.H.S.a. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. (HHS Publication No. SMA 15-4927, NSDUH Series H-50) 2015. [Google Scholar]
- 35.Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry. 2010;197(2):86–87. doi: 10.1192/bjp.bp.109.076604. [DOI] [PubMed] [Google Scholar]
- 36.Parker AJ, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med. 2001;31(8):1331–1345. doi: 10.1017/s0033291701004664. [DOI] [PubMed] [Google Scholar]
- 37.Hickie I, Kirk K, Martin N. Unique genetic and environmental determinants of prolonged fatigue: a twin study. Psychol Med. 1999;29(2):259–268. doi: 10.1017/s0033291798007934. [DOI] [PubMed] [Google Scholar]
- 38.Harvey SB, et al. The relationship between fatigue and psychiatric disorders: evidence for the concept of neurasthenia. J Psychosom Res. 2009;66(5):445–454. doi: 10.1016/j.jpsychores.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh EA. Fatigue in childhood multiple sclerosis. Dev Med Child Neurol. 2016;58(3):218. doi: 10.1111/dmcn.13055. [DOI] [PubMed] [Google Scholar]
- 40.Mahieu MA, et al. Fatigue, patient reported outcomes, and objective measurement of physical activity in systemic lupus erythematosus. Lupus. 2016 doi: 10.1177/0961203316631632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones EA. Fatigue complicating chronic liver disease. Metab Brain Dis. 2004;19(3–4):421–429. doi: 10.1023/b:mebr.0000043986.70972.3b. [DOI] [PubMed] [Google Scholar]
- 42.Minnock P, et al. Perceptions of the Cause, Impact and Management of Persistent Fatigue in Patients with Rheumatoid Arthritis Following Tumour Necrosing Factor Inhibition Therapy. Musculoskeletal Care. 2016 doi: 10.1002/msc.1136. [DOI] [PubMed] [Google Scholar]
- 43.Barroso J, et al. Fatigue in HIV-Infected People: A Three-Year Observational Study. J Pain Symptom Manage. 2015;50(1):69–79. doi: 10.1016/j.jpainsymman.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swain MG. Fatigue in chronic disease. Clin Sci (Lond) 2000;99(1):1–8. [PubMed] [Google Scholar]
- 45.van't Leven M, et al. Fatigue and chronic fatigue syndrome-like complaints in the general population. Eur J Public Health. 2010;20(3):251–257. doi: 10.1093/eurpub/ckp113. [DOI] [PubMed] [Google Scholar]
- 46.NIH. Unexplained Fatigue in Elderly. 2007. [Google Scholar]
- 47.Furberg H, et al. The prevalence of self-reported chronic fatigue in a U.S. twin registry. J Psychosom Res. 2005;59(5):283–290. doi: 10.1016/j.jpsychores.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinos S, et al. A systematic review of chronic fatigue, its syndromes and ethnicity: prevalence, severity, co-morbidity and coping. Int J Epidemiol. 2009;38(6):1554–1570. doi: 10.1093/ije/dyp147. [DOI] [PubMed] [Google Scholar]
- 49.Jason LA, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159(18):2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 50.Song S, Jason LA, Taylor RR. The Relationship Between Ethnicity and Fatigue in a Community-Based Sample. Journal of Gender, Culture and Health. 1999;4(4):255–268. [Google Scholar]
- 51.Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083:329–344. doi: 10.1196/annals.1367.023. [DOI] [PubMed] [Google Scholar]
- 52.Flores S, et al. Examining the impact of obesity on individuals with chronic fatigue syndrome. Workplace Health Saf. 2013;61(7):299–307. doi: 10.3928/21650799-20130617-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 54.Vgontzas AN, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82(5):1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 55.Lim W, et al. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch Intern Med. 2005;165(8):910–915. doi: 10.1001/archinte.165.8.910. [DOI] [PubMed] [Google Scholar]
- 56.Fukuda K, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 57.Echteld MA, et al. Multidimensional fatigue and its correlates in hospitalised advanced cancer patients. Eur J Cancer. 2007;43(6):1030–1036. doi: 10.1016/j.ejca.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Stauder MC, et al. Overall survival and self-reported fatigue in patients with esophageal cancer. Support Care Cancer. 2013;21(2):511–519. doi: 10.1007/s00520-012-1537-1. [DOI] [PubMed] [Google Scholar]
- 59.McCabe RM, et al. Fatigue as a Driver of Overall Quality of Life in Cancer Patients. PLoS One. 2015;10(6):e0130023. doi: 10.1371/journal.pone.0130023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flensner G, et al. Fatigue in relation to perceived health: people with multiple sclerosis compared with people in the general population. Scand J Caring Sci. 2008;22(3):391–400. doi: 10.1111/j.1471-6712.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 61.Seeborg FO, et al. Perceived Health in Patients with Primary Immune Deficiency. J Clin Immunol. 2015;35(7):638–650. doi: 10.1007/s10875-015-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Covinsky KE, et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med. 1999;106(4):435–440. doi: 10.1016/s0002-9343(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 63.Efficace F, et al. Patient- versus physician-reporting of symptoms and health status in chronic myeloid leukemia. Haematologica. 2014;99(4):788–793. doi: 10.3324/haematol.2013.093724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skeath P, et al. The nature of life-transforming changes among cancer survivors. Qual Health Res. 2013;23(9):1155–1167. doi: 10.1177/1049732313499074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masten AS, Coatsworth JD. The development of competence in favorable and unfavorable environments. Lessons from research on successful children. Am Psychol. 1998;53(2):205–220. doi: 10.1037//0003-066x.53.2.205. [DOI] [PubMed] [Google Scholar]
- 66.Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull. 2008;134(2):163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]