Summary
Options for people with severe paralysis who have lost the ability to communicate orally are limited. We describe a method for communication in a patient with late-stage amyotrophic lateral sclerosis (ALS), involving a fully implanted brain–computer interface that consists of subdural electrodes placed over the motor cortex and a transmitter placed subcutaneously in the left side of the thorax. By attempting to move the hand on the side opposite the implanted electrodes, the patient accurately and independently controlled a computer typing program 28 weeks after electrode placement, at the equivalent of two letters per minute. The brain–computer interface offered autonomous communication that supplemented and at times supplanted the patient’s eye-tracking device. (Funded by the Government of the Netherlands and the European Union; ClinicalTrials.gov number, NCT02224469.)
Locked-in syndrome is characterized by a loss of voluntary muscular control, resulting in quadriplegia and aphonia, with retention of normal cognition.1 The syndrome is typically linked to brainstem stroke, but degenerative disorders such as ALS, which affects approximately 5 persons per 100,000 population,2 progress to the same state.3 Despite their predicament, people in the locked-in state often report a high quality of life,4 which is correlated with the ability to communicate.5 Current strategies for communication depend mainly on eye movements that are followed by a camera, which enables selection of items on a computer screen (“eye tracker”); when that fails, communication may depend on eye movements or blinks in response to closed-ended questions, which limits the options for independent and private communication.
Recent developments in brain–computer interfaces capitalize on the neuroelectrical properties of the brain and the localized somatic activation by these brain regions.6,7 These systems take advantage of the observation that mental acts, such as an attempt to move a limb, lead to reproducible signals in corresponding cortical regions.8 The detection of signals from the cortex requires computational processing to separate (“decode”) them from background noise. Decoding of sufficient quality provides the input for a computer system that directs typing software, thereby enabling communication.9
Decoding of neuronal signals from brain implants has yielded achievements such as the control of a robotic or paralyzed limb by patients with quadriplegia.10–12 In spite of such achievements, it is difficult to implement independent communication for daily use at home. To accomplish this, a brain–computer interface must allow for user autonomy and continuous functionality and must reliably detect only intended actions.13 Furthermore, permanent availability of the interface for communication requires sensors that are connected to the cerebral cortex but do not cause discomfort or disfiguration.14
We report on a communication system that involves a totally implantable brain–computer interface for home use by a locked-in patient with ALS. The implant, combined with automated decoding software, enabled independent communication with the use of typing software.
Methods
Patient
The patient is a woman who was 58 years old at the time that informed consent was obtained in September 2015. She was in a locked-in state from late-stage ALS, which was diagnosed in 2008, and required positive-pressure mechanical ventilation through a tracheostomy. She was able to communicate using eye movements (with an eye tracker) and blinks denoting yes and no but was otherwise completely paralyzed (a score of 2 on the ALS functional rating scale, which rates functional impairment on a scale of 0 to 40, with higher scores indicating less disability). We report on the period from implantation of the brain–computer interface in October 2015 to the most recent observations in July 2016. The study was approved by the ethics committee at the University Medical Center Utrecht in accordance with the 2013 provisions of the Declaration of Helsinki. Informed consent was obtained (see the Supplementary Appendix, available with the full text of this article at NEJM.org). This is the first patient to be enrolled in this program. Since the inception and registration of this study, two additional patients have been evaluated, but they died from their underlying condition (ALS) before electrodes were implanted.
Medtronic provided all components of the implant, plus the antenna and receiver, free of charge to the University Medical Center Utrecht. Medtronic contributed funds to the Dutch government agency that funded this study but did not provide funds directly to the institution, the researchers, or the patient. The fifth author, who is an employee of Medtronic and holds shares in the company, directed the team that was responsible for developing the implanted parts and the receiver; he also wrote portions of the “Implant Components” subsection of the Supplementary Appendix. He was not involved in the interpretation of the results.
Implantation and Description of Electrodes and Device
On day 0 (October 27, 2015), four subdural electrode strips (Resume II, Medtronic), which were designed for epidural spinal cord stimulation but have been tested for acceptable endotoxin levels in cerebral subdural implantation, were implanted through burr holes (1 cm in diameter) by means of a frameless stereotaxic procedure, guided by functional magnetic resonance imaging (fMRI) and neuronavigation. There were four electrodes on each strip (each electrode was 4 mm in diameter, with a 1-cm interelectrode distance). Two strips were placed over each target region (see below). Extension leads were connected to the electrode leads, tunneled subcutaneously to the abdomen, and externalized through the abdominal skin for temporary connection to electrocorticography equipment (Micromed; sampling rate, 512 Hz; high-pass filter at 0.15 Hz and low-pass filter at 134.4 Hz). Three days later, the externalized extension leads were removed, and two of the electrode strips (Figs. S1 and S3 in the Supplementary Appendix) were connected to an amplifier and transmitter device (Activa PC+S, Medtronic) that was placed subcutaneously beneath the left clavicle. The device was designed for deep-brain stimulation and biopotential recording, which allows for long-term electrocorticography with two strips; it is currently certified according to Conformité Européene for use in patients with Parkinson’s disease, essential tremor, or epilepsy (Fig. 1, and Fig. S2 in the Supplementary Appendix). The leads of the other two strips were capped subcutaneously and left in place as a backup. During use of the system, an antenna is placed on the chest over the device.
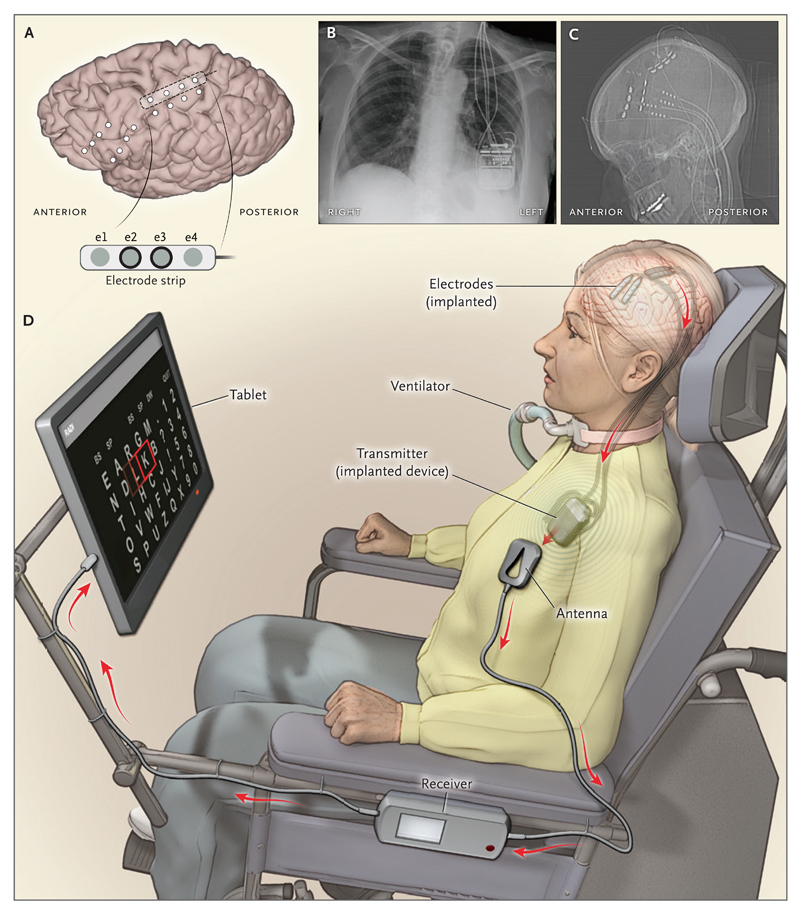
Figure 1. Electrode Placement and System Setup in the Brain–Computer Interface System.
Panel A shows the contact points of the electrode strips, which are indicated by white dots, over the sensorimotor and dorsolateral prefrontal cortex; the positions of electrodes were based on postoperative computed tomographic (CT) scans merged with the presurgical MRI. Electrodes e2 and e3 on the electrode strip were chosen for brain–computer interface feedback. Panel B shows a postoperative chest radiograph displaying the transmitter device (Activa PC+S, Medtronic), which was placed subcutaneously in the chest, and wires leading to the electrodes. Two of four wires were connected to the device. Panel C shows the postoperative CT scan with the locations of four electrode strips. The dots on the four wires are connectors. Panel D shows the components of the brain–computer interface system, including the transmitter, receiving antenna, receiver, and tablet.
The two procedures, which lasted 6.75 hours and 1.5 hours, respectively, were performed with the patient under general anesthesia. The patient became febrile on day 5, and the fever resolved without treatment. She was discharged from the hospital on day 6.
Determination of Activated Cortical Region
The subdural electrodes were placed over the hand region of the left motor cortex, on the basis of previous work showing that patients with quadriplegia can generate neuroelectrical activity by trying to move their hands.10–12 Since the motor cortex may be affected in ALS, as a backup, electrodes were also placed on a second region — the left prefrontal region, which is activated by mental calculation.15 Two strips were positioned on each target area to guarantee the best possible coverage of activated regions, the locations of which were determined with the use of fMRI.15–17 Electrocorticography was used to select the two optimal strips for further use. Details of the fMRI and electrode selection are provided in the Supplementary Appendix. We present results from the patient’s activation of the sensorimotor area, because it showed the best brain signals in early testing and yielded consistent control of the brain–computer interface. We have not investigated the prefrontal region extensively.
Decoding and Training
For 28 weeks after implantation, several computer tasks were used to test and improve the algorithms and parameters in the decoding software for control of the brain–computer interface. Each setting that yielded good control was fixed for the rest of the study. The patient then trained with the tasks to improve control over her brain signal.
First, the patient practiced activating the motor cortex with a task in which she attempted to hit a target on a video screen by trying to move her right hand to move a cursor upward and then relaxing her hand to move the cursor downward18 (target task) (Video 1, and Fig. S4A in the Supplementary Appendix). Next, she tried to regulate the magnitude and timing of her brain signal by moving an image of a ball up and down on the computer screen at specific moments indicated by the computer (ball task) (Fig. S4C in the Supplementary Appendix). She then learned to select specific items, shown in rows and columns on the screen, by generating “brain clicks,” which are analogous to mouse clicks (click task) (Video 2, and Fig. S4E in the Supplementary Appendix). The patient made a brain click by trying to move her hand for approximately 1 second. She had to withhold brain clicks until the correct item was highlighted. Details of these tasks and of the signal processing are provided in the Supplementary Appendix. During every research visit (two visits per week), the patient was asked to rate her mood and motivation on separate 21.5-cm visual-analogue scales, with higher scores on the motivation scale indicating higher motivation and higher scores on the mood scale indicating more depressive feelings.19
Communication and Home Use
Spelling was accomplished by the selection, with brain clicks, of individual or grouped letters that were highlighted automatically and sequentially (Video 3, and Fig. S4 in the Supplementary Appendix). Similar to the click task, spelling involved brain clicks being consciously withheld by the patient until the desired letter (or group of letters) was highlighted. To quantify performance, the patient was asked to spell dictated words. Further details of this task are provided in the Supplementary Appendix. After every run, mental effort was rated by the patient on a scale of 1 to 5 (with higher scores indicating greater mental effort).
When settings had been established that made reliable spelling possible, a home-use system was provided to the patient; the system included automated decoding, fixed settings, and a commercial spelling program (Communicator-5, Tobii Dynavox), all running on a tablet (Microsoft Surface Pro 4). On day 197, she started using the entire system without assistance from the investigators.
Home use of the system — and of the eye tracker, for comparison — were evaluated 7 to 9 months after surgery with the use of two user-satisfaction questionnaires: the modified Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST 2.0)20 and Psychosocial Impact of Assistive Devices Questionnaire (PIADS).21 Possible responses on QUEST 2.0 (12 items) range from very unsatisfied to very satisfied (5 options), and responses on PIADS (26 items) range from a very negative to a very positive effect of the device (7-point scale, ranging from −3 to 3).
Results
Determination of the Activated Cortical Region
The patient’s attempts to move her right hand resulted in consistent fMRI activity in the left sensorimotor hand area (Fig. S1 in the Supplementary Appendix). Electrocorticography revealed that in each electrode strip, two specific electrodes recorded a consistent signal when the patient attempted to move her hand (Figs. S1 and S3 in the Supplementary Appendix). The electrode strip located immediately over the hand motor area was selected for further use (Fig. 1, and Fig. S1 in the Supplementary Appendix).
Decoding and Training
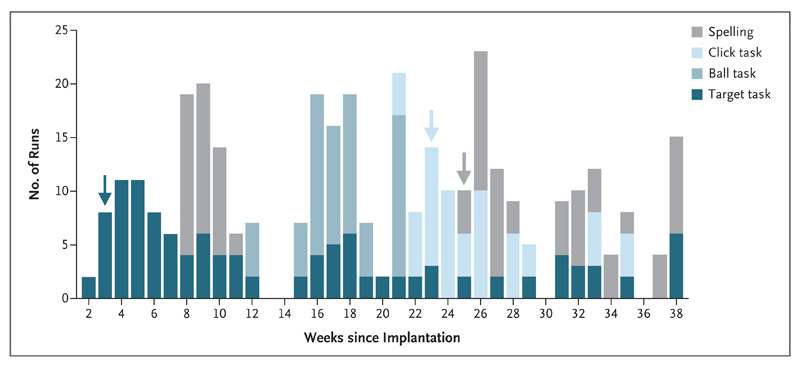
The patient performed 67 sessions (2 per week), each 2 hours in duration, at her home over the 262 days after surgery. Training and home-use sessions overlapped for several weeks. Sessions were used to test the algorithms that translated cortical activity to computer actions, to investigate the stability of the performance of the system over time, and to practice spelling (Fig. 2).
Figure 2. Overview of Training Sessions.
Two training sessions were conducted every week at the home of the patient. A total of 357 runs (with tasks that were 2 to 5 minutes in duration) were performed in 67 sessions. Shading indicates the types of tasks that were performed. Spelling was attempted in week 8, but the patient reported that excessive mental effort was required. Better decoding enabled spelling starting from week 25. Arrows with the same shading as the bars indicate the time at which parameter settings for the respective task were established, marking the onset of actual training on the task. The ball task was used only for parameter adjustment and was not subsequently used for training after the parameter settings had been fixed.
The patient’s performance on the target task was correct a mean (±SD) of 91±6% of the time (83 runs were performed between day 15 and day 262). Her performance on the click task was correct 87±7% of the time (37 runs were performed between day 157 and day 238). Inhibiting unintended brain clicks22 while preserving sensitivity to intended actions required several months of refining the algorithms. When the parameter testing was completed (day 157), the control signal was stable and the software settings were fixed. Additional details regarding the results of these tests and the setting of filter parameters are provided in Figs. S4, S5, and S6 in the Supplementary Appendix. Data from one target-task run and one click-task run are available at http://dx.doi.org/10.5061/dryad.k9f10.
Visual-analogue scores for mood, for which higher scores indicate greater depression, were consistently below 30% (the lower limit for mild depression).23 An exception occurred during a short period of unrelated medical problems, but the visual-analogue scores for motivation during this period remained above 80% (Fig. S7 in the Supplementary Appendix).
Communication and Independent Home Use
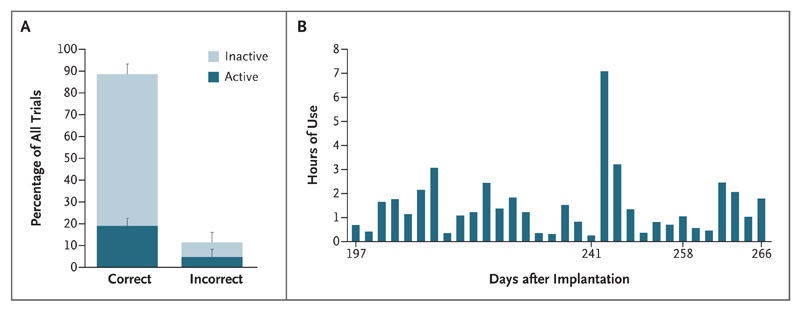
From day 168 to day 262, a total of 44 spelling runs were performed during research visits, and the patient’s performance was correct 89±6% of the time (Fig. 3, and Fig. S4 in the Supplementary Appendix). Half the incorrect clicks occurred just before or just after the target letter, which indicated that they were caused by small timing errors. The mental effort required during spelling, rated by the patient on a scale from 1 to 5, declined from an initial value of 5 to a mean of 2.8. Spelling initially took 52 seconds per letter; the time required dropped to 33 seconds per letter when word prediction was used.
Figure 3. Spelling Performance.
Panel A shows spelling performance aggregated over all 44 spelling runs, with the mean percentage of correct and incorrect responses indicated; T bars indicate standard deviations. Correct responses included the selection of the correct letters (“active” trial) and omission of a brain click when other letters were highlighted (“inactive” trial); more inactive than active trials were performed. Panel B shows the hours per day of use of the home-use system for spelling (the system was not used daily; therefore, days are not consecutive).
The home-use system was used at will with minimal assistance. Setting up the system required assistance in positioning the antenna, positioning and switching on the receiver and tablet, and connecting the cables; these tasks did not require any special skills (Fig. 1D). Logs from the patient’s system indicated that from day 197 (when she received the home-use system) through day 266, she used the system for communication on 32 days (Fig. 3B) and for 86 minutes per day on average. She used the system whenever she went outside, where lighting conditions made eye tracking impossible; in those circumstances, she relied on the system as the only means of communication and also used it to draw the attention of her caregiver by using the brain click to select a button that generated a sound.
Satisfaction with the home-use system was similar to that with the eye tracker. For both systems, the modified QUEST 2.0 scores for all aspects of use were “satisfied/very satisfied,” with the exception of “adaptability to your specific situation” for the eye tracker (for which the score was “unsatisfied”). PIADS items were grouped into three domains (competence, adaptability, and self-esteem); the resulting scores for the system and the eye tracker were 1.1 and 1.3, 2.2 and 1.5, and 1.0 and 0.6, respectively.
Discussion
We describe a method for autonomous communication, involving a fully implanted brain–computer interface, for use by a locked-in patient with late-stage ALS. By attempting to move her hand and by using software that automatically extracted electrocortical signal features, the patient was able to control commercial communication software that could type, albeit at a slow rate. She was able to use the system at home with minimal assistance; the assistance that was required mainly involved placement of the antenna in proximity to the implanted transmitter. The patient was able to use the system to replace an eye tracker when lighting conditions made the eye tracker impossible to use. She reported high levels of satisfaction with the device and used it multiple times per week. Scores for both systems were slightly lower than those previously reported for eye trackers by patients with ALS (a score of approximately 2 on the three PIADS domains and high satisfaction on the QUEST 2.0).24
These results show that the system is capable of meeting the requirements for independent communication and could be a method of communication for patients who are unable to use conventional communication tools such as eye trackers. Factors that may limit the use of the system by future patients include cortical damage, cognitive impairment, and unsupportive caregiving.
Supplementary Material
Acknowledgments
Supported by government grants from the Netherlands (UGT7685, Economic Affairs SSM06011 and STW 12803, cosponsored by Medtronic) and the European Union (ERC-Adv 320708).
We thank Tineke Gebbink and Bert Kamphuis for their help with the electrocorticographic recordings during and after the first surgery; Gerrit Melis and Guus Wisse for their help in setting up and performing the magnetic resonance imaging (MRI) experiments; Martine van Zandvoort for neuropsychological evaluation and monitoring of the psychological health of the patients in the study; Tom Snijders for preoperative and postoperative neurologic evaluation; Janine Ophorst for help in scheduling and for the postoperative medical evaluation; Jurgen de Graaff for providing anesthesiologic support during the design of the study and during the MRI scans and surgical procedures; Leo van Wolfswinkel for anesthesiologic support during the MRI scans and surgical procedures; Bert Jansen for placing the fiducial markers; Femke Nijboer for advice on the informed consent procedure with locked-in patients and for serving as an independent observer during this procedure; Ghislaine van Thiel for helpful discussions on the ethical aspects of implants for locked-in patients; Diederik van Dijk, Meggy Peters, Roland Burgers, and the intensive care unit nurses at University Medical Center Utrecht for hosting and care of our patient; Michael Gaytant for help and advice regarding mechanical ventilation; Herke Jan Noordmans for assistance in neuronavigational issues; Bon Verweij for helpful discussions about neurosurgery and brain–computer interfaces for locked-in patients; Gaetano Leogrande, Scott Stanslaski, and Randy Jensen for technical support before and during the implantation of the device; Mark Bruurmijn, Efraïm Salari, and Julia Berezutskaya for helpful discussion and for help with data analysis during the implantation week; Meron Vermaas for his help in developing a user-friendly software platform for training games and communication; Quido de Valk for advice on assistive technology to potential and excluded patients; and the patient in this study, for her time, effort, and helpful feedback.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.American Congress of Rehabilitation Medicine. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil. 1995;76:205–9. doi: 10.1016/s0003-9993(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 2.Chiò A, Logroscino G, Traynor BJ, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41:118–30. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laureys S, Pellas F, Van Eeckhout P, et al. The locked-in syndrome: what is it like to be conscious but paralyzed and voiceless? Prog Brain Res. 2005;150:495–511. doi: 10.1016/S0079-6123(05)50034-7. [DOI] [PubMed] [Google Scholar]
- 4.Bruno MA, Bernheim JL, Ledoux D, Pellas F, Demertzi A, Laureys S. A survey on self-assessed well-being in a cohort of chronic locked-in syndrome patients: happy majority, miserable minority. BMJ Open. 2011;1(1):e000039. doi: 10.1136/bmjopen-2010-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousseau MC, Baumstarck K, Alessandrini M, Blandin V, Billette de Villemeur T, Auquier P. Quality of life in patients with locked-in syndrome: evolution over a 6-year period. Orphanet J Rare Dis. 2015;10:88. doi: 10.1186/s13023-015-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolpaw JR. Brain-computer interfaces as new brain output pathways. J Physiol. 2007;579:613–9. doi: 10.1113/jphysiol.2006.125948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 8.Pfurtscheller G, Flotzinger D, Pregenzer M, Wolpaw JR, McFarland D. EEG-based brain computer interface (BCI): search for optimal electrode positions and frequency components. Med Prog Technol. 1995-1996;21:111–21. [PubMed] [Google Scholar]
- 9.Kennedy PR, Bakay RA. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998;9:1707–11. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 11.Collinger JL, Wodlinger B, Downey JE, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381:557–64. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouton CE, Shaikhouni A, Annetta NV, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–50. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- 13.Huggins JE, Wren PA, Gruis KL. What would brain-computer interface users want? Opinions and priorities of potential users with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:318–24. doi: 10.3109/17482968.2011.572978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nijboer F. Technology transfer of brain-computer interfaces as assistive technology: barriers and opportunities. Ann Phys Rehabil Med. 2015;58:35–8. doi: 10.1016/j.rehab.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Vansteensel MJ, Hermes D, Aarnoutse EJ, et al. Brain-computer interfacing based on cognitive control. Ann Neurol. 2010;67:809–16. doi: 10.1002/ana.21985. [DOI] [PubMed] [Google Scholar]
- 16.Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FS, Ramsey NF. Neurophysiologic correlates of fMRI in human motor cortex. Hum Brain Mapp. 2012;33:1689–99. doi: 10.1002/hbm.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siero JC, Hermes D, Hoogduin H, Luijten PR, Ramsey NF, Petridou N. BOLD matches neuronal activity at the mm scale: a combined 7T fMRI and ECoG study in human sensorimotor cortex. Neuroimage. 2014;101:177–84. doi: 10.1016/j.neuroimage.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 19.Kleih SC, Riccio A, Mattia D, et al. Motivation affects performance in a P300 Brain Computer Interface. Int J Bioelectromagn. 2011;13:46–7. [Google Scholar]
- 20.Kübler A, Holz EM, Riccio A, et al. The user-centered design as novel perspective for evaluating the usability of BCI-controlled applications. PLoS One. 2014;9(12):e112392. doi: 10.1371/journal.pone.0112392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day H, Jutai J. Measuring the psychosocial impact of assistive devices: the PIADS. Can J Rehabil. 1996;9:159–68. [Google Scholar]
- 22.Torres Valderrama A, Paclik P, Vansteensel MJ, Aarnoutse EJ, Ramsey NF. Error probability of intracranial brain computer interfaces under non-task elicited brain states. Clin Neurophysiol. 2012;123:2392–401. doi: 10.1016/j.clinph.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Bech P, Timmerby N, Martiny K, Lunde M, Soendergaard S. Psychometric evaluation of the Major Depression Inventory (MDI) as depression severity scale using the LEAD (Longitudinal Expert Assessment of All Data) as index of validity. BMC Psychiatry. 2015;15:190. doi: 10.1186/s12888-015-0529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caligari M, Godi M, Guglielmetti S, Franchignoni F, Nardone A. Eye tracking communication devices in amyotrophic lateral sclerosis: impact on disability and quality of life. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:546–52. doi: 10.3109/21678421.2013.803576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.