Abstract
Background
Trigeminal neuralgia (TN) is a chronic brain condition involving the trigeminal nerve and characterized by severe and recurrent facial pain. While the etiology of TN has been researched extensively, there is a lack of convergence on the exact physiological processes leading to pain symptoms. This review seeks to better elucidate the underlying pathophysiology of TN by analyzing the outcomes of studies that utilize magnetic resonance (MR) structural imaging and diffusion-weighted imaging (DWI) to examine nerve damage in patients with TN.
Methods
Performing a structured review of the literature, the authors included human MR anatomical and DWI studies aimed at visualizing the trigeminal nerve and/or measuring neural damage pertaining to TN. Studies that measured and compared nerve damage in the affected and unaffected sides in patients and/or patients and controls were analyzed for neural changes associated with TN.
Results
Twenty-five studies met inclusion criteria. Overall, the data from the anatomical and diffusion studies showed decreased volume and cross sectional area, decreased fractional anisotropy, and increased apparent diffusion coefficient and diffusivity associated with the affected side of patients compared to the unaffected side as well as in patients compared to controls.
Conclusion
A review of the included studies indicates that neural differences exist between the affected and unaffected sides in patients as well as between patients and controls in both structural and diffusion metrics. The amalgamated data suggests that damage of the trigeminal nerve tissue is commonly found in TN patients and could be a primary factor in TN pathophysiology.
Keywords: Diffusion Weighted Imaging, Magnetic Resonance Imaging, Nerve Damage, Trigeminal Neuralgia
Introduction and Etiology
Trigeminal neuralgia (TN) is a debilitating neuropathic brain condition, which classically involves the sensory branch of the trigeminal nerve. Primarily affecting the elderly population, TN involves severe, episodic facial pain characterized by extreme, sudden shock-like or burning sensations. [1, 2] Classically described as a sharp lancinating pain, this syndrome is often initially misdiagnosed and under-treated. Many patients ultimately require surgical intervention for long-term pain relief. [2] The economic aspects of TN are significant as well, with approximately 15,000 new patients being diagnosed in the United States annually [3] and an estimated healthcare cost exceeding $100 million per year for surgery alone. [1]
The physiological basis of TN has been an ongoing topic of research and study. [2, 4] Possible etiologies of the disease include neurovascular compression (NVC) [5], multiple sclerosis [6], tumors [7], arteriovenous malformations [8], and facial injury. [9] The most commonly accepted etiology in TN is thought to be related to NVC [1] as originally described by Dr. Janetta. [10] Mechanical compression of the trigeminal nerve can occur as the nerve leaves the brainstem pons and passes across the subarachnoid space toward Meckel’s cave. [9] It is believed that the nerve region is especially susceptible to pathologic changes from vascular contact in the Redlich-Obersteiner’s zone, also known as the root entry zone (REZ). Serving as a boundary between the central and peripheral nervous systems, the REZ is characterized by nerve axons ensheathed in central myelin and subsequently transitioning to peripheral myelin.
Proposed Neurophysiological Mechanisms
Vascular contact in the REZ region can lead to chronic demyelination and abnormal conduction in the nerve, resulting in structural disarray, electrical instability, and atrophy. [7] This theory, raised by Devor et al. and coined as the ignition hypothesis [11], is characterized by the discovery that injured sensory neurons often become electrically hyperexcitable and generate abnormal spike discharge. [12] It is believed that spontaneous occurrence of ectopic firing results in burning sensations and paresthesias, symptoms that are reported in some TN patients. [1] Other injured sensory neurons that are silent but have a hair-trigger threshold can give rise to a surge of spontaneous firing and neuronal afterdischarge. This is presumed to cause the pain paroxysms present in TN patients. [12] The sudden onset of the intense pain paroxysms experienced by some patients with TN points to a synchronization of the afterdischarge bursts, made possible by axon-to-axon cross-excitation or crossed after-discharge. Cross-excitation between neurons, also referred to as ephaptic crosstalk, can occur through close membrane apposition. [13] This pathology can arise from a lack of myelin sheaths and intervening glial processes. [14]
The second mechanism for synchronization, crossed after-discharge, is characterized by nonsynaptic and nonephaptic coupling occurring within sensory ganglia as well as at injured nerve regions. In this mechanism, impulse activity induces nonsynaptic release of potassium ions or a neurotransmitter(s) into the interstitial space, which move by diffusion and generate afterdischarge in neighboring neurons. [15] Both synchronization mechanisms operate such that even a brief stimulus at a trigger point could be sufficient to induce immense synchronous activity. [12] The ignition hypothesis explains the electrical mechanism by which aberrations from loss of nerve integrity can result in abnormal contacts between nerve fibers and underlie the symptomology of TN. [16]
Dissenting Views to Neurovascular Compression as Etiology
Although NVC is a commonly accepted cause for TN symptoms, some imaging studies have shown that TN can be present and recur in the absence of NVC. [17, 18] Since TN is considered a clinical description disease, magnetic resonance imaging (MRI) is not the standard of care in diagnosis and treatment and, consequently, proof of compression is not required nor often confirmed for treatment or surgery in the majority of cases. A review of autopsy studies shows NVC in 90%–100% of TN patients, yet also in 16%–58% of patients without TN. [19–22] Miller et al. [23] examined NVC in TN patients as well as patients without TN and found that NVC of the trigeminal nerve was more severe in patients with TN, although NVC also occurred in healthy patients. While a review of the literature indicates that a wide range of 11%–96% of TN patients have demonstrable NVC, this leaves 4%–89% of TN patients with no observable NVC [17, 20, 24, 25]. This wide range may be due to selection bias, inclusion of older studies, and/or use of poor quality MRI.
MRI Imaging in TN
MRI has been used to examine neural changes and atrophy at the REZ associated with TN. [16, 26, 27] These studies have been useful in investigating the potential pathophysiology of TN. This structured review examines the existing literature on whether damage to the trigeminal nerve has been identified in TN patients through advanced MRI techniques including structural imaging and diffusion-weighted imaging (DWI). These MRI techniques are used extensively in TN studies, since they have efficacious features for detecting nerve damage.
DWI allows for visualization of tissue microstructure and white matter tracts in the brain and spinal cord through tractography. [28] Neuronal structural connectivity can be estimated by measuring diffusivity of protons along axonal paths. Alterations in tissue microstructure can be quantified by measuring complementary changes in the diffusion of water within those tissues. DWI has been a successful tool with clinical applications including characterization of white matter in patients with brain tumors and characterization of acute ischemic stroke lesions in the central nervous system. [29] It has also been used to determine the microstructural neural changes associated with TN utilizing parameters such as fractional anisotropy (FA), diffusivity, and apparent diffusion coefficient (ADC) for quantitative analysis of nerve integrity. [6, 30] White matter neuropathology frequently causes a decrease in FA, but since this may result from increased radial diffusivity and/or decreased axial diffusivity, other parameters in addition to FA are needed to properly characterize the tissue changes. Moreover, measuring radial and axial diffusivities can provide specific information about the diffusion tensor and alterations in tissue architecture. [29] ADC is informative since it measures the magnitude of diffusion of water molecules within tissue, which can alter with changes in tissue microstructure. In addition, two main sequence types used in structural imaging for visualization of the nerve are SSFP-based (steady state free precession) sequences and T1 and T2-weighted imaging sequences utilized at high resolution. Mean cross sectional area and volume of the nerve are often measured to have a quantitative metric of nerve damage. [31, 32]
Exploring and understanding the neural changes and damage associated with TN can allow better identification of the source of pain and etiology of the disease, ultimately providing a framework for timely and accurate diagnosis as well as effective treatments addressing the root cause of the disease.
Methods
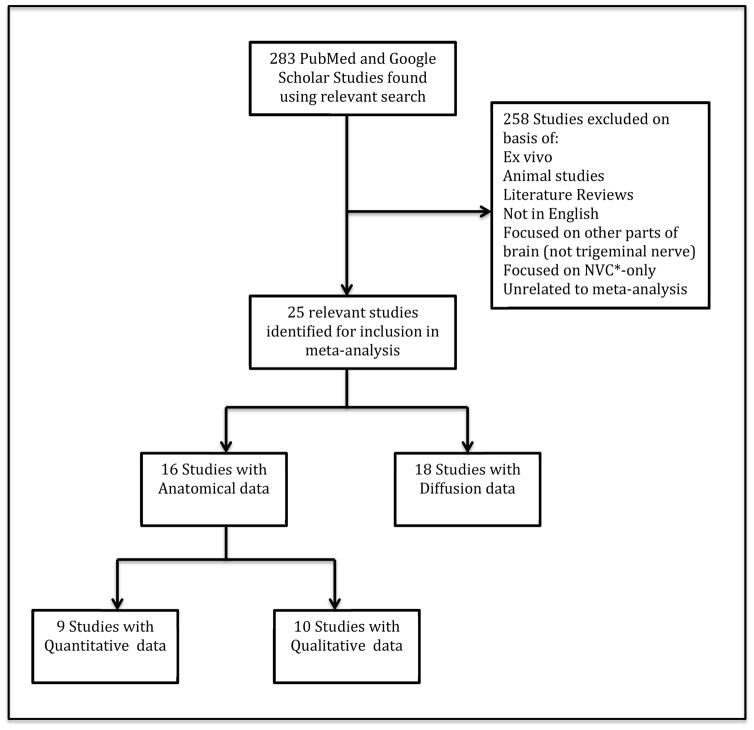
Searches were conducted using PubMed and Google Scholar. Of the 283 studies reviewed, 25 were relevant for inclusion (see Figure 1). The criteria determined for inclusion were: recent studies describing imaging techniques performed on humans for the purpose of tracking or visualizing the trigeminal nerve and/or measuring the degree of nerve damage or atrophy.
Figure 1.
Flowchart of study selection strategy
* Neurovascular Compression
Studies that met these inclusion criteria were separated into two categories: “Anatomical” and “Diffusion-Weighted Imaging” studies. Anatomical studies were further subdivided into studies that analyzed the nerve through “Qualitative” versus “Quantitative” measures. The parameters evaluated included neurovascular compression, fractional anisotropy, and mean cross-sectional area of the nerve, among others. Studies which went beyond delineating and tracking the trigeminal nerve itself and explored nerve damage, often did so by comparing the affected and unaffected sides of the nerve in patients and/or comparing affected and unaffected sides in patients and controls.
Studies that focused on NVC only, or were preclinical, such as those performed on primate models, or focused on other parts of the brain, such as the thalamus and grey matter, were not included. See Figure 1 for a flowchart of study inclusion strategy and a full list of study exclusion criteria.
Results
Included Studies
Of the titles and abstracts reviewed through the searches, 25 studies [6, 16, 26, 27, 30–50] were selected for inclusion in an effort to understand whether nerve damage is a key component of the pain in TN. Of the 25 included studies, there were seven anatomical-only studies, nine DWI-only studies, and nine studies that fell into both categories (see Figure 1). Four of the DWI-only studies performed trigeminal nerve visualization only [45–47, 49] while the remaining twenty-one studies in the review inspected the neural alterations associated with TN. Anatomical imaging and DWI results are discussed separately in the upcoming sections, with further distinction made between studies that compare TN patients to healthy controls and studies that compare the affected and unaffected sides of the trigeminal nerve. Thirteen studies compared TN patients to healthy controls (see Tables 1, 2a, and 2b). Twelve of the studies also identified neurovascular compression on the affected side of the trigeminal nerve (see Table 1). Two studies analyzed nerve changes by comparing pre- and post-surgery measurements (see Table 2b).
Table 1.
Summary of Anatomical Data for Qualitative and Quantitative Structural Nerve Damage, Neurovascular Compression, Volume, and Cross Sectional Area of the Trigeminal Nerve. N = patient number, H= healthy control number, SND = structural nerve damage, NVC = neurovascular compression, CSA = cross sectional area, REZ = root entry zone, Y = Yes, “
 ” = significant increase (on the affected side, in patients, or after surgery), “
” = significant increase (on the affected side, in patients, or after surgery), “
 ” = significant decrease (on the affected side, in patients, or after surgery), “
” = significant decrease (on the affected side, in patients, or after surgery), “
 ” = not significant, “–” = not studied; Sequence Acronyms: SSFP = steady-state free precession, bSSFP = balanced steady-state free precession, Drive = driven equilibrium, TOF = time of flight, TOF-MRA = time of flight angiography, Gad = gadolinium, FIESTA = fast imaging employing steady-state acquisition, ceMRA = contrast enhanced MR angiography, FLAIR = fluid-attenuated inversion recovery, ceFSPGR = contrast enhanced fast spoiled gradient echo, FLASH = fast low angle shot, TrueFISP/TRUFI = true fast imaging with steady state precession, CISS = constructive interference in steady state
” = not significant, “–” = not studied; Sequence Acronyms: SSFP = steady-state free precession, bSSFP = balanced steady-state free precession, Drive = driven equilibrium, TOF = time of flight, TOF-MRA = time of flight angiography, Gad = gadolinium, FIESTA = fast imaging employing steady-state acquisition, ceMRA = contrast enhanced MR angiography, FLAIR = fluid-attenuated inversion recovery, ceFSPGR = contrast enhanced fast spoiled gradient echo, FLASH = fast low angle shot, TrueFISP/TRUFI = true fast imaging with steady state precession, CISS = constructive interference in steady state
| Author | Sequence Type | N | H | SND Identified | NVC Identified | Structural Metric | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qualitative | Quantitative | Volume | CSA Proximal Nerve at REZ | |||||||
| Difference in Mean Volume: Affected vs. Unaffected | Difference in Mean Volume: Patients vs. Controls | Difference in Area: Affected vs. Unaffected | Difference in Area: Patients vs. Controls | |||||||
| Duan (2015) | SSFP | 26 | - | Y | Y | - | - | - |

|
- |
| Fujiwara (2010) | 3D-bSSFP | 13 | 14 | Y | Y | Y | - | - |

|

|
| Leal (2011) | T2-3D Drive, 3D TOF-MRA, 3D T1 with T1-Gad | 10 | 6 | Y | Y | Y |

|

|

|

|
| Lummel (2015) | 3D FIESTA, ceMRA, FLAIR, T2, 3D T1, ceFSPGR | 12 | 12 | - | - | Y | - | - | - | - |
| Lin (2014) | 3D FIESTA | 20 | 18 | - | - | Y | - | - | - | - |
| Wilcox (2013) | T1 | 9 | 26 | - | Y | - |

|

|

|

|
| Kress (2005) | T1 FLASH, T2 TrueFISP | 41 | 50 | Y | Y | Y |

|
- | - | - |
| Lutz (2015) | 3D FIESTA, ceMRA | 81 | - | - | - | Y | - | - | - | - |
| Lutz (2011) | T1, T2, FLAIR, FIESTA | 20 | - | - | - | Y | - | - | - | - |
| Liu (2013) | 3D TOF, FLAIR | 16 | 6 | - | - | Y | - | - | - | - |
| Leal (2014) | T2-3D Drive, 3D TOF-MRA, 3D T1 with T1-Gad | 50 | 20 | Y | Y | Y |

|

|

|

|
| Herweh (2007) | TRUFI-T2, FLAIR | 6 | 7 | Y | - | Y | - | - | - | - |
| Yuan (2015) | 3D FIESTA, TOF-MRA | 40 | 40 | Y | Y | Y |

|

|

|

|
| Antonini (2014) | 3D-CISS, 3D-TOF-MRA, 3D-T1-Gad | 24 | 24 | Y | - | Y | - | - | - | - |
| Park (2009) | T1, T2, CISS | 26 | - | Y | Y | - |

|
- |

|
- |
| Erbay (2006) | CISS | 31 | - | Y | Y | - | - | - |

|
- |
Table 2a.
Summary of Diffusion Data for FA and ADC values. N = patient number, H= healthy control number, FA = fractional anisotropy, ADC = apparent diffusion coefficient, “
 ” = significant increase (on the affected side, in patients, or after surgery), “
” = significant increase (on the affected side, in patients, or after surgery), “
 ” = significant decrease (on the affected side, in patients, or after surgery), “
” = significant decrease (on the affected side, in patients, or after surgery), “
 ” = not significant, “–” = not studied
” = not significant, “–” = not studied
| Author | N | H | FA | ADC | |||||
|---|---|---|---|---|---|---|---|---|---|
| Affected vs. Unaffected | Patients vs. Controls | Pre vs. Post Surgery | Affected vs. Unaffected | Patients vs. Controls | |||||
| Affected | Unaffected | Affected | Unaffected | ||||||
| Alonso (2014) | 10 | - |
 * *
|
- | - | - | - | - | - |
| DeSouza (2014) | 18 | 18 |

|

|

|
- | - | - | - |
| Fujiwara (2010) | 13 | 14 |

|

|

|
- |

|

|

|
| Herweh (2007) | 6 | 7 |
 † †
|
- | - | - | - | - | - |
| Leal (2011) | 10 | 6 |

|

|

|
- |

|

|

|
| Liu (2013) | 16 | 6 |

|

|

|
- | - | - | - |
| Lutz (2011) | 20 | - |

|
- | - | - |

|
- | - |
| Lutz (2015) | 81 | - |

|
- | - | - |
 ‡ ‡
|
- | - |
| Lummel (2015) | 12 | 12 |

|

|

|
- |

|

|

|
| Lin (2014) | 20 | 18 |

|

|

|
- | - | - | - |
| Wilcox (2013) | 9 | 26 |

|

|
- | - | - | - | - |
| Chen (2015) | 10 | 10 |
 § §
|

|

|
- | - | - | - |
| Hodaie (2012) | 5 | - | - | - | - |

|
- | - | - |
| DeSouza (2015) | 25 | 14 |

|

|
- |

|
- | - | - |
8/10 patients
3/6 patients
trend toward significantly higher values
decrease at the ipsilateral REZ segment, while increase in FA in cisternal segment (both ipsilateral to the side of the pain)
Table 2b.
Summary of Diffusion Data for Radial, Axial, and Mean Diffusivity. N = patient number, H= healthy control number, “
 ” = significant increase (on the affected side, in patients, or after surgery), “
” = significant increase (on the affected side, in patients, or after surgery), “
 ” = significant decrease (on the affected side, in patients, or after surgery), “
” = significant decrease (on the affected side, in patients, or after surgery), “
 ” = not significant, “–” = not studied
” = not significant, “–” = not studied
| Author | N | H | Radial Diffusivity | Axial Diffusivity | Mean Diffusivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients vs. Controls | Affected vs. Unaffected |
Pre vs. Post Surgery |
Patients vs. Controls | Affected vs. Unaffected |
Pre vs. Post Surgery |
Patients vs. Controls | Affected vs. Unaffected |
Pre vs. Post Surgery |
||||||
| Affected | Unaffected | Affected | Unaffected | Affected | Unaffected | |||||||||
| DeSouza (2014) | 18 | 18 |

|

|
 * *
|
- |

|

|

|
- |

|

|

|
- |
| Liu (2013) | 16 | 6 |

|

|

|
- |

|

|

|
- |

|

|

|
- |
| Lin (2014) | 20 | 18 |

|

|

|
- |

|

|

|
- |

|
- |

|
- |
| Wilcox (2013) | 9 | 26 | - | - | - | - | - | - | - | - |

|

|

|
- |
| Chen (2015) | 10 | 10 |

|

|

|
- |

|

|

|
- |

|

|

|
- |
| Hodaie (2012) | 5 | - | - | - | - |

|
- | - | - |

|
- | - | - | - |
| DeSouza (2015) | 25 | 14 |

|
- |

|

|
 † †
|
- |

|
 ‡ ‡
|

|
- |

|

|
trend towards significantly higher values
in effective treatment group
excluding left side post-treatment, which was lower than pre-treatment values but still higher than controls
Anatomical Studies
The main outcomes of the studies that performed anatomical evaluation are outlined in Table 1, with eleven studies identifying structural nerve damage. While some of the studies identified significant changes using either qualitative [31, 34] or quantitative [48] measures, the majority of the studies identified structural nerve damage both qualitatively and quantitatively (see Table 1).
Among those studies that performed a quantitative anatomical evaluation, mean volumes of the nerve and/or mean cross sectional area of the proximal nerve at the root entry zone were measured (see Table 1). Of the studies measuring the mean volume of the nerve, some studies found significant alterations in the mean volume either when comparing the affected side to the unaffected side of the nerve [26, 39] or when comparing patients to controls. [48] The rest of the studies [32, 40, 50] measured significant differences in both. All mean volumes measured were smaller in the affected side of the nerve and in patients compared to controls.
Of the studies examining mean cross sectional area, some studies measured significant differences either between the affected and unaffected sides [16, 26, 37] or between patients and controls [48], while the remaining studies [30, 32, 40, 50] identified significant changes in both comparisons. In all cases, the mean cross sectional area was found to be smaller in the affected side than the unaffected side and smaller in patients than controls. See Table 1 for a summary of all anatomical data.
DWI Studies
Four of the DWI studies [45–47, 49] tracked the trigeminal nerve for visualization purposes. Visualizing the trigeminal nerve is important for understanding the nerve’s microanatomy and understanding where abnormalities and damage in the nerve can occur when looking at the nerve in TN patients.
As shown in Table 2a, fourteen DWI studies examined FA and five studies examined ADC. A majority of the studies examining FA by comparing the affected side and unaffected sides, found a significant decrease in FA in the affected side. Of the studies examining FA by comparing the affected and unaffected sides in patients and controls, two [6, 35] found significant decreases in FA on both sides while four of the studies [27, 32, 36, 42] found a significantly lower FA only in the affected side. Hodaie et al. [38] and DeSouza et al. [27] compared FA values as measured before and after surgery, with Hodaie’s study recording a significant decrease in FA after surgery and DeSouza’s study describing a significant increase in FA after surgery.
ADC values were examined by Lummel et al. [6] and Leal et al. [32] with significantly higher ADC values found in the affected side compared to the unaffected side. These studies also found ADC to be higher in the affected side of patients compared to controls. The remaining three studies that examined ADC did not find significance in either of these categories, as seen in Table 2a.
Seven of the 25 studies in this review (displayed in Table 2b) examined diffusivity of the nerve, including radial, axial and/or mean diffusivity. Of those looking at radial diffusivity (RD), three studies [27, 35, 42] found a significant increase when comparing affected to unaffected sides of the nerve, one study [36] found it trending towards significance, while the last study [41] did not find any significance. Two studies [35, 36] found significant increases in the RD when comparing patients to controls in both their affected and unaffected sides, while another two studies [27, 42] measured significance on the affected side of the patients compared to controls, and another study [41] did not find a significant difference in either category. Hodaie et al. [38] and DeSouza et al. [27] found significant differences in RD when comparing the affected side before and after surgery; Hodaie found a significant increase while DeSouza found a significant decrease.
For axial diffusivity (AD), only DeSouza et al. [27] measured significance when comparing the affected and unaffected sides. Two studies [35, 36] found significant increases in both the affected and unaffected sides when comparing patients to controls, one study [27] measured a significant increase on the affected side in patients compared to controls, while another two studies [41, 42] found the measurements for both categories insignificant. When comparing before and after surgery, DeSouza et al. [27] found a significant decrease in AD while Hodaie et al. [38] found no significant changes.
In regards to mean diffusivity (MD), the results were scattered with one study [36] finding a significant increase in the affected and unaffected sides when comparing patients to controls, three studies [27, 35, 42] finding a significant increase only in the affected side when comparing patients to controls, and two studies [27, 35] finding a significant increase when comparing the affected side to the unaffected side. Two studies [41, 48] found no significance in any of the categories. DeSouza et al. [27] found a significant decrease in MD in comparing patients pre- and post-surgery.
Discussion
Several trends have emerged through the analysis of the structural and diffusion data informing nerve changes that occur in TN.
Volume and cross sectional area Decrease
All of the studies that examined the mean volume of the nerve and mean cross sectional area of the proximal nerve at the root entry zone in TN patients found significant decreases in both parameters, both when comparing the affected and unaffected sides and comparing patients to controls. One study that measured the mean diameter of the trigeminal nerve found that it was smaller on the affected side than the unaffected side. Similarly, another study, which measured the length of the cisternal segment of the trigeminal nerve, found that it was smaller on the affected side than the unaffected side. The overall trend of these data suggests that depletion of the trigeminal nerve volume is correlated with TN.
Neurovascular Compression Commonly Seen
In these studies, NVC was generally determined through a qualitative visual inspection using T2-weighted images. Five of the studies measuring quantitative structural nerve damage indicated NVC of the nerve on the affected side and seven of the studies pointing to qualitative structural nerve damage found neurovascular compression of the nerve on the affected side. This supports the idea that TN is accompanied by mechanical compression of the nerve and thereby may result in nerve atrophy. These findings are consistent with those in previous studies, which agree that persistent and severe NVC can lead to atrophy of the trigeminal nerve and tends to induce demyelination and axonal loss in the affected nerve. [51] Moreover, some studies have suggested the ignition hypothesis as an explanation, specifically that damages to the nerve may induce hyper-excitability in the sensory afferents due to ephaptic transmission and synchronized activity between axons after discharge. [52]
Nevertheless, some studies dispute the role of NVC in the etiology for TN, pointing to TN patients without NVC while confirming the presence of NVC in healthy controls. [17, 20, 24, 25] A possible theory is that NVC is not seen in many cases due to insufficient field strength and the resolution and contrast benefits it provides. It may also be helpful to investigate the nature and causes of NVC itself in order to better understand its role in trigeminal neuralgia.
Fractional Anisotropy
Eleven of the studies found significant differences in FA. This is a clear indicator of microstructural changes in the nerve and, perhaps, demyelination, since FA is a measure of tissue microstructure. [4] The overall trend was that decreased FA values were found when comparing patients and controls as well as the affected and unaffected sides. This is consistent with the finding that nerve atrophy is associated with decreased FA values and that degradation of nerve structure indicates not only a compressive effect but also cellular changes. [32]
Apparent Diffusion Coefficient
Of the five studies looking at ADC, two of the studies found a significantly higher ADC in the affected side compared to the unaffected side and in the affected side of patients compared to controls. This is significant for understanding the etiology of TN, because higher ADC values are associated with nerve atrophy. [32]
Diffusivity
Five of the studies examining diffusivity identified significant changes in radial, axial, or mean diffusivity. With the exception of one study that found decreased diffusivity, the remaining studies that found significant differences in diffusivity identified an increase in one or more directions, either when comparing patients to controls, affected and unaffected sides, or pre- and post-surgery outcomes. While all types of diffusivities can be telling, MD (like FA) has been identified as a marker of more subtle changes in tissue microstructure. These changes may emerge from tissue shrinkage and densely packed myelinated fibers as a consequence of neurodegeneration. [53] This suggests that diffusivity, especially the mean diffusivity, is a good indicator of neural microstructure and is useful for identifying the nerve damage accompanied with TN.
Limitations and Biases of this study
Limitations of this review include the small number of studies and small number of patients analyzed, precluding any statistical analysis for substantiating aggregated results. Biases may be introduced by some studies, which included only patients who were surgical candidates or had already undergone surgery, as well as studies which compared patients and controls who were not necessarily age or gender-matched.
In this review we investigated MRI-detectable trigeminal nerve changes, which may serve to identify the source of pain in TN and therefore guide treatment. However, study of the nerve on the cellular and functional levels could also serve to unravel the many layers of TN pathology.
Conclusions and Future Directions
This review reports the overall trigeminal nerve differences observed between patients and controls in both diffusion metrics and structural metrics.
The aggregated results of TN structural and diffusion imaging studies suggest that the etiology of TN may involve mechanical compression of the trigeminal nerve resulting in intrinsic tissue loss and structural changes in the trigeminal nerve. In twelve out of sixteen cases, NVC, as revealed through imaging, was found in combination with trigeminal nerve atrophy indicated by structural and diffusion metrics.
Future studies could be focused on utilizing additional measures for the anatomical basis of TN such as enhanced vascular imaging, higher resolution structural imaging, and higher resolution DWI, in order to more accurately characterize nerve loss and identify NVC in TN. [54, 55] In addition, it may be useful to have a separate analysis looking at the correlation between structural nerve damage and NVC, in order to determine how the two factors may be linked in the etiology of TN. Metabolic nerve studies may also prove useful. One avenue for accomplishing this may be to use higher field strengths such as 7T MRI. [56]
Gaining further insight into the pathophysiology of TN can help in treatment selection, therapeutic development, and surgical planning and can hopefully improve clinical patient outcomes in the future. [12]
Highlights.
Neural differences are observed between patients with TN and controls as well as between the affected and unaffected sides of the nerve in patients
Nerve damage is found to be associated with TN
Nerve damage may play a role in the etiology of TN
Acknowledgments
The authors thank Thomas Barrett for his assistance with the literature review process. This work was supported by National Institutes of Health under NINDS R00 NS070821 and NCI R01 CA202911, and the Icahn School of Medicine Capital Campaign, Translational and Molecular Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai. Portions of this work were presented as part of the first author’s Masters thesis at the School of Engineering of City College of New York.
Abbreviations
- AD
axial diffusivity
- ADC
apparent diffusion coefficient
- DWI
diffusion-weighted imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- NVC
neurovascular compression
- RD
radial diffusivity
- REZ
root entry zone
- SSFP
steady state free precession
- TN
trigeminal neuralgia
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollock BE, Ecker RD. A prospective cost-effectiveness study of trigeminal neuralgia surgery. Clin J Pain. 2005;21(4):317–22. doi: 10.1097/01.ajp.0000125267.40304.57. [DOI] [PubMed] [Google Scholar]
- 2.Harsha KJ, et al. Imaging of vascular causes of trigeminal neuralgia. J Neuroradiol. 2012;39(5):281–9. doi: 10.1016/j.neurad.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Katusic S, et al. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Annals of neurology. 1990;27(1):89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- 4.Montano N, et al. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015;11:289–99. doi: 10.2147/TCRM.S37592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pamir MN, et al. Microvascular decompression in the surgical management of trigeminal neuralgia. Neurosurg Rev. 1995;18(3):163–7. doi: 10.1007/BF00383719. [DOI] [PubMed] [Google Scholar]
- 6.Lummel N, et al. Diffusion tensor imaging of the trigeminal nerve in patients with trigeminal neuralgia due to multiple sclerosis. Neuroradiology. 2015;57(3):259–67. doi: 10.1007/s00234-014-1463-7. [DOI] [PubMed] [Google Scholar]
- 7.Samadian M, et al. Trigeminal Neuralgia Caused by Venous Angioma: A Case Report and Review of the Literature. World Neurosurg. 2015;84(3):860–4. doi: 10.1016/j.wneu.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 8.Isik S, et al. Trigeminal Neuralgia Caused by Cerebellopontine Angle Arteriovenous Malformation Treated With Gamma Knife Radiosurgery. J Craniofac Surg. 2016;27(1):e55–7. doi: 10.1097/SCS.0000000000002310. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal SM, Kambalimath DH. Trigeminal neuralgia involving supraorbital and infraorbital nerves. Natl J Maxillofac Surg. 2010;1(2):179–82. doi: 10.4103/0975-5950.79226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jannetta PJ. Neurovascular compression in cranial nerve and systemic disease. Annals of surgery. 1980;192(4):518. doi: 10.1097/00000658-198010000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. The Clinical journal of pain. 2002;18(1):4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96(3):532–43. doi: 10.3171/jns.2002.96.3.0532. [DOI] [PubMed] [Google Scholar]
- 13.Amir R, Devor M. Axonal cross-excitation in nerve-end neuromas: comparison of A-and C-fibers. Journal of neurophysiology. 1992;68(4):1160–1166. doi: 10.1152/jn.1992.68.4.1160. [DOI] [PubMed] [Google Scholar]
- 14.Hilton DA, et al. Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery. 1994;35(2):299–303. doi: 10.1227/00006123-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. The Journal of neuroscience. 1996;16(15):4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erbay SH, et al. Nerve atrophy in severe trigeminal neuralgia: noninvasive confirmation at MR imaging--initial experience. Radiology. 2006;238(2):689–92. doi: 10.1148/radiol.2382042214. [DOI] [PubMed] [Google Scholar]
- 17.Lee A, et al. Trigeminal neuralgia occurs and recurs in the absence of neurovascular compression: Clinical article. Journal of neurosurgery. 2014;120(5):1048–1054. doi: 10.3171/2014.1.JNS131410. [DOI] [PubMed] [Google Scholar]
- 18.Ko AL, et al. Trigeminal neuralgia without neurovascular compression presents earlier than trigeminal neuralgia with neurovascular compression. Journal of neurosurgery. 2015;123(6):1519–1527. doi: 10.3171/2014.11.JNS141741. [DOI] [PubMed] [Google Scholar]
- 19.Haines SJ, Jannetta PJ, Zorub DS. Microvascular relations of the trigeminal nerve: an anatomical study with clinical correlation. Journal of neurosurgery. 1980;52(3):381–386. doi: 10.3171/jns.1980.52.3.0381. [DOI] [PubMed] [Google Scholar]
- 20.Hamlyn PJ, King TT. Neurovascular compression in trigeminal neuralgia: a clinical and anatomical study. Journal of neurosurgery. 1992;76(6):948–954. doi: 10.3171/jns.1992.76.6.0948. [DOI] [PubMed] [Google Scholar]
- 21.Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. 1. Review of the literature and development of a new method of vascular injection-filling in cadaveric controls. Clinical Anatomy. 1997;10(6):371–379. doi: 10.1002/(SICI)1098-2353(1997)10:6<371::AID-CA1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Hamlyn PJ. Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. 2. Neurovascular compression of the trigeminal nerve in cadaveric controls and patients with trigeminal neuralgia: quantification and influence of method. Clinical Anatomy. 1997;10(6):380–388. doi: 10.1002/(SICI)1098-2353(1997)10:6<380::AID-CA2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Miller JP, et al. Radiographic evaluation of trigeminal neurovascular compression in patients with and without trigeminal neuralgia: Clinical article. Journal of neurosurgery. 2009;110(4):627–632. doi: 10.3171/2008.6.17620. [DOI] [PubMed] [Google Scholar]
- 24.Adams C, Kaye AH, Teddy P. The treatment of trigeminal neuralgia by posterior fossa microsurgery. Journal of Neurology, Neurosurgery & Psychiatry. 1982;45(11):1020–1026. doi: 10.1136/jnnp.45.11.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia*. Journal of neurosurgery. 1967;26(1part2):159–162. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- 26.Park SH, et al. Nerve atrophy and a small cerebellopontine angle cistern in patients with trigeminal neuralgia. J Neurosurg. 2009;110(4):633–7. doi: 10.3171/2008.8.JNS08522. [DOI] [PubMed] [Google Scholar]
- 27.DeSouza DD, Davis KD, Hodaie M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain. 2015;156(6):1112–23. doi: 10.1097/j.pain.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 28.Jones DK. Diffusion mri. Oxford University Press; 2010. [Google Scholar]
- 29.Alexander AL, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara S, et al. High-resolution diffusion tensor imaging for the detection of diffusion abnormalities in the trigeminal nerves of patients with trigeminal neuralgia caused by neurovascular compression. J Neuroimaging. 2011;21(2):e102–8. doi: 10.1111/j.1552-6569.2010.00508.x. [DOI] [PubMed] [Google Scholar]
- 31.Herweh C, et al. Loss of anisotropy in trigeminal neuralgia revealed by diffusion tensor imaging. Neurology. 2007;68(10):776–8. doi: 10.1212/01.wnl.0000256340.16766.1d. [DOI] [PubMed] [Google Scholar]
- 32.Leal PR, et al. Structural abnormalities of the trigeminal root revealed by diffusion tensor imaging in patients with trigeminal neuralgia caused by neurovascular compression: a prospective, double-blind, controlled study. Pain. 2011;152(10):2357–64. doi: 10.1016/j.pain.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Alonso P, et al. Trigeminal neuralgia: functional study using high density diffusion tensor imaging as a diagnostic tool. Rev Argent Radiol. 2013;79(2):65–71. [Google Scholar]
- 34.Antonini G, et al. Magnetic resonance imaging contribution for diagnosing symptomatic neurovascular contact in classical trigeminal neuralgia: a blinded case-control study and meta-analysis. Pain. 2014;155(8):1464–71. doi: 10.1016/j.pain.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Chen DQ, et al. Diffusivity signatures characterize trigeminal neuralgia associated with multiple sclerosis. Mult Scler. 2015 doi: 10.1177/1352458515579440. [DOI] [PubMed] [Google Scholar]
- 36.DeSouza DD, Hodaie M, Davis KD. Abnormal trigeminal nerve microstructure and brain white matter in idiopathic trigeminal neuralgia. Pain. 2014;155(1):37–44. doi: 10.1016/j.pain.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 37.Duan Y, et al. Degree of distal trigeminal nerve atrophy predicts outcome after microvascular decompression for Type 1a trigeminal neuralgia. J Neurosurg. 2015:1–7. doi: 10.3171/2014.12.JNS142086. [DOI] [PubMed] [Google Scholar]
- 38.Hodaie M, et al. Tractography delineates microstructural changes in the trigeminal nerve after focal radiosurgery for trigeminal neuralgia. PLoS One. 2012;7(3):e32745. doi: 10.1371/journal.pone.0032745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kress B, et al. MRI volumetry for the preoperative diagnosis of trigeminal neuralgia. Eur Radiol. 2005;15(7):1344–8. doi: 10.1007/s00330-005-2674-4. [DOI] [PubMed] [Google Scholar]
- 40.Leal PR, et al. Atrophic changes in the trigeminal nerves of patients with trigeminal neuralgia due to neurovascular compression and their association with the severity of compression and clinical outcomes. J Neurosurg. 2014;120(6):1484–95. doi: 10.3171/2014.2.JNS131288. [DOI] [PubMed] [Google Scholar]
- 41.Lin W, Chen YL, Zhang QW. Vascular compression of the trigeminal nerve in asymptomatic individuals: a voxel-wise analysis of axial and radial diffusivity. Acta Neurochir (Wien) 2014;156(3):577–80. doi: 10.1007/s00701-013-1970-z. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, et al. Microstructural abnormalities in the trigeminal nerves of patients with trigeminal neuralgia revealed by multiple diffusion metrics. Eur J Radiol. 2013;82(5):783–6. doi: 10.1016/j.ejrad.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Lutz J, et al. Trigeminal neuralgia due to neurovascular compression: high-spatial-resolution diffusion-tensor imaging reveals microstructural neural changes. Radiology. 2011;258(2):524–30. doi: 10.1148/radiol.10100477. [DOI] [PubMed] [Google Scholar]
- 44.Lutz J, et al. Microstructural alterations in trigeminal neuralgia determined by diffusion tensor imaging are independent of symptom duration, severity, and type of neurovascular conflict. J Neurosurg. 2015:1–8. doi: 10.3171/2015.2.JNS142587. [DOI] [PubMed] [Google Scholar]
- 45.Mamata H, et al. High-resolution line scan diffusion tensor MR imaging of white matter fiber tract anatomy. AJNR Am J Neuroradiol. 2002;23(1):67–75. [PMC free article] [PubMed] [Google Scholar]
- 46.Moayedi M, et al. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain. 2012;153(7):1467–77. doi: 10.1016/j.pain.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Rousseau A, et al. Diffusion tensor magnetic resonance imaging of trigeminal nerves in relapsing herpetic keratouveitis. PLoS One. 2015;10(4):e0122186. doi: 10.1371/journal.pone.0122186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcox SL, et al. Trigeminal nerve anatomy in neuropathic and non-neuropathic orofacial pain patients. J Pain. 2013;14(8):865–72. doi: 10.1016/j.jpain.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Wilcox SL, et al. Anatomical changes at the level of the primary synapse in neuropathic pain: evidence from the spinal trigeminal nucleus. J Neurosci. 2015;35(6):2508–15. doi: 10.1523/JNEUROSCI.3756-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan W, et al. Microstructural abnormalities of the trigeminal nerve correlate with pain severity and concomitant emotional dysfunctions in idiopathic trigeminal neuralgia: A randomized, prospective, double-blind study. Magn Reson Imaging. 2015 doi: 10.1016/j.mri.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Sarlani E, Balciunas BA, Grace EG. Orofacial pain--Part I: Assessment and management of musculoskeletal and neuropathic causes. AACN Clin Issues. 2005;16(3):333–46. doi: 10.1097/00044067-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Montano N, et al. Percutaneous balloon compression for the treatment of trigeminal neuralgia in patients with multiple sclerosis. Analysis of the potentially prognostic factors. Acta Neurochir (Wien) 2012;154(5):779–83. doi: 10.1007/s00701-012-1301-9. [DOI] [PubMed] [Google Scholar]
- 53.Sierra A, et al. Diffusion tensor MRI with tract-based spatial statistics and histology reveals undiscovered lesioned areas in kainate model of epilepsy in rat. Brain Struct Funct. 2011;216(2):123–35. doi: 10.1007/s00429-010-0299-0. [DOI] [PubMed] [Google Scholar]
- 54.Duyn JH. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage. 2012;62(2):1241–1248. doi: 10.1016/j.neuroimage.2011.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McNab JA, et al. High resolution diffusion-weighted imaging in fixed human brain using diffusion-weighted steady state free precession. Neuroimage. 2009;46(3):775–785. doi: 10.1016/j.neuroimage.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Balchandani P, Naidich T. Ultra-high-field MR neuroimaging. American Journal of Neuroradiology. 2015;36(7):1204–1215. doi: 10.3174/ajnr.A4180. [DOI] [PMC free article] [PubMed] [Google Scholar]