Abstract
Many tissues develop coordinated patterns of cell polarity that align with respect to the tissue axes. This phenomenon refers to planar cell polarity (PCP) and is controlled by multiple conserved PCP modules. A key feature of PCP proteins is their asymmetric localization within the tissue plane, whose orientation is guided by global directional cues. Here, we highlight current models and recent findings on the role of tissue-level gradients, local organizer signals, and mechanical forces in establishing the global patterns of PCP.
Introduction
Planar cell polarity (PCP) refers to the collective polarization of cells along a common tissue axis. Notable examples of planar polarized structures are the bristles covering the insect body, the stereocilia bundles that line the vertebrate inner ear, and the hairs, scales and feathers that decorate the vertebrate epidermis [1–4]). Planar polarity is also apparent in the collective cell movements that drive embryo gastrulation and axis elongation, where large collections of cells coordinately migrate in a common direction [5–7] The defining feature of these planar polarized structures and behaviors is their uniform orientation across the tissue axis, a feature that strongly suggests the existence of long-range directional cues that bias polarity. The identification of these global cues has been both a challenge and a priority for the field of planar polarity. Using a combination of experimental approaches and mathematical modeling significant progress has been made towards identifying and elucidating the mechanisms by which global cues orient planar polarity. In this review, we highlight some recent advances in understanding global PCP cues. For comprehensive and historical perspectives of global PCP inputs, we refer the reader to excellent reviews by [8–12].
Framework of a PCP module
Similar to apical-basal and or individual cell polarity, PCP establishment requires 1) a tissue level directional cue to orient polarity relative to the tissue axes; 2) asymmetric segregation of polarity components and feedback amplification to reinforce asymmetry; and 3) transduction of the polarity cue, often to cytoskeletal regulatory proteins, to produce polarized cell behaviors. What sets planar polarized systems apart from apical-basal or individual cell polarity, is the direct physical coupling and propagation of polarity between adjacent cells, which enables local coordination of polarity. This intercellular coupling is achieved through the formation of asymmetric bridges between the transmembrane PCP components, which form heterodimers in trans, across the extracellular space [13–19]. The formation of asymmetric bridges appears to be an essential and conserved feature across different planar polarity systems, three of which we discuss here: the core PCP pathway, the Fat-Dachsous PCP pathway, and germ band extension pathway. In the core PCP pathway, Frizzled (Fz) and Vang Gogh (Vang) form an asymmetric bridge whose interaction requires the homodimeric cadherin, Flamingo/Celsr [13–15,18–20]. Heterotypic binding between the large protocadherins Fat (Ft) and Dachsous (Ds) form the intercellular bridges for the Ft-Ds pathway [16,17], while heterotypic binding between different Toll receptors bridges collectively migrating cells in the Drosophila germ band [21]. Importantly, an excess of one transmembrane PCP component preferentially recruits its binding partner to the interface between neighboring cells [14,16–18,22]. This imbalance of PCP proteins between neighbors is thought to be central to the mechanism by which global cues orient PCP vectors [12,13,15,23].
Downstream of the asymmetric bridges are cytoplasmic factors that play dual functions in connecting the transmembrane components to downstream outputs and establishing feedback loops to amplify and stabilize PCP protein asymmetry. For the core PCP system, these factors include Disheveled, Prickle, and Diego [2,4]. In the Fat-Ds system, the functions of the cytoplasmic factors are less understood but include the asymmetrically localized myosin Dachs [10,24]. Finally, there are the downstream effectors, which are highly diverse and cell type specific depending on the planar polarized behavior [25].
Axial versus vectorial asymmetry
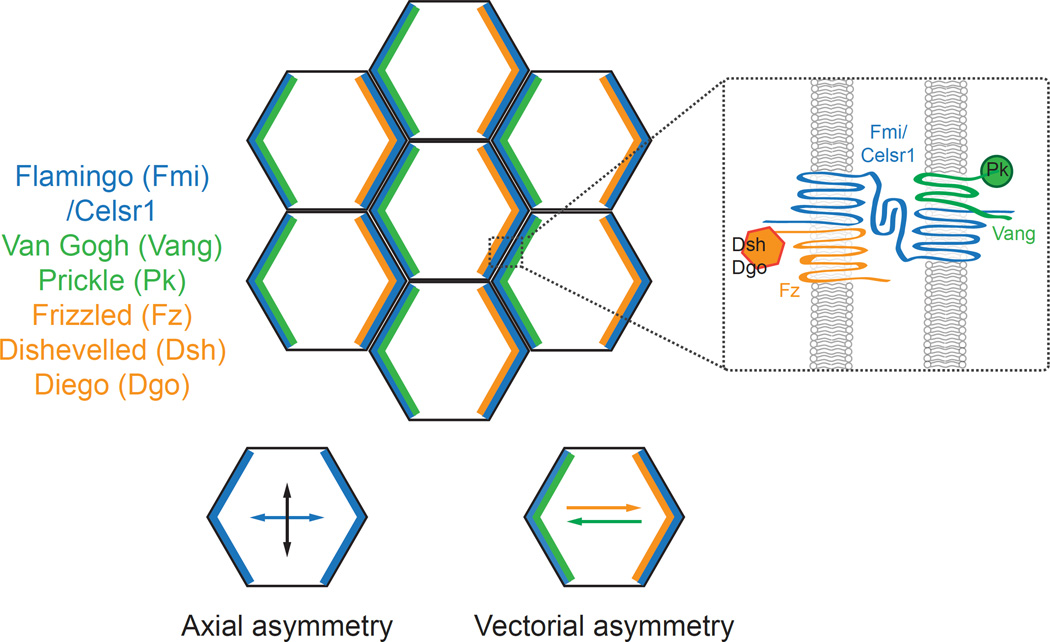
Planar polarity proteins display two types of polarity, which we term axial and vectorial. For example, in the core PCP system, Frizzled (Fz) and Vang Gogh (Vang) display vectorial, or unipolar, asymmetry, localizing preferentially to one pole of each cell (Figure 1). Flamingo (Fmi; Celsr in vertebrates), by contrast, displays axial, or bipolar, asymmetry where it localizes to both poles along one planar axis and is excluded from the junctions oriented along the orthogonal planar axis (Figure 1). Global cues that orient PCP localization must bias both of these asymmetries, and current evidence suggests that the PCP axis and the PCP vector may be biased and aligned, at least in part, by different types of directional cues.
Figure 1.
Asymmetric distribution of core planar cell polarity proteins. Core PCP proteins localize asymmetrically along cell boundaries as indicated (inset). Fmi or Celsr1 displays axial asymmetry where it localizes to intercellular junctions oriented along one tissue axis but is excluded from orthogonal junctions. In contrast, Vang-Prickle and Frizzled-Dishevelled-Diego complexes adopt vectorial asymmetries where each complex localizes to opposite poles of the cell.
Global cues that bias vectorial asymmetry
The global cues that bias vectorial asymmetry must promote the unipolar localization of PCP proteins at the individual cell level in a coordinate fashion across entire tissues. Factors expressed in tissue-wide gradients along the axis of polarity are therefore ideal candidates for long-range vectorial cues. Several graded factors are proposed to act as global PCP signals, but here we focus on recent progress understanding how the 1) Fat-Dachsous-Fourjointed (Ft-Ds-Fj), 2) Wnt/Wingless and 3) early patterning systems function to orient vectorial asymmetry mainly in the Drosophila wing.
Ft-Ds-Fj
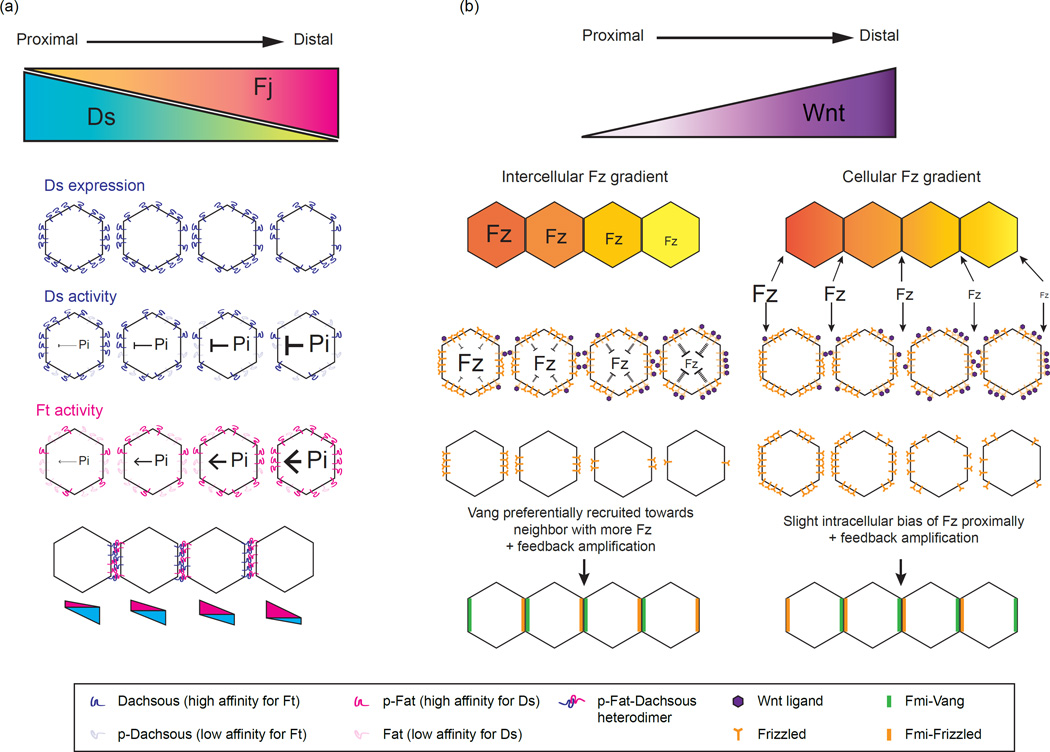
The orientation of cuticular hairs in Drosophila relies on both the core PCP and the Ft-Ds-Fj systems, which are thought to be independent but intersecting PCP pathways [9,10,24]. Ft and Ds are large protocadherins that localize to opposite sides of the cell where they form intercellular heterodimers [17,26–28]. Their interaction is regulated by the Golgi-associated kinase, Fj, which phosphorylates the extracellular domains of Ft and Ds and modulates their binding [22,29,30]. Ds and Fj are expressed in tissue wide gradients in several Drosophila tissues [16,31–33], and their transcriptional gradients are thought to act as global cues that are converted into Ft-Ds subcellular asymmetries (Figure 2). Because phosphorylation by Fj has opposite effects on Ft and Ds activity - it increases the affinity of Ft for Ds, but decreases the affinity of Ds for Ft - the Fj gradient generates complementary affinity gradients of Ft and Ds (Figure 2) [22,29]. The imbalance of Ds and Ft activity between neighboring cells has been proposed to bias their localization to opposite cell edges [11,13,26–28,34].
Figure 2.
Converting morphogen gradients into positional information. (a) Dachsous (Ds) and Four-jointed (Fj) are expressed in opposing gradients in the fly wing. Fat (Ft) and Dachsous cadherins interact heterotypically across neighboring cells. Fj phosphorylates the extracellular cadherin domains of both Ft and Ds. Fj phosphorylation increases Ft affinity for Ds and decreases Ds affinity for Ft. The transcriptional gradient of Ds and Fj generates opposing gradients of Ft and Ds binding affinity along the tissue axis, which is converted to subcellular asymmetries of Ft and Ds heterodimers. Adapted from [35]. (b) Two possible mechanisms by which can convert an extracellular Wnt morphogen gradient into intercellular or intracellular Frizzled (Fz) gradients [46]. In the first model (left), Wnt reduces the level of available or active Fz receptors in a given cell indirectly, via signaling transduction or other intracellular mechanism. Because neighboring cells are exposed to slightly different levels of ligand, an intercellular gradient of available Fz receptors is generated across the junction between neighboring cells. Each proximal cell has a higher concentration of available Fz receptors than its distal neighbor leading to the preferential recruitment of Vang to the cell interface with more available extracellular Fz. Combined with feedback amplification, this should bias Fz localization distally and recruit Vang proximally. In the second model (right), Wnt generates a gradient of active Fz across the length of each cell by directly antagonizing Fz extracellularly. In this direct model, there is no difference in Fz concentration across the junction between adjacent cells, instead the difference lies on either side of the same cell - Fz levels or activity are lower on the distal side than on the proximal side. This is expected to provide a slight proximal bias in Fz localization and distal recruitment of Vang, a reversal of their observed localization patterns in vivo. Thus, an indirect, intercellular model may be more likely to explain the mechanism by which an inhibitory Wnt gradient orients core PCP vectors.
Support for this model comes from a recent experiment in silico, which successfully generated Ft-Ds subcellular asymmetry by simulating complementary Ft and Ds activity gradients and making phospho Ft-Ds heterodimers more stable over other dimer combinations (Figure 2) [35]. Notably a 2–3% difference in Fj expression between neighbors gave rise to modest Ft and Ds asymmetries in the absence of any feedback amplification, indicating that small differences in the level of phosphorylated Ft or Ds are sufficient to bias their localization. Testing the model in vivo, Hale et al reasoned that lateral mobility within the membrane would be reduced upon heterodimer formation, and used fluorescence recovery after photobleaching (FRAP) as a novel in vivo assay to monitor the effects of phosphorylation on Ft-Ds binding. Consistent with the model and with previous binding assays, mutations in Ft that blocked phosphorylation by Fj reduced heterodimer stability while phospho-mutants in Ds enhanced heterodimer stability [35]. Thus, the Fj gradient acts, in a sense, as a morphogen, altering Ft and Ds activities in a dose-dependent manner. The resulting polarity vector arises from the local difference in activity between neighboring cells and preferred binding between phospho-Ft-Ds heterodimers.
The Ft-Ds-Fj system also intersects with the core PCP system influencing the orientation of Fz and Vang vectors [10]. In the proximal region of the wing, Ft and Ds promote the formation of polarized microtubule networks upon which PCP complexes (Fmi-Fz-Dsh) are transcytosed and trafficked preferentially towards one side of the cell [36–38]. In a simulated model where Dsh travels towards microtubule plus ends that are aligned by gradients of Ds and Ft, Dsh asymmetry arises under a variety of Ds gradients, suggesting that a microtubule-based polarizing cue is relatively insensitive to the shape or slope its input gradient, as long as it is in the right direction [37]. The transport-based bias in Fz-Dsh concentration is then thought to be amplified by feedback interactions, stabilizing Fz-Dsh and Vang-Pk unipolar localizations [39,40]. However, because hair polarity is mostly unaffected by Ft and Ds near the wing margin, and because Ft and Ds mutant phenotypes are largely rescued by uniform expression [17,37,41,42], the extent to which Ft-Ds-Fj gradients act as upstream global cues for the core system continues to be debated. Nevertheless, polarized microtubules have also been shown to align with PCP vectors in mouse airway epithelial cells and Xenopus gastrulae, suggesting that microtubule-based transport may be a broadly used mechanism to bias PCP asymmetry [43,44].
Wnt
Given their ability to bind to Fz receptors and their graded expression patterns in many developing tissues, Wnt/Wingless (Wg) secreted ligands have long been hypothesized to act as global PCP cues. Drosophila wing hairs point towards the source of Wnt along the wing margin, and recent work demonstrated that Wg and dWnt4 are required redundantly for wing hair polarity. Wnts appears to act instructively, as clonal ectopic expression of either ligand non-autonomously reorients hairs towards high levels of Wnt [45]. This pattern resembles the effect of loss-of-function Fz clones on neighboring wild type cells suggesting that Wnt acts antagonistically towards Fz. Consistently, addition of Wnt conditioned media to Drosophila S2 cells inhibits the intercellular interaction between Fz and Vang in a dose dependent manner [45], suggesting that Wg may compete with Vang for Fz binding. While the idea that a Fz activity gradient directs PCP orientation has been around for some time [15,19,23], Wu et al propose a mechanism where Wnt generates the Fz gradient by antagonizing Fz-Vang binding [4,45]. Note that, unlike the Fj gradient in the Ft-Ds system, this direct inhibitory mechanism is predicted to generate differences in Fz activity across cells, but not across the junctions/boundaries between adjacent cells (the concentration of Wnt, and therefore Fz activity, would be equivalent on both sides of an intercellular space). Interestingly, in the theoretical model by Abley et al, an extracellular gradient that generates activity differences within each cell produces a Fz vector pointing in the opposite direction compared to a gradient that generates activity differences across neighboring cells (see Figure 2 and legend for detail) [46]. Based on this model and the directions of Wnt gradients in vivo, a mechanism that produces differences in Fz activity between adjacent cells, perhaps by indirectly antagonizing Fz-Vang binding, may be more likely to produce the observed, margin-oriented, Fz vector.
A Wnt gradient has also been proposed to orient PCP in the mouse embryonic limb, but through a mechanism that positively regulates Vangl2, rather than inhibits Fz. In the limb, a distal-toproximal Wnt5a gradient induces dosage sensitive phosphorylation of Vangl2, generating different levels of phospho-Vang between cells [47]. Phosphorylation of Vang on equivalent residues is required for Vang function and asymmetry in the fly wing, suggesting phospho-Vang may represent an active form of the protein [48]. While the precise mechanisms by which Wnt gradients orient core PCP vectors still need to be deciphered, the polarity defects resulting from the loss of both Wg and dWnt4 are relatively minor, suggesting the existence of additional cues to orient polarity.
Patterning and morphogenesis
The earliest PCP patterns in the Drosophila wing are established long before metamorphosis, in the larval wing imaginal disc. In the disc, core PCP vectors closely follow the expression domains of the major wing patterning organizers, orienting towards Wg and Notch along the dorsal-ventral (DV) boundaries, Hedgehog (Hh) and Decapentaplegic (Dpp) along the anteriorposterior (AP) boundary, and Dachsous near the wing hinge [49]. Though these morphogens are graded in their expression, flattening the gradient of Wg, Hh, or Dpp does not reduce PCP asymmetry [49]. Instead, regional shifts in the orientation of PCP vectors are observed that are consistent with underlying changes in tissue growth [49]. Sanger et al suggest that morphogen gradients orient PCP vectors through their effects on growth and morphogenesis rather than through their graded expression. Moreover, they propose that if PCP patterns are established when a tissue is small, cells could specify their vector orientation by interpreting combinations of local patterning signals in the absence of gradients, and later maintain and refine the pattern during growth and morphogenesis. This is similar to what is proposed in Drosophila germ band extension in which periodic expression of A-P patterning genes generates an overlapping expression pattern of Toll-like receptors, such that each cell along a segment contains a unique positional code of Toll-like receptors [21]. This allows cells to compare their identity with each other and specify the orientation of myosin contractility and junctional remodeling across the tissue. These are appealing mechanism for tissues that acquire PCP among a small number of cells, but whether similar local compartment boundaries can be applied to PCP in large tissues, such as the mammalian skin, remains to be determined.
Global cues that bias axial asymmetry
The global cues that bias axial asymmetry must promote the bipolar localization of PCP proteins along one tissue axis and exclusion from the orthogonal axis. Mechanical tension, which arises during tissue morphogenesis, can act over many cell distances and bias the PCP axis. Here we focus on recent studies revealing how physical forces exerted during tissue morphogenesis act as directional cues for axial PCP asymmetry.
Fmi/Celsr in the core PCP system
The atypical cadherin, Fmi (or Celsr in vertebrates), is a central component of the core PCP system that forms intercellular homodimers and connects the vectorial polarity of Fz and Vang between neighboring cells. Unlike Fz and Vang, however, Fmi/Celsr displays axial asymmetry where it localizes preferentially to cell borders along one tissue axis and is excluded from cell borders along the orthogonal axis (Figure 1) [50,51]. This asymmetry gives rise to the characteristic zigzag pattern of Fmi localization observed in the Drosophila pupal wing [51]. While the mechanisms that ensure Fmi/Celsr homodimers orient along only one tissue axis is relatively a poorly understood phenomenon, directed physical forces have recently emerged as global cues that coordinate the Fmi/Celsr axis.
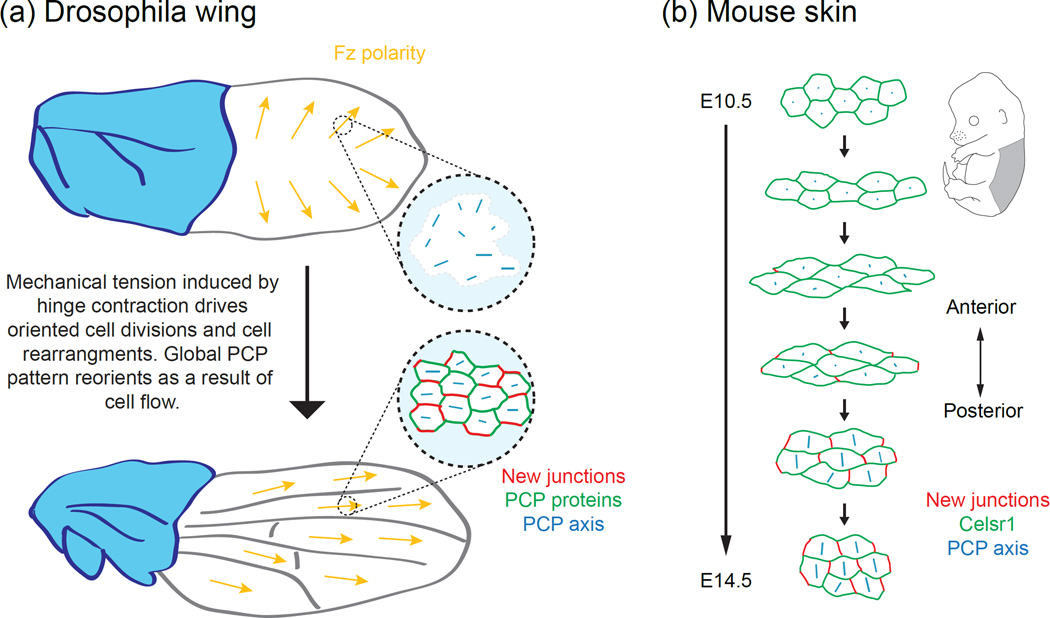
During Drosophila wing morphogenesis, the epithelium changes shape dramatically, elongating along the proximal-distal axis as the wing hinge contracts. During this process the PCP axis is completely remodeled, where it rotates from a radial to proximal-distal orientation (Figure 3). This shift in polarity is driven by shear-induced cell rearrangements, during which cells dissolve and assemble intercellular junctions as they exchange neighbors [52,53]. Because PCP proteins are preferentially retained at persistent junctions and are slow to accumulate at newlyformed junctions, neighbor exchanges establish a new axis of asymmetry [52].
Figure 3.
Interdependent relationship between the orientation of junctional remodeling and the Fmi/Celsr1 polarity axis. (a) The global axis of PCP across the wing blade reorients with hinge contraction, during which oriented cell divisions and cell rearrangements occur. These morphogenetic changes reorient the PCP axis from a radial to proximal-distal orientation. The new PCP axis aligns parallel to the orientation of new cell-cell junctions. Schematic adapted from [52]. (b) Schematic depiction of how oriented junction formation, functioning to relax tissue strain during skin morphogenesis, could define Celsr1 polarity axis in developing mouse embryonic skin.
A similar mechanism also coordinates the initial Celsr1 axis during murine skin morphogenesis [54]. Early in epidermal development, skin epithelial cells are mechanically deformed along the one tissue axis, inducing oriented cell divisions and cell rearrangements (Figure 3). Concomitantly, Celsr1 redistributes from a disordered to highly polarized and aligned localization. Live imaging of fluorescently-tagged Celsr1 in skin organotypic cultures showed that Celsr1 asymmetry emerges spontaneously during neighbor exchange upon the formation of new intercellular junctions. Celsr1 is slow to accumulate at nascent junctions yet stably associates with persistent junctions, resulting in the spontaneous breaking of Celsr1 symmetry [54].
The vectorial asymmetry of PCP proteins is considered to provide the vectorial information for downstream cellular structures, but the function of axial asymmetry is less clear. We speculate that axial polarity may both restrict and enhance feedback propagation of PCP vectors. By generating cell edges that are depleted for Celsr1, Fz and Vangl vectorial information is restricted along one tissue axis, limiting feedback propagation along the orthogonal axis. This would in turn refine the position at which downstream structures like wing hairs and hair follicle can form. It would be interesting to generate models of PCP establishment with and without axial asymmetry and compare the rate and precision of emerging PCP patterns.
Acknowledgments
Research in the laboratory is funded by NIH/NIAMS grants R01AR066070 and R01AR068320 as well as the Searle Scholars Program and the Vallee Foundation. W.Y.A. is supported by the American Heart Association Predoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell's compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Mlodzik M. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt) Annu Rev Cell Dev Biol. 2015;31:623–646. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tada M, Heisenberg CP. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development. 2012;139:3897–3904. doi: 10.1242/dev.073007. [DOI] [PubMed] [Google Scholar]
- 7.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–971. doi: 10.1016/j.semcdb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence PA, Casal J. The mechanisms of planar cell polarity, growth and the Hippo pathway: some known unknowns. Dev Biol. 2013;377:1–8. doi: 10.1016/j.ydbio.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes Dev. 2013;27:2207–2220. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strutt D. Gradients and the specification of planar polarity in the insect cuticle. Cold Spring Harb Perspect Biol. 2009;1:a000489. doi: 10.1101/cshperspect.a000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- 16.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 17.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 18.Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Struhl G, Casal J, Lawrence PA. Dissecting the molecular bridges that mediate the function of Frizzled in planar cell polarity. Development. 2012;139:3665–3674. doi: 10.1242/dev.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pare AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA. A positional Toll receptor code directs convergent extension in Drosophila. Nature. 2014;515:523–527. doi: 10.1038/nature13953. This is the first demonstration that the planar polarized cell behaviors that drive Drosphila germ band extension are established by the formation of asymmetric bridges, not by the known PCP pathway members, but between different Toll receptors. The striped expression of Toll receptors and hetertypic binding between neighboring cells provides a direct mecahistic link between A-P patterning genes and the downstream planar polarized cell behaviors they control
- 22.Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat:dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- 24.Thomas C, Strutt D. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Dev Dyn. 2012;241:27–39. doi: 10.1002/dvdy.22736. [DOI] [PubMed] [Google Scholar]
- 25.Devenport D. Tissue morphodynamics: Translating planar polarity cues into polarized cell behaviors. Semin Cell Dev Biol. 2016;55:99–110. doi: 10.1016/j.semcdb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambegaonkar AA, Pan G, Mani M, Feng Y, Irvine KD. Propagation of Dachsous-Fat planar cell polarity. Curr Biol. 2012;22:1302–1308. doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, Marchand R, Bardet PL, Marcq P, Graner F, et al. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–727. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- 28.Brittle A, Thomas C, Strutt D. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr Biol. 2012;22:907–914. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 32.Zeidler MP, Perrimon N, Strutt DI. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr Biol. 1999;9:1363–1372. doi: 10.1016/s0960-9822(00)80081-0. [DOI] [PubMed] [Google Scholar]
- 33.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence PA, Struhl G, Casal J. Do the protocadherins Fat and Dachsous link up to determine both planar cell polarity and the dimensions of organs? Nat Cell Biol. 2008;10:1379–1382. doi: 10.1038/ncb1208-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hale R, Brittle AL, Fisher KH, Monk NA, Strutt D. Cellular interpretation of the long-range gradient of Four-jointed activity in the Drosophila wing. Elife. 2015;4 doi: 10.7554/eLife.05789. This paper presents experimental and computational evidence that a tissue-level gradient of Four-jointed activity is convereted into subcellular Fat and Dachsous asymmetries. Using FRAP as an in vivo binding assay, this paper provides in vivo evidence that phosphorylation of Fat and Dachsous by Four-jointed modulates their binding. The computational model makes interesting predictions about the dominant role of Four-jointed on Ft over Dachsous and about the role of feedback in generating asymmetries.
- 36.Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matis M, Russler-Germain DA, Hu Q, Tomlin CJ, Axelrod JD. Microtubules provide directional information for core PCP function. Elife. 2014;3:e02893. doi: 10.7554/eLife.02893. Matis et al follow up up previous findings from Shimada, Harumoto and colleagues demonstrating a close spatial and temporal correlation between Fat-Dachsous-Four-jointed gradients, microtubule polarity, polarized vesicle trafficking and PCP vector orientation. The paper provides additional evidence for the model that the Fat-Dachsous-Four-jounted module provides a global input into the core PCP system by orienting microtubule polarity and core PCP transport.
- 38.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- 40.Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 41.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 42.Matakatsu H, Blair SS. Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development. 2012;139:1498–1508. doi: 10.1242/dev.070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chien YH, Keller R, Kintner C, Shook DR. Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr Biol. 2015;25:2774–2784. doi: 10.1016/j.cub.2015.09.015. This is the first paper to detect core PCP asymmetries in the Xenopus non-neural ectoderm at the gastrula stage. The PCP axis correlates with strain-induced microtubule alignment that arises from large-scale gastrulation movements.
- 44.Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Curr Biol. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–1055. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abley K, De Reuille PB, Strutt D, Bangham A, Prusinkiewicz P, Maree AF, Grieneisen VA, Coen E. An intracellular partitioning-based framework for tissue cell polarity in plants and animals. Development. 2013;140:2061–2074. doi: 10.1242/dev.062984. [DOI] [PubMed] [Google Scholar]
- 47.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelly LK, Wu J, Yanfeng WA, Mlodzik M. Frizzled-Induced Van Gogh Phosphorylation by CK1epsilon Promotes Asymmetric Localization of Core PCP Factors in Drosophila. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.06.010. Kelly et al demonstrate that Vang is phosphorylated at the plasma membrane in a Frizzled-dependent manner by the kinase CK1episilon. The specific function of Vang phosphorylation is unclear but it is required for polarized localization of Vang and other core PCP proteins in the Drosophila wing. This is related to the mechanism by which Wnt5a regulates Vang phosphorylation and planar polarity in the developing mouse limb.
- 49.Sagner A, Merkel M, Aigouy B, Gaebel J, Brankatschk M, Julicher F, Eaton S. Establishment of global patterns of planar polarity during growth of the Drosophila wing epithelium. Curr Biol. 2012;22:1296–1301. doi: 10.1016/j.cub.2012.04.066. [DOI] [PubMed] [Google Scholar]
- 50.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 52.Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 53.Eaton S, Julicher F. Cell flow and tissue polarity patterns. Curr Opin Genet Dev. 2011;21:747–752. doi: 10.1016/j.gde.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 54. Aw WY, Heck BW, Bradley J, Devenport D. Transient tissue-scale deformation coordinates alignment of planar cell polarity junctions in the mammalian skin. Curr Biol. 2016 doi: 10.1016/j.cub.2016.06.030. In press. Aw et al demonstrate that the PCP axis in the mouse epidermis arises concomitantly with tissue-scale deformations that induce neighbor exchanges and new junction formation. They describe a spontaneously polarizing in vitro model system where planar polarity arises de novo with the formation of new intercellular junctions due to the stable association of Celsr1 homodimers at persistent cell interfaces and slow accumulation at new cell interfaces.