In a recent edition of Annals of Translational Medicine, Doran and Voora are giving a commentary entitled “Circulating extracellular vesicles containing miRNAs may have utility as early biomarkers for cardiac injury” (1), a perspective on our recent work on the release of extracellular vesicles (EVs) after myocardial injury (MI) (2). Current standard biomarkers for MI are circulating creatinine Kinase MB (CKMB) and cardiac troponin, which are released within 2–3 hours after the onset of cardiac injury. Although extremely powerful in a daily clinical setting, a continuous search for new markers is warranted since both an early rule-in or rule-out of MI is associated with improved outcomes and lower health-care costs. A novel direction for new biomarkers is their association with small carriers, including EVs, which are present in human serum and can carry a variety of proteins, RNAs and microRNAs (miRNAs) in higher quantities then free in plasma. Nature is, however, not generating EVs as biomarkers but is using these mediators as powerful signaling molecules to activate and stimulate cells, thereby potentially including reparative signals to induce inflammation or maybe regeneration (3).
Especially in cancer, detection of tumor derived vesicles is often reported (4) which might serve as a diagnostic tool in the detection and possibly the prediction of tumor development. Extracellular microvesicles from tumors are involved in tumor progression (5); they promote angiogenesis and facilitate tumor growth and metastasis or they suppress tumor directed immune responses. In our recent cardiac work (2), we have described that more EVs are present in plasma after myocardial infarction, of which part is derived from the cardiomyocytes. Although we demonstrated that these vesicles contain several different miRNAs, a clear mechanistic understanding or role of these miRNAs is still lacking. Additionally, we have not studied if these EVs can have any biological function, hence e.g., stimulate regenerative processes upon MI. In this correspondence, we would like to speculate further on a possible role for these EVs.
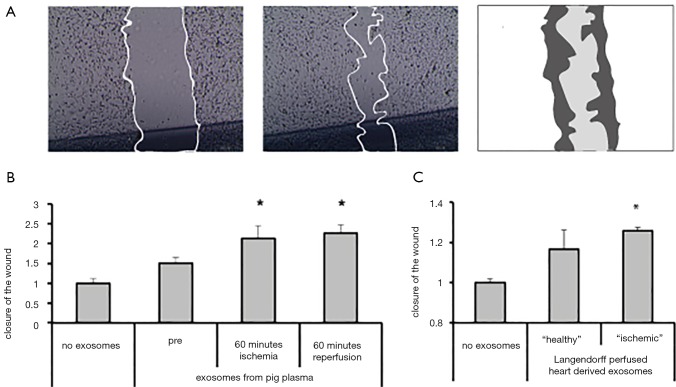
Exosomes (subfraction of EV) derived from different cells, including cardiac-derived progenitor cells (CPC) (6), have been reported to stimulate angiogenesis powerfully and can protect the heart against myocardial ischemia. Recently, we demonstrated that CPC-derived EVs stimulate angiogenic responses in endothelial cells via extracellular matrix metalloproteinase inducer (EMMPRIN) (7). Inspired by this observation, we hypothesized that cardiac-derived EVs might also be able to stimulate similar effects upon myocardial infarction, thereby potentially promoting a pro-angiogenic effect in endothelial cells (ECs) and induce a reparative signal. To explore this, we used human microvascular endothelial cells (HMECs) in a scratch wound migration assay in vitro, as described before (see Figure 1A) (6), and circulating EVs after myocardial damage (2). By exposing the in vitro cultured HMECs to these isolated EVs that were obtained from our porcine MI model, we observed that HMECs were activated. Circulating EVs, collected at baseline, showed a 1.5±0.2-fold increase in closure of the wound compared to controls. Furthermore, cardiac ischemia followed by reperfusion, led to further enhancement of EV release and thereby increased HMEC activation, (closure of the wound is further increased: ischemia—2.1±0.3, reperfusion—2.3±0.2 fold increase, respectively; see Figure 1B). Subsequently, we aimed to identify if this response is originating from the myocardial tissue itself by using a murine heart Langendorff set-up. Results demonstrated that hypoxia induced EVs were able to activate the HMECs to a higher degree than normoxic derived EVs (closure of the wound increased by 17% in normoxia, and by 26% in hypoxia, compared to non-stimulated ECs; see Figure 1C). These results indicate that the myocardium is able to release EVs, which are enhanced upon cardiac injury, and thereby could potentially trigger other cell types in their function, here represented by cultured ECs.
Figure 1.
Circulating extracellular vesicles can activate cultured endothelial cells. (A) Example of a scratch wound migration assay. Endothelial cells repopulate a scratch upon injury, which effect is enhanced upon exposure to extracellular vesicles (EVs); (B) extracellular vesicles collected after cardiac ischemia and reperfusion injury from a porcine model stimulate endothelial cell migration (HUVECs) in a wound closure assay in vitro. Vesicles derived after reperfusion enhanced this effect further; (C) extracellular vesicles from Langendorff perfused mouse hearts stimulate endothelial cell migration in a wound closure assay in vitro. Extracellular vesicles derived from ischemic hearts enhanced this effect. *, P<0.05 EVs vs. no exosomes.
Since the content of EVs reflects part of the cellular compartment, the content is depending on the original cell type and cellular state, and profiling the composition and cargo of exosomes could provide insights in possible mechanisms (8). In addition, EVs that are released from a cell type, but have a different composition and cargo due to stress exposures provide a potential diagnostic tool (9). For example hypoxia and oxidative stress changed the proteomic and RNA content of endothelial and mast cells (9-11). In addition to proteins and mRNA, EVs contain selectively packaged miRNAs, including miR-1, which can be efficiently shuttled to target cells. Previously, we reported that the angiogenic differentiation of CPCs can be enhanced by the upregulation of miR-1, thereby targeting Spred1, which may indicate a different therapeutic direction enhancing angiogenesis and potentially to improve cardiac regeneration (12).
For a therapeutic approach of exosomes only, engineering of exosomes by manipulating their contents or binding specificity is very interesting and holds true potential for targeted in vivo delivery. Moreover, the use of systems biology to analyze EV content and their effects creates an opportunity to increase our understanding and paves the way for designing optimized vesicles to enhance their effect on cardiac regeneration (13).
Acknowledgements
Funding: This work was supported by a grant from the ZonMw-TAS program (116002016) and by the HUSTCARE grant from the Netherlands Cardiovascular Research Initiative (CVON): the Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences.
Provenance: This is a Guest Correspondence by Section Editor Zhijun Han, MD (Department of Laboratory Medicine, Wuxi Second Hospital, Nanjing Medical University, Wuxi, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Doran B, Voora D. Circulating extracellular vesicles containing miRNAs may have utility as early biomarkers for cardiac injury. Ann Transl Med 2016;4:S60. 10.21037/atm.2016.10.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deddens JC, Vrijsen KR, Colijn JM, et al. Circulating Extracellular Vesicles Contain miRNAs and are Released as Early Biomarkers for Cardiac Injury. J Cardiovasc Transl Res 2016;9:291-301. 10.1007/s12265-016-9705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sluijter JP, Verhage V, Deddens JC, et al. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res 2014;102:302-11. 10.1093/cvr/cvu022 [DOI] [PubMed] [Google Scholar]
- 4.Baran J, Baj-Krzyworzeka M, Weglarczyk K, et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother 2010;59:841-50. 10.1007/s00262-009-0808-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883-91. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vrijsen KR, Sluijter JP, Schuchardt MW, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 2010;14:1064-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrijsen KR, Maring JA, Chamuleau SA, et al. Exosomes from Cardiomyocyte Progenitor Cells and Mesenchymal Stem Cells Stimulate Angiogenesis Via EMMPRIN. Adv Healthc Mater 2016;5:2555-65. 10.1002/adhm.201600308 [DOI] [PubMed] [Google Scholar]
- 8.Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 2012;83:1484-94. 10.1016/j.bcp.2011.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong OG, Verhaar MC, Chen Y, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012;12:421. 10.1186/1471-2407-12-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldh M, Ekström K, Valadi H, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One 2010;5:e15353. 10.1371/journal.pone.0015353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Mil A, Vrijsen KR, Goumans MJ, et al. MicroRNA-1 enhances the angiogenic differentiation of human cardiomyocyte progenitor cells. J Mol Med (Berl) 2013;91:1001-12. 10.1007/s00109-013-1017-1 [DOI] [PubMed] [Google Scholar]
- 13.Sluijter JP, van Rooij E. Exosomal microRNA clusters are important for the therapeutic effect of cardiac progenitor cells. Circ Res 2015;116:219-21. 10.1161/CIRCRESAHA.114.305673 [DOI] [PubMed] [Google Scholar]