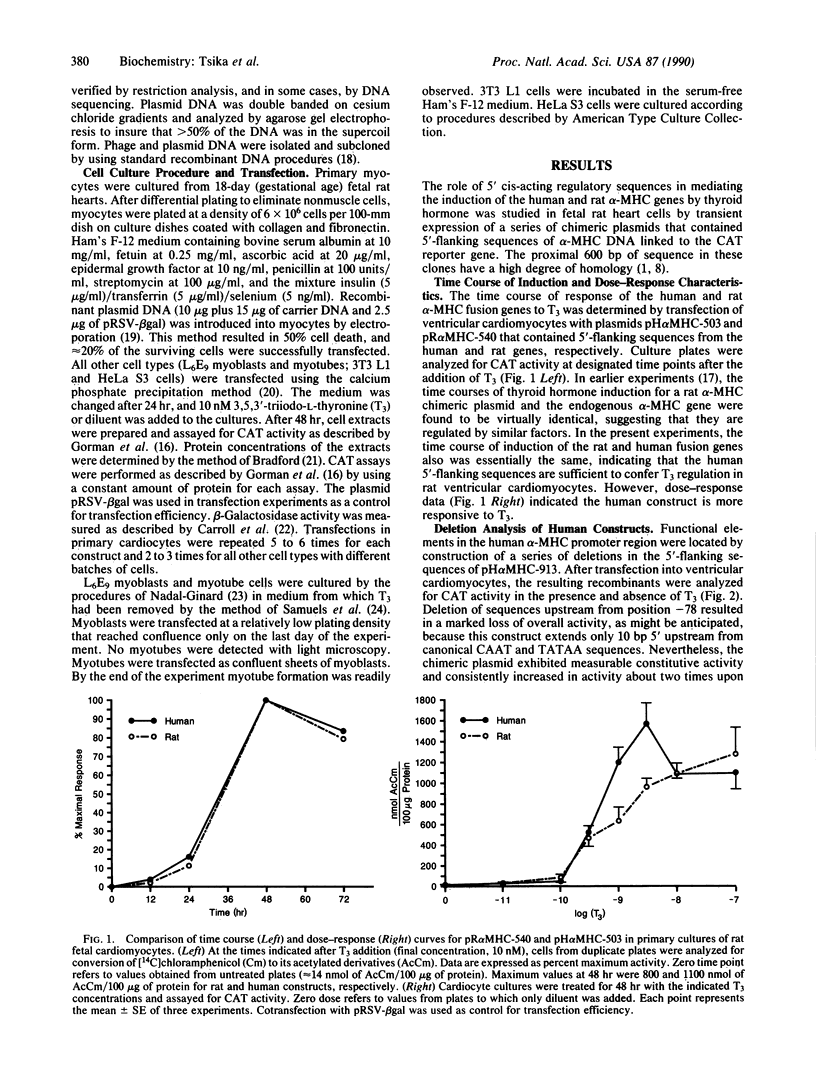
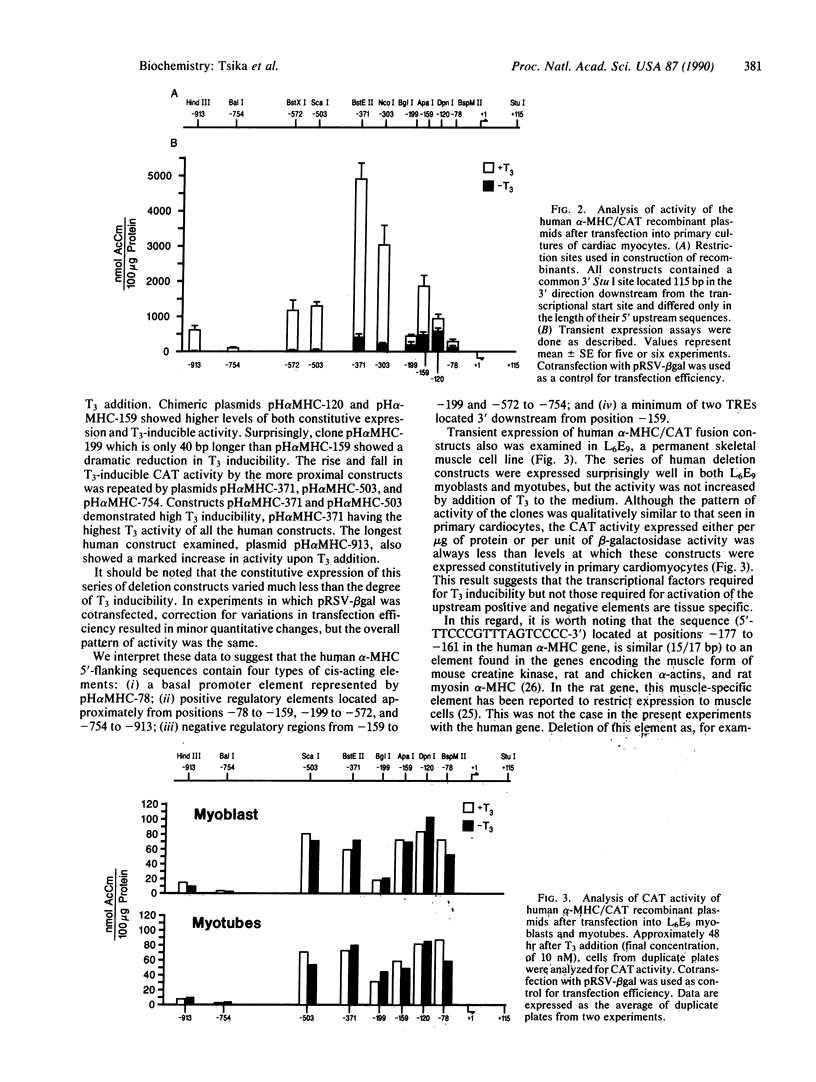
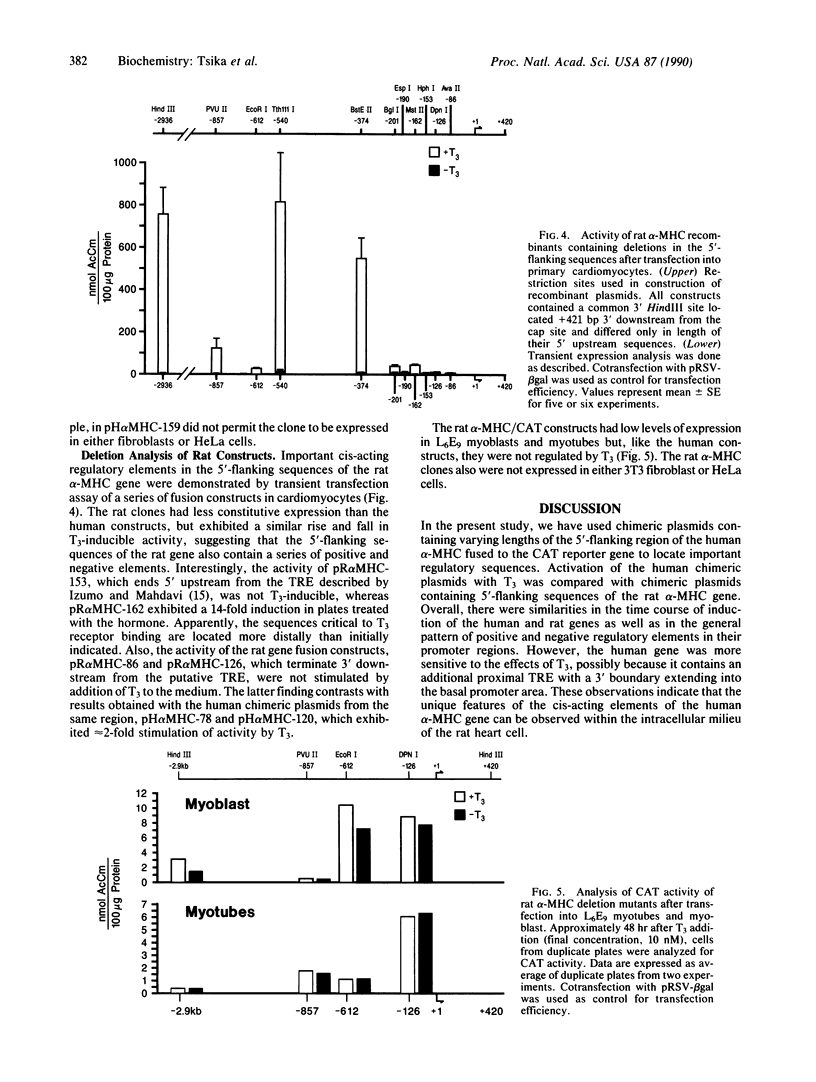
Abstract
The rat alpha-myosin heavy-chain (alpha-MHC) gene is regulated by 3,5,3'-triiodo-L-thyronine (T3) in ventricular myocardium and is constitutively expressed in atrial tissue. Less is known about regulation of the human gene, but conservation of sequences in the 5'-flanking region between the rat and human alpha-MHC genes suggests that the human gene may be regulated similarly. Accordingly, T3-responsiveness and tissue-specific expression of human and rat alpha-MHC/chloramphenicol acetyltransferase fusion constructs have been compared in rat fetal heart cells, L6E9 myoblasts and myotubes, 3T3 fibroblasts, and HeLa cells. Transient transfection assays revealed a complex series of cis-regulatory elements in the 5'-flanking sequences in the human genes, including a basal promoter element with canonical TATAA and CAAT sequences, two positive regulatory element(s), and two negative regulatory elements, which markedly diminished both constitutive and T3-inducible activity. Interestingly, the human gene seemed to contain a proximal thyroid-hormone response element(s) not found in the rat gene. In L6E9 myoblasts and myotubes, the human constructs were constitutively expressed but not T3-regulated; none of the constructs were active in 3T3 or HeLa cells. We propose that interactions among the thyroid hormone responsive elements and other cis-acting elements in the human alpha-MHC 5'-flanking sequences may be sufficient to explain the characteristic features of expression of this gene in cardiac tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvagnet P., Leger J., Pons F., Dechesne C., Leger J. J. Fiber types and myosin types in human atrial and ventricular myocardium. An anatomical description. Circ Res. 1984 Dec;55(6):794–804. doi: 10.1161/01.res.55.6.794. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll S. L., Bergsma D. J., Schwartz R. J. A 29-nucleotide DNA segment containing an evolutionarily conserved motif is required in cis for cell-type-restricted repression of the chicken alpha-smooth muscle actin gene core promoter. Mol Cell Biol. 1988 Jan;8(1):241–250. doi: 10.1128/mcb.8.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Franco R., Weinberger C., Albert V. R., Evans R. M., Rosenfeld M. G. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987 Oct 22;329(6141):738–741. doi: 10.1038/329738a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Bahl J. J., Morkin E. Thyroid hormone regulates expression of a transfected alpha-myosin heavy-chain fusion gene in fetal heart cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3122–3126. doi: 10.1073/pnas.84.10.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Markham B. E., Morkin E. Effects of thyroid hormone on alpha-actin and myosin heavy chain gene expression in cardiac and skeletal muscles of the rat: measurement of mRNA content using synthetic oligonucleotide probes. Circ Res. 1986 Aug;59(2):194–201. doi: 10.1161/01.res.59.2.194. [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987 Mar;79(3):970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Jandreski M. A., Liew C. C. Construction of a human ventricular cDNA library and characterization of a beta myosin heavy chain cDNA clone. Hum Genet. 1987 May;76(1):47–53. doi: 10.1007/BF00283049. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., Chamberlain J. S., Buskin J. N., Johnson J. E., Hauschka S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol Cell Biol. 1986 Aug;6(8):2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurabayashi M., Tsuchimochi H., Komuro I., Takaku F., Yazaki Y. Molecular cloning and characterization of human cardiac alpha- and beta-form myosin heavy chain complementary DNA clones. Regulation of expression during development and pressure overload in human atrium. J Clin Invest. 1988 Aug;82(2):524–531. doi: 10.1172/JCI113627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Norman M. F., Lavin T. N., Baxter J. D., West B. L. The rat growth hormone gene contains multiple thyroid response elements. J Biol Chem. 1989 Jul 15;264(20):12063–12073. [PubMed] [Google Scholar]
- Saez L. J., Gianola K. M., McNally E. M., Feghali R., Eddy R., Shows T. B., Leinwand L. A. Human cardiac myosin heavy chain genes and their linkage in the genome. Nucleic Acids Res. 1987 Jul 10;15(13):5443–5459. doi: 10.1093/nar/15.13.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi-Takihara K., Sole M. J., Liew J., Ing D., Liew C. C. Characterization of human cardiac myosin heavy chain genes. Proc Natl Acad Sci U S A. 1989 May;86(10):3504–3508. doi: 10.1073/pnas.86.10.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]



