Abstract
Introduction: Application of lactic acid bacteria for synthesis of silver (AG) nanoparticles (NPs) could be a good ecological friendly alternative to chemical and physical methods. The objective of this study was to investigate the biosynthesis of silver NPs using Lactobacillus strains and to compare their monosaccharide composition of capsular exopolysaccharides and the antibacterial activity of synthesized nanoparticles.
Methods: The washed cells of 22 Lactobacillus strains were used for in vitro silver nanoparticle biosynthesis from silver nitrate solution. The NPs formation was confirmed by UV-visible spectroscopy and transmission electron microscopy (TEM) analysis. TEM micrographs were used for the evaluation of NPs size. The monosaccharide composition of capsular exopolysaccharides was determined using GC/MS analysis. The antimicrobial activity was determined by agar well diffusion assay.
Results: The capsular layers of Lactobacillus strains contained heteropolysaccharides that were composed mostly of glucose, mannose, galactose and rhamnose in a different molar ratio. It was found that Ag NPs with large size (30.65 ± 5.81 nm) obtained from L. acidophilus 58p were more active against S. epidermidis, E. coli, K. pneumonia,S. flexneri and S. sonnei compared with Ag NPs from L. plantarum 92T (19.92 ± 3.4 nm).
Conclusion: The size and antibacterial activities of Ag NPs were strain-dependent and such characteristics may be due to the capsular biopolymer composition of Lactobacillus strains used for Ag NPs synthesis.
Keywords: Antibacterial activity, Lactic acid bacteria, Nanobiotechnology, Silver nanoparticles, Opportunistic pathogens
Introduction
The genus Lactobacillus includes rod-shaped bacteria that are generally recognized as safe (GRAS). They have many strains are commercially available as probiotics with health-promoting properties. It belongs to the group of lactic acid bacteria (LAB).1,2 One of the new and prospect areas of LAB applications is nanobiotechnology field. Biological methods of nanoparticle synthesis using bacteria have offered an ecologically friendly and reliable alternative to chemical and physical methods.4 The LAB strains also were used for the synthesis of silver (Ag) nanoparticles (NPs).5,6 Ag NPs are considered as a valuable alternative for ionic silver and have been widely used as an effective bactericidal agent against pathogenic bacteria, including antibiotic resistant strains.3 At the same time, little is known about the antibacterial function of Ag NPs that are produced using Lactobacillus strains. Furthermore, the mechanisms involved in Ag NPs biosynthesis by lactobacilli are not elucidated yet. To the best of our knowledge, there has been no report untill now on the monosaccharide composition of LAB strains with the ability to reduce silver ions and NPs formation.
The objective of this work was to investigate the biological synthesis of Ag NPs using strains of different Lactobacillus species and to compare the monosaccharide composition of capsular exopolysaccharides between LAB strains. The synthesized NPs were characterized and their antibacterial activity were evaluated.
Materials and methods
Bacterial strains and synthesis of silver NPs
The capacity to precipitate silver and synthetize NPs was evaluated for 22 different strains (Table 1). Bacteria were cultured in the MRS medium at 37°C for 24 h. After incubation, the bacterial biomass was harvested by centrifugation at 1.500 g for 10 min and washed several times with sterile deionized water. For the synthesis of Ag NPs, 1 mM AgNO3 was added to bacterial pellet to give an optical density of 1.0 at 630 nm.
Table 1. Size of Ag NPs synthesized by Lactobacillus strains used in the study .
| No | Strain | Ag NPs size, nm | |
| Mean±SD | Min-Max | ||
| 1 | L. acidophilus 58p UCM B-2637 | 30.65±5.81a | 20.30-40.60 |
| 2 | L. acidophilus CCM 4833Т | 25.11±5.89 | 12.69-43.17 |
| 3 | L. acidophilus G1/1(3) UCM B-2691 | -* | - |
| 4 | L. acidophilus ncs | 36.99±7.71 | 25.31-56.96 |
| 5 | L. acidophilus 1660 | - | - |
| 6 | L. fermentum 32GI UCM B-2665 | - | - |
| 7 | L. fermentum 90TC UCM B-2696 | - | - |
| 8 | L. fermentum 23p | - | - |
| 9 | L. fermentum 32/4(16) UCM B-2661 | 32.04-10.10ad | 15.82-60.91 |
| 10 | L. fermentum 215 UCM B-2675 | 22.23±7.68b | 10.06-45.28 |
| 11 | L. fermentum CCM 7192T | 21.88±3.22b | 15.22-28.48 |
| 12 | L. plantarum 337D UCM B-2627 | - | - |
| 13 | L. plantarum RA UCM B-2693 | - | - |
| 14 | L. plantarum G3/3(13) UCM B-2705 | 33.37±6.76d | 21.09-63.29 |
| 15 | L. plantarum 92T UCM B-2629 | 19.92±3.4c | 12.6-27.77 |
| 16 | L. plantarum 11/16 UCM B-2694 | - | - |
| 17 | L. plantarum 2209 UCM B-2709 | - | - |
| 18 | L. plantarum 93T | - | - |
| 19 | L. plantarum CCM 7039T | - | - |
| 20 | L. casei CCM 7088T | 27.12-9.39 | 8.17-46.61 |
| 21 | L. rhamnosus CCM 1825T | 19.21±4,62c | 10.12-32.99 |
| 22 | L. bulgaricus CCM 7190T | - | - |
Means with the same letter superscript were not significantly different (p > 0.05).
*no Ag NP formation
UCM: Ukrainian Collection of Microorganisms (Institute Microbiology and Virology NASU, Kiev, Ukraine), CCM: Czech Collection of Microorganisms (Masaryk University, Brno, Czech Republic).
Characterization of silver NPs
The UV–Vis spectrum was determined using a SPECORD M-400 UV-Vis spectrophotometer (Japan). The absorbance was measured in the range of 400-800 nm. Deionized water was used as the blank.
Transmission electron microscopy (TEM) measurements were performed using a JEOL JEM 1400 instrument operated at an accelerating voltage of 80 kV. Size of the NPs was determined using TEM micrographs. A total of 100 particles were evaluated for each strain.
Capsular exopolysaccharides isolation
For isolation of capsular exopolysaccharide (CPS), lactobacilli strains were grown in MRS medium (1000 mL) at 37°C for 24 h. The cultures were centrifuged at 12 000 g for 15 min at 4°C and the cell pellets were collected for the extraction of CPS. The cells were washed with saline solution and further treated with 0.5% phenol for 4 h at room temperature.7 After centrifugation, to remove the cells, the supernatant was precipitated with three-fold (v/v) of chilled ethanol. After standing overnight at 4°C, the precipitate was collected by centrifugation (12 000 g, 20 min at 4°C), suspended in 10 mL of distilled water, and dialyzed against 3 L of distilled water for 2 days with 3 water changes per day. The dialysate was lyophilized and the total sugar concentration was determined by the phenol-sulfuric method, using glucose as a standard.
Monosaccharide composition of capsular exopolysaccharides
The CPS samples were hydrolyzed with 1 mL of 2 M trifluoroacetic acid (TFA) at 100°C for 6 h. TFA was removed by vacuum evaporation. A volume of 0.3 mL derivatization reagent (32 mg hydroxylamine hydrochloride mL-1, 40 mg 4-dimethylaminopyridine mL-1 in pyridine/methanol [4:1 v/v]) was added to each dried sample, and then the samples were incubated at 75°C for 25 min. After incubation, the tubes were cooled down to the room temperature and 0.3 mL of acetic anhydride was subsequently added and incubated at 75°C for 25 min. To the reaction mixture, 1 mL of dichloroethane was added. Excess reagents were removed by double extraction using 1M hydrochloric acid and followed by H2O washing (×3) steps. Dichloroethane phase was removed, dried, and redissolved in 300 μL of heptane/methyl acetate mixture (1:1 v/v).8
The GC/MS analysis of acetylated aldononitriles was carried out using Agillent 6890N/5973inert (Agillent Technologies, Inc., Richardson, TX USA). A capillary column НР-5MS (30 m ×0.25 mm (i.d), film thickness 0.25 μm) (J&W Scientific, USA) was used. The temperatures of injector and MS interface were 250°С and 280°С, respectively. The oven start temperature, 160°C, was held for 8 min, ramped up to 240°C at the rate of 5°C/min, and held for 6 min. The injection volume was 1 μL, and the split ratio was 1:50. For detection, the EI of samples were scanned in the range of 38-5400 m/z. Helium was used as mobile phase (flow rate of 1.2 mL min-1). Derivatised monoschacarides were identified by comparing their retention times with standard mixtures of monosacharides (galactose, mannose, xylose, glucose, fructose, arabinose, rhamnose) and their mass fragmentation spectra with NIST02 MS library.
Antimicrobial activity of silver NPs
The antimicrobial action was determined by agar well diffusion assay. Antibacterial action was tested for synthesized Ag NPs and cell free supernatant against opportunistic pathogens of Gram-positive bacteria (Staphylococcus aureus ATCC 25923, S. epidermidis ATCC 12228, Bacillus cereus ATCC 11778), Gram-negative bacteria (Pseudomonas aeruginosa ATCC 9027, Proteus vulgaris ATCC 6896, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 10031, Salmonella enterica NCTC 6017, Shigella sonnei GICK 337, S. flexneri GICK 233169), and yeast (Candida albicans UCM Y-1918) on Mueller–Hinton (MH) agar plates. Agar wells of 6 mm diameter were prepared and loaded with 50 μL of Ag NPs solution and 50 μL of bacterial cell suspension without AgNO3 as control. The plates were incubated at 37°C for 24 h and the diameter of zone of inhibition was measured as indicated by clear area devoid of growth of test-strains. Each experiment was done twice.
Statistical analysis
A one-way analysis of variance (ANOVA) was performed using Statistica Software version 7.0. Mean values were compared using LSD test (least-significant difference, p<0.05) and different letters were used to label values with statically significant differences among them.
Results and discussion
Silver NPs synthesis
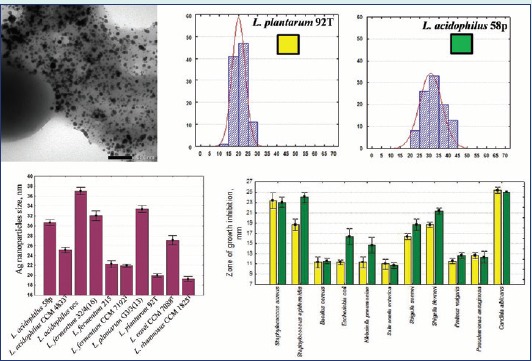
Only ten from 22 of Lactobacillus strains had the potential to reduce the silver ions to silver NPs (Table 1). The white bacterial biomass of these strains changed to yellowish brown after incubation with 1 mM aqueous AgNO3 solution suggesting the formation of silver NPs. The UV-VIS absorption spectra of all bacterial suspensions were monitored and a strong peak in the visible region typical for the Ag NPs (Fig. 1A) was obtained only for 10 bacterial cell suspensions.
Fig. 1.
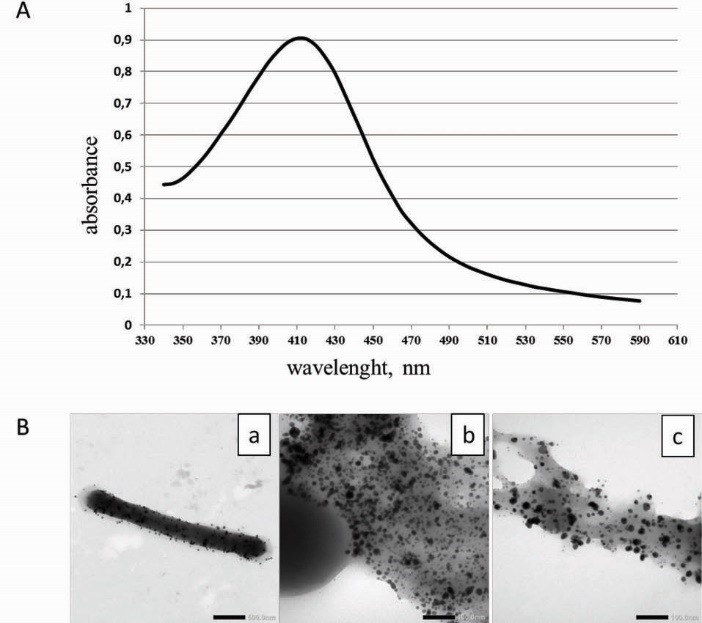
A- UV/VIS absorption spectra of the Ag NPs prepared using L. plantarum 92T cell suspension. B- Transmission of electron micrographs of Ag NPs obtained with lactobacilli after 24 h of incubation in AgNO3 solution. (a) – cell of strain L. fermentum 215 with Ag NPs on the surface (scale bar corresponds to 500 nm); (b, c) – accumulation of Ag NPs in capsular polymers of L. casei strain (scale bar corresponds to 100 nm).
The results were confirmed with the TEM analysis of the samples (Fig. 1B), which showed the presence of spherical nano-sized particles in the same 10 samples (Table 1). Thus, the ability to reduce the silver ions was strain-specific and was not dependent to the Lactobacillus species. In most cases Ag NPs had a diameter between 10 and 40 nm (89.6%), whereas 0.7 % of Ag NPs had a diameter smaller than 10 nm and 9.7% of NPs were greater than 40 nm. Therefore, the NPs in this work (Table 1) were in the same approximate range of 5–60 nm reported by authors for Ag NPs produced from LAB and other microorganisms.6,9 The Ag NPs produced by L. rhamnosus CCM 1825T and L. plantarum 92T had the smallest mean particle size. The NPs with the largest size were synthesized by strain L. acidophilus NCS. The particle size distribution in case of L. casei CCM 7088T was larger than that in case of other Lactobacillus strains. The narrowest size distribution was in case of strains L. plantarum 92T and L. fermentum CCM 7192T.
Capsular exopolysaccharides analysis
We investigated the CPS of five Lactobacillus strains that produced NPs with different size. The CPSs of two strains that did not produce Ag NPs were investigated for comparison. The monosaccharide composition of CPS from different Lactobacillus strains varied significantly (Table 2). Most of them were composed of galactose, glucose, mannose, and rhamnose in different molar ratios. The capsular exopolysaccharide of L. plantarum 11/16 contains only glucose and galactose. This could explain the ability to reduce the silver ions and produce nanoparticle and its different sizes and size distribution. As shown by other authors,10 the difference in the structure of monosaccharides and disaccharides influences the Ag NPs size.
Table 2. Monosaccharide composition of capsular polysaccharides from different Lactobacillus strains (in approximate molar ratios) .
| Strain | Monosaccharide | |||
| Rhamnose | Mannose | Glucose | Galactose | |
| L. acidophilus 58p | 3.62 | 0.65 | 19.50 | 1.00 |
| L. acidophilus G1/1(3)* | 0.23 | 1.72 | 6.11 | 1.00 |
| L. plantarum 92T | 1.00 | - | 3.42 | 1.00 |
| L. plantarum G3/3(13) | - | 0.44 | 3.12 | 1.00 |
| L. plantarum 11/16* | - | - | 2.50 | 1.00 |
| L. fermentum 32/4(16) | 0.43 | 0.50 | 1.07 | 1.00 |
| L. casei CCM 7088T | 0.82 | 13.60 | 10.50 | 1.00 |
*No Ag NP formation.
Antimicrobial action of silver NPs
Antimicrobial properties of Ag NPs from two Lactobacillus strains was studied (Fig. 2). The highest antimicrobial activity was observed against Candida albicans, followed by S. epidermidis and S. aureus. It was found that Ag NPs with large size (30.65±5.81 nm) obtained from L. acidophilus 58p were more active against S. epidermidis, E. coli, K. pneumonia, S. flexneri, and S. sonnei compared with Ag NPs from L. plantarum 92T (19.92±3.4 nm) (p<0.05) (Fig. 2).
Fig. 2.
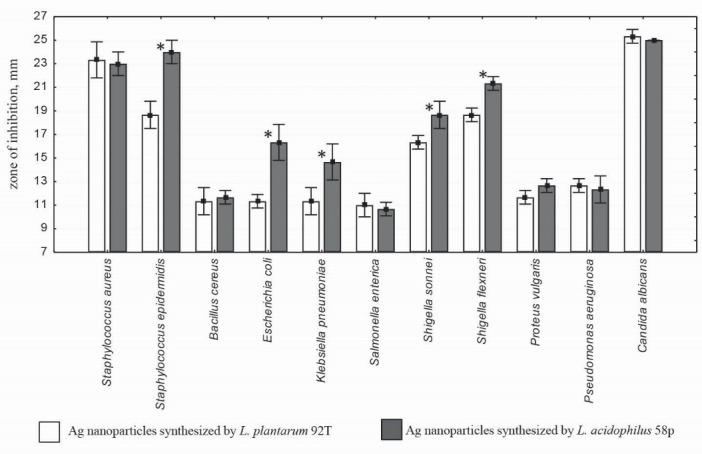
Size of the inhibition zone for Ag NPs synthesized by L. plantarum 92T and L. acidophilus 58p strains against the tested opportunistic pathogens. * Indicates a significant difference (p < 0.05) in the size of inhibition zones.
Antibacterial activity of Ag NPs depends on the reducing agents and stabilizers used for synthesis.3,11 Smaller particles having the larger surface area available for interaction would give more bactericidal effect than the larger particles.3 As was shown by Panacek et al.10 Ag NPs synthesized using disaccharides have a higher antibacterial activity than those synthesized using monosaccharides. The sizes of the colloidal Ag particles were smaller for disaccharide than for monosaccharide and thus may be responsible for the observed antibacterial activity. However, there was some exceptions, for example, galactose had the largest Ag NPs (50 nm), and had higher antibacterial activity against methicillin-susceptible S. epidermidis than those synthesized using glucose (44 nm). In addition, there were equal MIC values for NPs with different sizes; for example, MIC of Ag NPs synthesized via reduction by glucose (44 nm), maltosa (25 nm), and lactose (35 nm) against S. aureus, was 6.75 μg/mL.8 Thus, the size, size distribution, and differences in the antibacterial activity of Ag NPs produced by L. plantarum 92T and L. acidophilus 58p strains may be due to their capsular biopolymers composition, but further work is required to characterize these differences and other biological activities of synthesized Ag NPs.
At the present work, Ag NPs are extracellular-synthesized by the biomass of the Lactobacillus strains of different species. Therefore, the components produced by these strains, causing the absorption and reduction of silver ions to Ag NPs are most likely associated with the surface of bacterial cells. This may indicate the location of Ag NPs on the surface of cells, preferably in the capsular layer (Fig. 1B). Application of the LAB for the synthesis of Ag NPs has already been reported, and the ability to accumulate Ag NPs on their surfaces and inside the cells has been reported.12 However, the exact reaction mechanism leading to the formation of Ag NPs by LAB needs to be elucidated.
Conclusion
The ability to reduce the silver ions was strain-specific. The antibacterial activity of Ag NPs with different sizes from two Lactobacillus strains was compared for the first time. It was found that the monosaccharide composition of capsular heteropolysaccharides from different Lactobacillus strains varied significantly. This could explain the ability to reduce the silver ions and produce NPs with different sizes and size distribution. Further experiments should be performed to elucidate the role of capsular polysaccharides in the synthesis of Ag NPs.
Ethical issues
There is none to be declared.
Competing interests
Authors declare no conflict of interests.
Acknowledgments
The authors wish to thank Vasyliuk O.M. for skillful technical assistance in the determination of antibacterial activity.
Report Highlights
What is current knowledge?
√ Nanobiotechnology is a new area of application of the LAB.
√ AG NPs are known to have a broad spectrum of antimicrobial activities.
√ Antibacterial activity of Ag NPs may depend on the method of synthesis.
What is new here?
√ Ag NPs synthesized using biomass of Lactobacillus strains have a high antibacterial activity against opportunistic human pathogens in vitro.
√ The monosaccharide composition of capsular heteropolysaccharides from different Lactobacillus strains could explain the ability to reduce the silver ions and produce nanoparticles with different sizes and size distribution.
References
- 1.van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013;34:208–15. doi: 10.1016/j.it.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Kaur IP, Chopra K, Saini A. Probiotics, potential pharmaceutical applications. Eur J Pharm Sci. 2002;15:1–9. doi: 10.1016/S0928-0987(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 3.Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Korbekandi H, Iravani S. and Abbasi S Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp casei. J Chem Technol Biotechnol. 2012;87:932–937. doi: 10.1002/jctb.3702. [DOI] [Google Scholar]
- 6.Sintubin L, De Windt W, Dick J, Mast J, van der Ha D, Verstraete W. et al. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol. 2009;84:741–749. doi: 10.1007/s00253-009-2032-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol. 2014;63:133–139. doi: 10.1016/j.ijbiomac.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Xie MY, Wang YX, Nie SP, Li C. Analysis of the monosaccharide composition of purified polysaccharides in Ganoderma atrum by capillary gas chromatography. Phytochem Anal. 2009;20:503–510. doi: 10.1002/pca.1153. [DOI] [PubMed] [Google Scholar]
- 9.Kalimathu K, Suresh Babu R, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65:150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N. et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 11.Sukdeb P, Yu Kyung T, Joon Myong S. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Z, Wu J, Xue R, Yong Y. A further insight into the mechanism of Ag+ biosorption by Lactobacillus sp strain A09. Spectrochi Acta Part A. 2005;61:761–765. doi: 10.1016/j.saa.2004.06.041. [DOI] [PubMed] [Google Scholar]


