Abstract
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disease that can affect multiple end organs. Kidney and brain are two of the organs most commonly involved in SLE. Past studies have suggested the importance of macrophages in the pathogenesis of lupus nephritis (LN). Furthermore, as the immune effectors of the brain, microglia have been implicated in pathways leading to neuropsychiatric SLE (NPSLE).
We depleted macrophages and microglia using GW2580, a small colony stimulating factor-1 receptor (CSF-1R) kinase inhibitor, in MRL-lpr/lpr (MRL/lpr) mice, a classic murine lupus model that displays features of both LN and NPSLE. Treatment was initiated before the onset of disease, and mice were followed for the development of LN and neurobehavioral dysfunction throughout the study.
Treatment with GW2580 significantly ameliorated kidney disease, as evidenced by decreased proteinuria, BUN, and improved renal histopathology, despite equivalent levels of IgG and C3 deposition in the kidneys of treated and control mice. We were able to confirm macrophage depletion within the kidney via IBA-1 staining. Furthermore, we observed specific improvement in the depression-like behavioral deficit of MRL/lpr mice with GW2580 treatment. Circulating antibody and autoantibody levels were, however, not affected.
These results provide additional support for the role of macrophages as a potentially valuable therapeutic target in SLE. Inhibiting CSF-1 receptor signaling would be more targeted than current immunosuppressive therapies, and may hold promise for the treatment of renal and neuropsychiatric end organ disease manifestations.
Keywords: SLE, Lupus nephritis, Neuropsychiatric lupus, Macrophages, CSF-1R
1. INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that typically affects multiple organ systems. Kidney involvement, or lupus nephritis (LN), appears in 40-60% of individuals with SLE, and is one of the leading causes of morbidity and mortality in these patients [1]. Additionally, about 40% of SLE patients will display central nervous system (CNS) manifestations, known as neuropsychiatric lupus (NPSLE) [2]. Diffuse NPSLE manifests with a diverse array of symptoms that include headaches, cognitive dysfunction, and depression, all of which negatively affect patients’ quality of life [3]. The exact pathogenesis of LN and NPSLE are not yet known, although cytokines, immune cell infiltrates, and autoantibodies are believed to play an important role in both of these common end organ pathologies.
Macrophages are an important cellular component of the innate immune system. Present in nearly every tissue, macrophages can assume a myriad of phenotypes allowing them to perform various functional roles ranging from contributing to inflammation, to trophic scavenging and even aiding in the resolution of inflammation [4, 5]. The phenotype of a macrophage is dependent upon its microenvironment, which with it can interact through its large repertoire of surface receptors [6]. Within the brain, the resident macrophage population is known as microglia. Microglia are the predominant immune cell in the central nervous system (CNS), and are potent cytokine producers.
An important receptor in regulating both macrophage and microglia function is the colony stimulating factor 1 receptor (CSF-1R). Signaling through the CSF-1R (by either CSF-1 or IL-34) is crucial for macrophage and microglia development, survival, function, and activation [7]. Additionally, CSF-1R signaling is important for migration and proliferation of macrophages [7].
Macrophages are thought to be important in the pathogenesis of LN. In both LN patients and mouse models of SLE, elevated levels of CSF-1 are found in the serum, urine, and kidneys, with urine and serum levels correlating with LN disease activity [8-11]. An influx of macrophages with a unique activated phenotype can be seen in both murine [12, 13] and human LN [14, 15], with macrophages accumulating both periglomerularly and intraglomerularly [16-18]. However, while the presence of macrophages within kidneys displaying active LN is consistent with a role of macrophages in disease pathogenesis, it does not prove that they are necessary to cause disease.
Within the brain, cytokines such as TWEAK, IL-6, IP-10, TNF, MCP-1, and G-CSF are significantly upregulated in the cerebral spinal fluid (CSF) of NPSLE patients, and may be directly linked to disease pathogenesis. These cytokines can all be released by activated microglia, and additionally may modulate microglia activity. Activated microglia can be found in both human and murine lupus brains [19, 20]. As the major immune effector cell in the brain, it has been surmised that microglia may be relevant to NPSLE pathogenesis.
Targeting a specific cell type, rather than the broad cytotoxicity exhibited by some of the pharmacological agents currently used to treat lupus, may improve efficacy and decrease off target toxicity. Thus, evidence that macrophages and microglia are important to the pathogenesis of LN and NPSLE, respectively, needs to be further investigated. We previously had demonstrated the importance of macrophages in the pathogenesis of nephritis in an inducible mouse model of antibody mediated kidney disease known as nephrotoxic serum nephritis (NTN), which models LN [17]. In this study, we sought to expand upon these studies by assessing the effect of macrophage depletion in a spontaneous (and more severe) model of SLE known as the MRL/lpr mouse.
The MRL/lpr strain is a classic, validated model for the severe crescentic glomerulonephritis seen in LN patients, as well as for NPSLE, as the mice exhibit depression-like behavior and cognitive deficits [21, 22]. We utilized an orally administered kinase inhibitor specific for the CSF-1R kinase, known as GW2580, to inhibit CSF-1R signaling. We assessed treatment with GW2580 in MRL/lpr mice, and evaluated its effects on brain and kidney disease.
2. MATERIALS and METHODS
2.1 Mice
Eight week old, female MRL/lpr mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed at the Albert Einstein College of Medicine animal facility (Bronx, NY). All animal studies were approved by the Institutional Animal Care Committee. Mice were aged to 10 weeks, at which point they began receiving daily treatment with either GW2580 (n=9) or vehicle control (n=10). GW2580 was administered at a dose of 100 mg/kg suspended in PBS via oral gavage until the mice reached 16 weeks of age. The mice underwent neurobehavioral testing at 17 and 18 weeks, and were sacrificed immediately afterwards (at 18 weeks of age). After initiation of treatment, urine was collected every 1 to 2 weeks, and serum was collected every 3 weeks; both were additionally collected at sacrifice.
2.2 Assessment of kidney disease
Proteinuria was assessed semiquantitively via Uristix (Siemens Healthcare Diagnostic, Tarrytown, NY). Additionally, albumin was measured in the urine obtained at sacrifice using the Mouse Albumin ELISA Quantitation Set from Bethyl Laboratories (Montgomery, TX). Urinary creatinine levels were measured using the Quantichrom Creatinine Assay kit (BioAssay Systems, Hayward, CA) and blood urea nitrogen (BUN) levels were measured with the DIUR 500 kit (BioAssays).
2.3 Renal histopathology
Kidney sections were deparaffinized and stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) by the Histology and Comparative Pathology Core at the Albert Einstein College of Medicine. Sections were then scored by an experienced nephropathologist (L.H.) who was blinded to the treatment groups. The scoring system used has been described previously [17]. Briefly, the sections were assigned a score of 0-4 (where 0 is no disease, and 4 is severe disease) within different categories that pertained to glomerular or tubular pathology (glomerular deposits, endocapillary proliferation, glomerular crescent formation, interstitial inflammation, and tubular casts and dilatation).
2.4 Kidney IBA-1 staining
Kidney sections were deparaffinized and rehydrated, followed by antigen retrieval in a pH 6 citrate buffer for 5 minutes, at 90-95°C. After washing the slides with wash buffer (0.05% Tween20 in PBS), slides were blocked for 1-2 hours at room temperature with 20% normal horse serum in PBS. Primary antibody (anti-IBA-1, WAKO, Osake, Japan) was then added at a 1:250 dilution in 2% normal horse serum in PBS, and incubated overnight at 4°C. For the secondary antibody, AF488 conjugated anti-rabbit IgG (Jackson Immuno Research, West Grove, PA) was used at a dilution of 1:250 in PBS, and incubated for one hour at room temperature. Slides were then washed, stained with DAPI, and then mounted in Fluoromount-G (Southern Biotech). Images were taken using a Zeiss AxioObserver CLEM.
2.5 Kidney IgG and complement deposition
Kidney sections were deparaffinized and rehydrated, followed by antigen retrieval in a pH 9 Tris buffer for 5 minutes, at 90-95°C. After washing the slides with wash buffer (0.05% Tween20 in PBS), slides were blocked for 2 hours at room temperature with 20% normal horse serum in PBS. Sections were then incubated with anti-mouse C3 antibody (MP Biomedicals, Santa Ana, CA) at a 1:100 dilution in 2% normal horse serum in PBS, and incubated overnight at 4°C. Slides were washed three times, and stained with the secondary AF594 conjugated anti-goat IgG antibody (Jackson Immuno Research), which was used at a dilution of 1:250 in PBS. Additionally, the slides were simultaneously incubated with AF488 labelled anti-mouse IgG antibody (Jackson Immuno Research), for 2 hours at room temperature. The slides were then washed 5 times, stained with DAPI, and mounted using Fluoromount G (Southern Biotech). Sections were imaged using a Zeiss AxioObserver CLEM.
2.6 Total IgG ELISA
Plates were coated with goat anti-mouse IgG (Southern Biotech) at a concentration of 1 μg/ml overnight at 4°C, washed, and blocked with 2% BSA in PBS at 37°C for one hour. Samples and standards were added to the plate, serially diluted, and incubated for 2 hours at 37°C. The plates were washed and then incubated with a goat anti-mouse AP secondary antibody (Southern Biotech) for one hour at 37°C. After washing, the substrate solution was added, and the absorbance read at 405 nm.
2.7 Anti-dsDNA and anti-chromatin ELISA
For the anti-dsDNA ELISA, plates were coated with 0.1 mg/ml salmon sperm DNA (ThermoFisher Scientific, Waltham, MA) in PBS overnight in a warm room, and then rinsed once the next day with water. Plates were then blocked with 1% BSA in PBS for 1 hour at 37°C, and samples added at a dilution of 1:100. Samples were incubated for 2 hours at 37°C and then washed from the plate. Secondary antibody was then added (goat anti-mouse IgG AP, Southern Biotech), incubated for 1 hour at 37°C, and then washed off before the substrate solution was added. After developing, the plate was read at 405 nm.
The anti-chromatin ELISA was performed as previously described [23]. Briefly, plates were coated with 5 μg/ml of chromatin overnight at 4°C, and then blocked for 1 hour at 37°C with 2% BSA in PBS. Samples were added at a dilution of 1:100 for 2 hours at 37°C. After washing, secondary antibody (goat anti-mouse IgG AP, Southern Biotech) was added at a dilution of 1:1000, and incubated on the plate for 1 hour at 37°C. Substrate solution was then added, and the absorbance read at 405 nm. Sample ODs in both the anti-dsDNA and anti-chromatin ELISAs were normalized to the ODs of serum from healthy mice.
2.8. Neurobehavioral testing
To evaluate whether GW2580 treatment modulated neuropsychiatric manifestations, a comprehensive battery of neurobehavioral tests were conducted on GW2580 and control treated mice at 17-18 weeks of age. The detailed methods have been published previously [24-27]. Briefly, in the Porsolt swim test, animals were put in a glass beaker filled with water at a temperature of 27°C for 4 minutes. The first minute was used for the mice to adjust to the water environment, and the following 3 minutes were employed as the test session where the floating time, referred as immobility, was manually scored. The immobility percentage was calculated by the total floating time (in seconds)/total time scored (180 seconds). In addition, the open field assay was used to evaluate locomotor activity. Animals were put in an open field arena (40 cm × 40 cm) with a center zone (15 cm × 15 cm) for 6 minutes. Total track length, center track length, center visit, and center time was automatically tracked by Biobserve Viewer III software.
A standardized object placement (OP) test was employed to assess spatial recognition memory, as described [26, 27]. In brief, mice were given 5 minutes to explore two identical objects which had been placed in different locations within a cage. After a 25-minute retention interval during which time one object was moved to a new location, mice were returned to the same arena. The preference score (%) for the object placement test was calculated as ([exploration time of the novel location]/[exploration time of both locations)])×100, with the scorer of the test blinded to the experimental groups.
2.9 Brain histology
At 18 weeks of age, mice were anesthetized and perfused with PBS, and the brains extracted and divided through the midline. The right hemisphere of the brain was fixed in 4% paraformaldehyde (PFA) at 4°C overnight. Brains were sectioned in a sagittal plane. H&E staining was performed by the histopathology core facility at the Albert Einstein College of Medicine (Bronx, NY). Immune cell infiltration was observed on H&E stained slides under a Zeiss microscope (Zeiss Axioscope II). One random section from each mouse brain was analyzed.
2.10 Brain immunofluorescent staining
For IBA-1 and albumin staining, paraffin sections were incubated with primary antibody rabbit anti-mouse IBA-1 (DAKO, Chesterfield, VA) or rabbit anti-mouse albumin (Bethyl Laboratories, Montgomery, TX), respectively, followed by the secondary antibody donkey anti-rabbit IgG Alexa Fluor 594 (Jackson ImmunoResearch, West Grove, PA). TUNEL staining was performed using the in situ cell death detection kit-flurorescein (Roche, Indianapolis, IN) according to the kit instructions. Fluorescent slides were analyzed under a Zeiss microscope (Zeiss AxioObserver CLEM). For TUNEL staining, the number of TUNEL positive cells was counted in each section. Intensity scoring of IBA-1 was performed using ImageJ software.
2.11 Cytokine protein concentrations
Kidneys and brains were snap frozen at the time of sacrifice, and the T-PER extraction reagent (ThermoFisher Scientific) was used to isolate tissue proteins according to the manufacturer’s instructions. Total protein concentration was measured using the Pierce Coomassie (Bradford) Protein Assay (ThermoFisher Scientific). Concentrations were then adjusted, and equal amounts of total protein were used in the Biolegend LEGENDplex Mouse Inflammation Panel (Biolegend, San Diego, CA).
2.12 Statistics
All data sets were analyzed using Graph Pad Prism. Specifically, the data were checked for normal distributions. Normally distributed data were compared using a parametric t-test, while non-normally distributed data was analyzed using a Mann-Whitney test. A value of p<0.05 was considered significant.
3. RESULTS
3.1 MRL/lpr mice treated with GW2580 have attenuated kidney disease
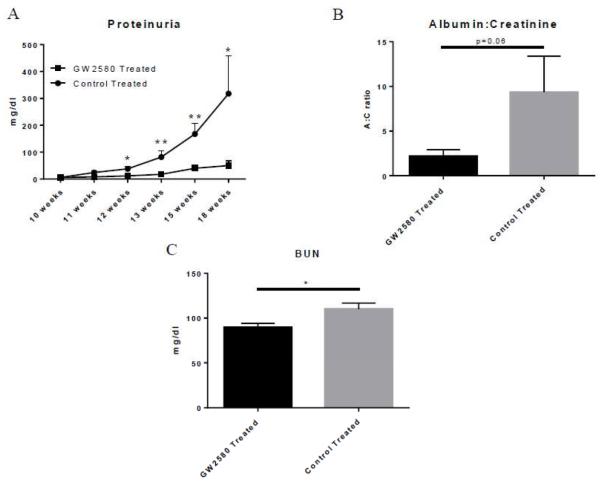
MRL/lpr mice began receiving daily treatments of GW2580 at 10 weeks of age, and were subsequently monitored for proteinuria at various time points until sacrifice at 18 weeks of age. As seen in Figure 1A, GW2580 treated mice were protected from developing the increasing levels of proteinuria seen in control treated mice. The albumin:creatinine (A:C) ratio calculated from urine obtained at sacrifice showed a similar trend (p=0.06) (Figure 1B).
Fig. 1.
Renal function in GW2580 treated mice. Proteinuria levels were measured over time via uristix (A). Urine albumin levels were measured via ELISA and normalized to urine creatinine levels to adjust for varying volumes of urinary output (B). BUN was measured via ELISA to assess kidney function (C). (GW2580 treated, n=9; Control treated, n=10) (*p<0.05, **p<0.01).
We also assessed kidney function by measuring blood urea nitrogen levels (BUN) in terminal serum, with GW2580 mice having significantly lower BUN levels than control treated mice (p=0.02) (Figure 1C). Overall, the decreased proteinuria and lower BUN indicate that macrophage depletion protected MRL/lpr lupus mice from the development of kidney damage and loss of kidney function.
3.2 GW2580 treatment improves renal histopathology
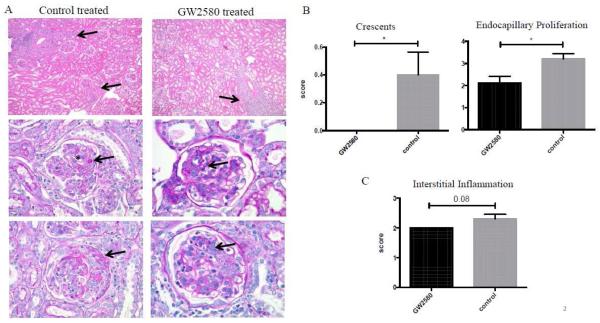
GW2580 treated MRL/lpr mice exhibited improved renal histopathology compared to control treated mice (Figure 2A). Specifically, GW2580 treated mice had decreased glomerular crescent formation and endocapillary proliferation (Figure 2B). Furthermore, treated mice trended towards less interstitial inflammation as well (Figure 2C). Overall, the renal histopathology findings were consistent with the protective effect demonstrated by analysis of proteinuria and BUN levels, indicating a significant benefit of macrophage depletion with GW2580 in MRL/lpr mice.
Fig. 2.
Renal histopathology. The left side of panel A shows representative images of control treated mice with interstitial inflammation (arrows, top left), deposits (arrow) with endocapillary proliferation (*) (middle left), and necrotizing crescent (arrow, bottom left). GW2580 treated mice displayed improved histopathology, although still displayed patchy interstitial inflammation (arrow, top right), mesangial deposits (arrow, middle right), and less extensive endocapillary proliferation (arrow, bottom right). Scores for crescents, endocapillary proliferation, and interstitial inflammation are shown in B and C. (GW2580 treated, n=9; Control treated, n=10) (*p<0.05).
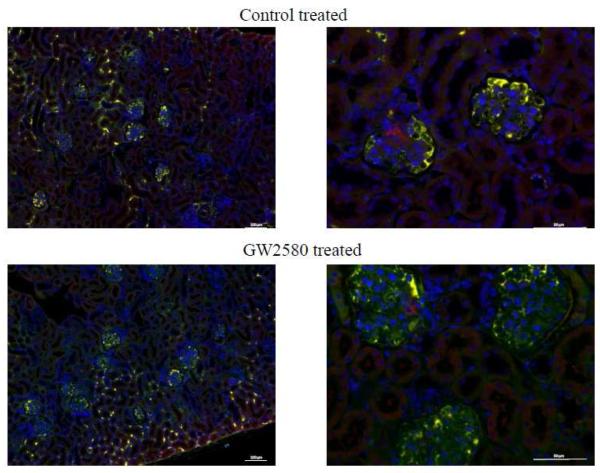
We further co-stained for IgG and complement (C3) deposition within the kidneys. Staining revealed no major differences in deposition of immunoglobulin or C3 between the control and the GW2580 treated mice (Figure 3).
Fig. 3.
Renal IgG and C3 deposition. Kidney sections were stained for IgG deposition (green) and C3 deposition (red). Co-staining of both IgG and C3 appears in yellow. Both control treated and GW2580 treated mice showed similar levels of deposition of both IgG and C3, as seen in these representative images (GW2580 treated, n=6; Control treated, n=6).
3.3 GW2580 depleted macrophages in the kidney
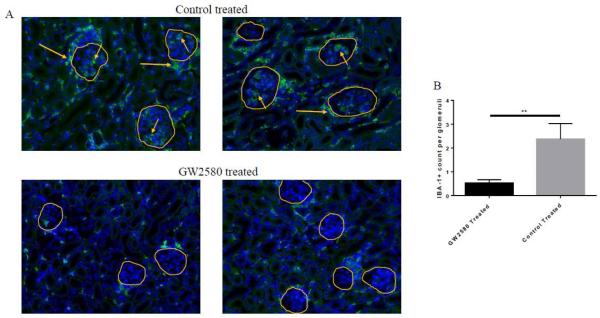
To confirm that the protective effect of GW2580 on nephritis was mediated via a local effect on macrophages, paraffin embedded sections of kidney were stained with IBA-1, a macrophage marker [28]. GW2580 treatment decreased macrophages within the kidney (Figure 4A). Specifically, control treated mice displayed a large influx of macrophages into the interstitium, as well as periglomerular accumulation and intraglomerular infiltration. GW2580 treated mice were not completely depleted of macrophages within the kidney; however, they did have considerably less IBA-1+ cells infiltrating the glomeruli when compared to control treated mice (Figure 4A,B).
Fig. 4.
IBA-1+ macrophages in the kidney. Kidney sections were stained with the macrophage marker IBA-1 to assess macrophage numbers within the kidney. (A) Control treated mice have more numerous IBA-1+ cells than GW2580 treated mice. Glomeruli are circled in yellow, and are infiltrated by macrophages in the control treated mice (short arrows). Additionally, periglomerular accumulation of macrophages can also be seen in control treated mice (long arrows) (GW2580 treated, n=9; Control treated, n=10) (**p<0.01). Intraglomerular macrophages were quantified in (B) (GW2580 treated, n=7, 73 total glomeruli counted; Control treated, n=9, 91 total glomeruli counted).
3.4 GW2580 treatment does not alter circulating autoantibody levels
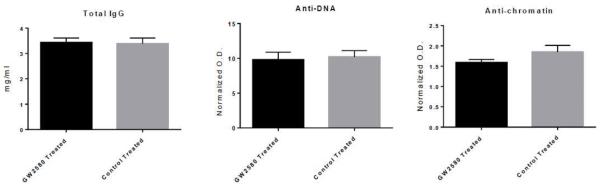
To assess the systemic effect of GW2580 on circulating antibody and autoantibody levels, total IgG, anti-dsDNA IgG, and anti-chromatin IgG levels were measured in GW2580 and control treated MRL/lpr mice at 18 weeks of age. As seen in Figure 5, GW2580 and control treated mice had comparable levels of all the aforementioned antibodies.
Fig. 5.
Serum antibody and autoantibody levels. Total IgG levels were measured in the serum from 18 week old mice (A). Additionally, anti-DNA antibodies (B) and anti-chromatin antibodies (C) were measured in serum from the same time point. Shown is the O.D. normalized to healthy mouse serum. (GW2580 treated, n=9; Control treated, n = 10)
3.5 GW2580 treatment attenuates depression-like behavior in MRL/lpr mice
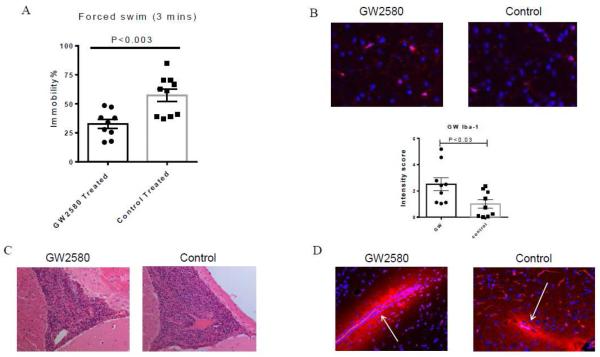
One early and profound neuropsychiatric deficit in the MRL/lpr strain is depression-like behavior [29]. In order to assess whether GW2580 treatment could modulate this neurobehavioral deficit, the Porsolt swim test, a robust assay to evaluate for the presence of a depressive phenotype in rodents by quantifying behavioral despair, was performed. Interestingly, GW2580 treated mice displayed significantly less time floating in the water (i.e. immobility, an indication of despair-like behavior) compared to the control treated group (Figure 6A). Thus, GW2580 treatment ameliorated depression-like behavior.
Fig. 6.
GW2580 treated mice display ameliorated depression-like behavior and increased microglial activation. (A) Depression-like behavior was assessed in the Porsolt swim test for 3 minutes. (B) Representative images of IBA-1 staining in the cortex from the PBS and GW2580 treated groups is shown, along with the intensity of IBA-1 staining as measured by ImageJ. (C) Representative images of H&E staining in the third ventricles of PBS and GW2580 treated mice. (D) Representative images of albumin staining are shown, with arrows indicating the blood vessels. The number of animals in the forced swim test in the PBS and GW2580 treated groups is 10 and 10, respectively. For IBA-1, H&E, and albumin staining, the number is 10 and 9, respectively.
3.6 Locomotor activity is unaltered in the GW treated mice
To exclude the possibility that the differences seen in the Porsolt swim test were a result of the control mice moving less due to sickness behavior or the presence of musculoskeletal disease, the open field test was carried out to evaluate locomotive activity. Total track length (control vs GW2580 treated: 2339.1 ± 110.1 cm vs 2197.7 ± 118.7 cm), center track length (control vs GW2580 treated: 458.0 ± 36.2 cm vs 381.6 ± 43.5 cm), center entries (control vs GW2580 treated: 29.6 ± 2.0 vs 26.1 ± 3.4), and center time (control vs GW2580 treated: 55.4 ± 6.2 s vs 40.5 ± 5.0 s) were calculated in this test, respectively. However, none of these parameters exhibited significant differences between GW2580 treated and the control groups. This latter experiment indicates that GW2580 had no effect on general locomotive activity or anxiety-like behavior, and that the behavioral deficit observed in the swim test was specific.
3.7 GW2580 treated mice exhibited spatial memory deficits similar to the control group
Besides depression-like behavior, cognitive dysfunction (abnormal spatial memory) is another characteristic neuropsychiatric manifestation in the MRL/lpr strain [24]. To evaluate the therapeutic effect of GW2580 administration on cognitive function, an object placement (spatial memory) test was conducted. Generally, animals possess an innate preference to explore objects in novel locations, and a preference score of more than 53% indicates preserved memory [26]. However, mice treated with GW2580 exhibited a preference score of 44.1% ± 6.4 %, suggesting that they are incapable of recalling the original placement of the object, similar to the control treated animals (52.3% ± 6.6%) (p=NS). Thus, GW2580 treated mice demonstrated spatial memory deficits which were no different from the control group.
3.8 GW2580 treated mice demonstrate an increase in microglia activation compared to PBS treated mice
To investigate whether GW2580 treatment had an effect on the number of brain microglia, we sacrificed the mice after the behavioral testing was completed and performed IBA-1 staining. Increased intensity of brain IBA-1 staining was observed in GW2580 treated mice as compared with the control group (Figure 6B).
3.9 Immune cell infiltration, blood brain barrier integrity, and brain cell apoptosis are unaltered after GW2580 administration
Major histopathological brain findings in the MRL/lpr strain are immune cell infiltration, compromised blood brain barrier integrity, and brain cell apoptosis [2, 20]. To investigate whether GW2580 treatment affects these histopathological characteristics, cellular infiltrates were observed on H&E stained slides, and albumin was used as a tracer to detect BBB leakage. In addition, TUNEL staining was performed to evaluate brain cell apoptosis. However, after GW2580 treatment, cellular infiltrates persisted in the third (Figure 6C) and fourth ventricles (data not shown). Albumin accumulated around the blood vessels in the periventricular areas, cortex, and cerebellum similar to the control treated group (Figure 6D), indicative of persistent BBB leakage. Furthermore, there were no significant differences in the number of TUNEL positive cells between the groups (data not shown). Therefore, several histological features present in the brains of MRL/lpr lupus mice were not significantly affected by GW2580 administration.
3.10 GW2580 treatment modulates cytokine expression in the kidneys and brains of treated mice
To assess the effect of GW2580 treatment on cytokine levels in both the brain and kidney, total protein was extracted from these tissues, and the isolated protein probed using the LEGENDplex Mouse Inflammation panel. As can be seen in Figure 7A, treatment with GW2580 significantly modulated the expression of several proinflammatory cytokines, including IL-1β, IL-27, and GM-CSF. There were also near significant changes in TNF and MCP-1. Within the brain, significant reductions in IL-12p70, IL-10, and IL-27 levels were present in GW2580 as compared to control treated mice, while changes in TNF and IL-1β were nearly significant (Figure 7B).
Fig. 7.
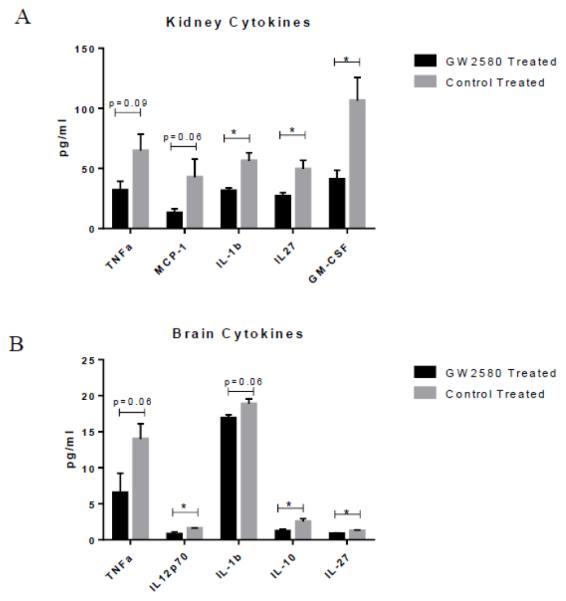
GW2580 treatment modulates cytokine expression. GW2580 treated mice have significantly decreased concentrations of several inflammatory cytokines as compared to control treated mice in both kidneys (A) and brain (B) (GW2580 treated, n=4; Control treated, n = 4) (*p<0.05).
4. DISCUSSION
SLE can negatively affect multiple different organ systems, including the kidneys and the brain which are the major organs affected by this disease. Current treatment options are insufficient, and generally depend upon broadly non-specific immunosuppression. Such treatment approaches have no guarantee of success, and are associated with many serious side effects. Patients would greatly benefit from more specific therapies, and limiting cellular targeting to macrophages may be a potentially promising therapeutic approach.
GW2580 is a kinase inhibitor specific for CSF-1R [30], a receptor important for macrophage and microglia function and survival. We have confirmed the ability of GW2580 to deplete both macrophages [17] and microglia (unpublished data). Specifically, we described the effect of GW2580 on kidney and splenic macrophages, as well as on circulating monocytes [17]. Furthermore, additional studies confirmed the ability of GW2580 to systemically deplete macrophages, including in organs such as the liver [30, 31]. We were able to use this drug to target macrophages and microglia before the onset of disease in the MRL/lpr strain, a mouse model validated for the study of SLE, including LN and NPSLE. Prophylactic treatment with GW2580 ameliorated kidney disease, as assessed by decreased proteinuria, improved BUN levels, and improved renal histopathology. Moreover, GW2580 treatment improved depression-like behavior.
However, despite this improvement in end organ pathologies, we did not note a difference in circulating autoantibody levels. Additionally, there was no difference in IgG deposition within the glomeruli. These findings, in the context of improved renal disease, highlight the importance of macrophage effector functions in LN pathogenesis.
Macrophages have been previously studied in the context of LN [5, 32]. Activated macrophages not seen in healthy kidneys are found in active LN, both in murine models and human disease [12, 14, 15]. Additionally, CSF-1 is a validated biomarker for LN disease activity, in both the serum and urine [9]. Past studies have looked at targeting the CSF-1/CSF-1R signaling pathway in the MRL/lpr model. Congenital deletion (knockout) of either of these interacting molecules protected MRL/lpr mice from the development of severe nephritis; however, because macrophages play an important role in development, knockout mice suffered developmental defects including compromised bone marrow compartments, confounding interpretation of the study [11]. We previously showed the importance of macrophages in an inducible model of LN [17]. Depleting macrophages prophylactically with GW2580 ameliorated kidney damage and improved renal function. Furthermore, we found that GW2580 given after the onset of disease in the NTN model could still provide protective benefits – highlighting the therapeutic potential of targeting macrophages.
Our present study examined the effects of depleting macrophages in a spontaneous model of LN, while also circumventing any developmental defects by avoiding genetic manipulation. Although the mice were sacrificed before the control mice reached extremely high levels of proteinuria, we believe the results still indicate the promising potential of this drug in the context of LN. Although serum GW2580 levels were not measured, we have effectively used this dose of GW2580 in previous experiments, and we confirmed the efficacy of treatment by assessing macrophage depletion via IBA-1 (a marker for macrophages [28]) staining and flow cytometry analysis.
Mechanistically, GW2580 treatment modulated cytokine expression within the kidney. We noted a decrease in the levels of TNF, MCP-1, IL-1β, IL-27, and GM-CSF in GW2580 treated mice. Decreased levels of these proinflammatory cytokines likely contributed to the attenuated proteinuria and improved renal functioning. In our previous study in an inducible model of LN, we similarly noted that GW2580 treatment decreased kidney cytokine expression [17], underscoring the importance of macrophage associated cytokines in LN pathogenesis.
In addition to the effect on cytokines, it is worth pointing out that downstream effects of GW2580 could also have affected podocyte function. This would help explain the significant improvement in proteinuria levels despite the fact that treated mice did not have complete reversal of the glomerular pathology. This is a potential area to explore in future studies.
Lupus patients exhibit neuropsychiatric manifestations that can occur at any stage of disease, even before the onset of other organ damage [24, 25]. Thus, NPSLE is believed to be a primary manifestation of SLE [25]. Cytokines are believed to be pivotal mediators in NPSLE [33], while microglia are an important source of cytokines in the brain. The use of GW2580 to deplete microglia allowed us to assess neurobehavioral manifestations after the administration of the monospecific kinase inhibitor. Excitingly, we found GW2580 treated mice exhibited significantly less depression-like behavior.
Macrophages have been implicated as potential effector cells in the pathogenesis of various forms of depression, and it is thought macrophages may contribute to depression via expression of proinflammatory cytokines [34, 35]. Cytokine dysregulation has been proven to be directly associated with depression by numerous studies [33, 36]. Intracerebroventricular injection of microglia-derived cytokines such as TWEAK and IL-6 directly result in depression-like behavior in vivo [26, 37]. In addition, systemic inflammatory mediators may also induce negative behavior outcomes by accessing the brain via the BBB, or directly acting on the vagus nerve [38]. We found that GW2580 treatment decreased the levels of several proinflammatory cytokines in the brain, including TNF, IL-12p70, IL-1β, IL-10, and IL-27. Therefore, considering the known contribution of cytokines to depression, our data in this paper highlight a potential mechanism underlying the attenuated depression-like phenotype in GW2580 treated mice.
However, we did not find that GW2580 affects visual memory, suggesting perhaps that different mechanisms may account for individual behavioral phenotypes in the MRL/lpr strain. Indeed, MRL/lpr mice display depression-like behavior as early as 5 weeks of age, while spatial memory deficits do not occur until 16 weeks of age [24]. In addition, different sub-regions of the brain are involved in depression and cognitive function. Furthermore, depleting microglia in healthy C57/Bl6 mice does not affect normal cognition suggesting that this function may not be not dependent on microglia [38], as would be consistent with our results.
Although we expected that mice receiving the GW2580 compound would exhibit decreased microglia number and/or activation in the brain, we actually observed increased IBA-1 staining intensity in the GW2580 treated group. This perhaps can be explained by the fact that the brains were extracted 2 weeks after the treatment ended, and by that time point the microglial population may have already recovered. Indeed, Elmore et al. also found that microglia can rapidly repopulate one week after the cessation of treatment with PLX3397 [39], a drug with a similar mechanism of action in its inhibition of CSF-1R. Indeed, the cessation of GW2580 two weeks prior to neurobehavioral testing may also be an alternative explanation why treated mice exhibited no improvement in spatial memory deficits. In addition, we did not find any histopathological differences between the GW2580 and control treated group, indicating that these NPSLE-associated phenomena are mediated by other factors besides, or in addition to, microglia. Analyzing the timing of microglia depletion and repopulation and any differential effect on microglial sub-phenotypes (M1 or M2), as well as cytokine expression during or right after cessation of the GW2580 treatment, will be a priority in future studies.
In conclusion, this study highlights macrophages as a promising therapeutic target for multiple end organ manifestations of SLE. Targeting this cell type both in the kidney and in the brain leads to amelioration of LN and NPSLE related depression. With similar drugs, such as PLX3397, already being used to treat humans in cancer clinical trials, this class of kinase inhibitors monospecific for the CSF-1R may hold realistic promise for future therapeutic applications in treating SLE.
Highlights.
Macrophages have been implicated in the pathogenesis of kidney and brain disease in SLE;
GW2580 is a specific inhibitor of CSF-1R;
Treatment with GW2580 significantly ameliorated kidney disease in MRL/lpr mice;
GW2580 treated mice MRL/lpr mice displayed less depression-like behavior;
Circulating antibody and autoantibody levels were not affected by GW2580 treatment.
ACKNOWLEDGEMENTS
These studies were supported by a research grant from the NIH (R01 AR065594) to C. Putterman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- [1].Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nature Reviews: Nephrology. 2015;11:329–341. doi: 10.1038/nrneph.2015.33. [DOI] [PubMed] [Google Scholar]
- [2].Stock AD, Wen J, Putterman C. Neuropsychiatric Lupus, the Blood Brain Barrier, and the TWEAK/Fn14 Pathway. Front Immunol. 2013;4:484. doi: 10.3389/fimmu.2013.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat. Rev. Rheumatol. 2014;10:338–347. doi: 10.1038/nrrheum.2014.15. [DOI] [PubMed] [Google Scholar]
- [4].Murray Peter J., Allen Judith E., Biswas Subhra K., Fisher Edward A., Gilroy Derek W., Goerdt S, Gordon S, Hamilton John A., Ivashkiv Lionel B., Lawrence T, Locati M, Mantovani A, Martinez Fernando O., Mege J-L, Mosser David M., Natoli G, Saeij Jeroen P., Schultze Joachim L., Shirey Kari A., Sica A, Suttles J, Udalova I, van Ginderachter Jo A., Vogel Stefanie N., Wynn Thomas A. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Orme J, Mohan C. Macrophage Subpopulations in Systemic Lupus Erythematosus. Discov. Med. 2012;69:151–158. [PubMed] [Google Scholar]
- [6].Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- [8].Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR. Circulating CSF-1 Promotes Monocyte and Macrophage Phenotypes that Enhance Lupus Nephritis. J. Am. Soc. Nephrol. 2009;20:2581–2592. doi: 10.1681/ASN.2009050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Menke J, Amann K, Cavagna L, Blettner M, Weinmann A, Schwarting A, Kelley VR. Colony-Stimulating Factor-1: A Potential Biomarker for Lupus Nephritis. J. Am. Soc. Nephrol. 2015;26:379–389. doi: 10.1681/ASN.2013121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lenda DM, Stanley ER, Kelley VR. Negative Role of Colony-Stimulating Factor-1 in Macrophage, T Cell, and B Cell Mediated Autoimmune Disease in MRL-Faslpr Mice. J. Immunol. 2004;173:4744–4754. doi: 10.4049/jimmunol.173.7.4744. [DOI] [PubMed] [Google Scholar]
- [11].Lenda DM, Kikawada E, Stanley ER, Kelley VR. Reduced Macrophage Recruitment, Proliferation, and Activation in Colony-Stimulating Factor-1-Deficient Mice Results in Decreased Tubular Apoptosis During Renal Inflammation. J. Immunol. 2003;170:3254–3262. doi: 10.4049/jimmunol.170.6.3254. [DOI] [PubMed] [Google Scholar]
- [12].Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, Madaio MM, Bottinger EP, Davidson A. Activated Renal Macrophages Are Markers of Disease Onset and Disease Remission in Lupus Nephritis. J. Immunol. 2008;180:1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, Bottinger EP, Ivashkiv L, Kretzler M, Davidson A. A Unique Hybrid Renal Mononuclear Phagocyte Activation Phenotype in Murine Systemic Lupus Erythematosus Nephritis. J. Immunol. 2011;186:4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hill GS, Delahousse M, Nochy D, Remy P, Mignon F, Mery J-P, Bariety J. Predictive power of the second renal biopsy in lupus nephritis: Significance of macrophages. Kidney Int. 2001;59:304–316. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- [15].Ikezumi Y, Suzuki T, Hayafuji S, Okubo S, Nikolic-Paterson DJ, Kawachi H, Shimizu F, Uchiyama M. The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrology Dialysis Transplantation. 2005;20:2704–2713. doi: 10.1093/ndt/gfi105. [DOI] [PubMed] [Google Scholar]
- [16].Bethunaickan R, Sahu R, Davidson A. Analysis of Renal Mononuclear Phagocytes in Murine Models of SLE. In: Perl A, editor. Autoimmunity. Humana Press; 2012. pp. 207–232. [DOI] [PubMed] [Google Scholar]
- [17].Chalmers SA, Chitu V, Herlitz LC, Sahu R, Stanley ER, Putterman C. Macrophage depletion ameliorates nephritis induced by pathogenic antibodies. J. Autoimmun. 2014;57:42–52. doi: 10.1016/j.jaut.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chalmers S, Doerner J, Wen J, Putterman C. Macrophage depletion attenuates skin and kidney disease in lupus mice (BA7P.143) J. Immunol. 2015;194:115–113. [Google Scholar]
- [19].Meszaros ZS, Perl A, Faraone SV. Psychiatric symptoms in systemic lupus erythematosus: a systematic review. J Clin Psychiatry. 2012;73:993–1001. doi: 10.4088/JCP.11r07425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wen J, Doerner J, Weidenheim K, Xia Y, Stock A, Michaelson JS, Baruch K, Deczkowska A, Gulinello M, Schwartz M, Burkly LC, Putterman C. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J Autoimmun. 2015;60:40–50. doi: 10.1016/j.jaut.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xia Y, Herlitz LC, Gindea S, Wen J, Pawar RD, Misharin A, Perlman H, Wu L, Wu P, Michaelson JS, Burkly LC, Putterman C. Deficiency of Fibroblast Growth Factor-Inducible 14 (Fn14) Preserves the Filtration Barrier and Ameliorates Lupus Nephritis. Journal of the American Society of Nephrology : JASN. 2015;26:1053–1070. doi: 10.1681/ASN.2014030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gulinello M, Putterman C. The MRL/lpr Mouse Strain as a Model for Neuropsychiatric Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2011;2011:207504. doi: 10.1155/2011/207504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xia Y, Pawar RD, Nakouzi AS, Herlitz L, Broder A, Liu K, Goilav B, Fan M, Wang L, Li Q-Z, Casadevall A, Putterman C. The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti-DNA antibodies. J. Autoimmun. 2012;39:398–411. doi: 10.1016/j.jaut.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wen J, Xia Y, Stock A, Michaelson JS, Burkly LC, Gulinello M, Putterman C. Neuropsychiatric Disease in Murine Lupus is Dependent on the TWEAK/Fn14 Pathway. J. Autoimmun. 2013;43:44–54. doi: 10.1016/j.jaut.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stock AD, Wen J, Doerner J, Herlitz LC, Gulinello M, Putterman C. Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice. J. Neuroinflammation. 2015;12:205. doi: 10.1186/s12974-015-0423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wen J, Chen CH, Stock A, Doerner J, Gulinello M, Putterman C. Intracerebroventricular administration of TNF-like weak inducer of apoptosis induces depression-like behavior and cognitive dysfunction in non-autoimmune mice. Brain. Behav. Immun. 2016;54:27–37. doi: 10.1016/j.bbi.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wen J, Doerner J, Chalmers S, Stock A, Wang H, Gullinello M, Shlomchik MJ, Putterman C. B cell and/or autoantibody deficiency do not prevent neuropsychiatric disease in murine systemic lupus erythematosus. J. Neuroinflammation. 2016;13:73. doi: 10.1186/s12974-016-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004;88:844–856. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- [29].Gao HX, Campbell SR, Cui MH, Zong P, Hee-Hwang J, Gulinello M, Putterman C. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J Neuroimmunol. 2009;207:45–56. doi: 10.1016/j.jneuroim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, Jansen M, Lin P, Payne A, Crosby RM, Johnson JH, Frick L, Lin M-HJ, Depee S, Tadepalli S, Votta B, James I, Fuller K, Chambers TJ, Kull FC, Chamberlain SD, Hutchins JT. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16078–16083. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-Resident Macrophage Necroptosis Orchestrates Type 1 Microbicidal Inflammation and Type-2-Mediated Tissue Repair during Bacterial Infection. Immunity. 42:145–158. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- [32].Chalmers SA, Chitu V, Ramanujam M, Putterman C. Therapeutic targeting of macrophages in lupus nephritis. Discov. Med. 2015;20:43–49. [PubMed] [Google Scholar]
- [33].Okamoto H, Kobayashi A, Yamanaka H. Cytokines and Chemokines in Neuropsychiatric Syndromes of Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2010;2010:8. doi: 10.1155/2010/268436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith R. The macrophage theory of depression. Med Hyptheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- [35].Roman A, Kreiner G, Nalepa I. Macrophages and depression - a misalliance or well-arranged marriage? Pharmacol. Rep. 2013;65:1663–1672. doi: 10.1016/s1734-1140(13)71528-7. [DOI] [PubMed] [Google Scholar]
- [36].Fragoso-Loyo H, Atisha-Fregoso Y, Llorente L, Sánchez-Guerrero J. Inflammatory profile in cerebrospinal fluid of patients with headache as a manifestation of neuropsychiatric systemic lupus erythematosus. Rheumatology. 2013;52:2218–2222. doi: 10.1093/rheumatology/ket294. [DOI] [PubMed] [Google Scholar]
- [37].Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, Brandon NJ. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Translational Psychiatry. 2012;2:e199. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bluthe R-M, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. Neuroreport. 1996;7:2823. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- [39].Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]