Abstract
In the last decade, in vivo oxygen-17 (17O) MRS has evolved into a promising MR technique for noninvasively studying oxygen metabolism and perfusion in aerobic organs with the capability of imaging the regional metabolic rate of oxygen and its changes. In this chapter, we will briefly review the methodology of the in vivo 17O MRS technique and its recent development and applications; we will also discuss the advantages of the high/ultrahigh magnetic field for 17O MR detection, as well as the challenges and potential of this unique MRS method for biomedical research of oxygen metabolism, mitochondrial function and tissue energetics in health and disease.
Introduction
Compared to well-established in vivo magnetic resonance (MR) spectroscopy (MRS) methodologies such as phosphrous-31 (31P) or carbon-13 (13C) MRS [1], in vivo oxygen-17 (17O) MRS has had a relatively short history and is less commonly employed in biomedical research. As one of the most abundant elements on earth, molecules containing oxygen exist in all forms and levels of life. Thus, efforts to develop and utilize 17O-based MR technology to obtain valuable information of biological systems are ongoing. Perhaps the most intriguing use of in vivo 17O MRS is to study the cellular oxygen metabolism in living organs. The original idea to use 17O MRS for in vivo studies appeared in the late 1980s [2–7], and it was likely inspired by the 15O-based positron emission tomography (PET) technique developed for imaging the cerebral metabolic rate of oxygen (CMRO2), a critically important physiological parameter of the brain [8, 9].
Among all neuroimaging modalities capable of providing CMRO2 information, 15O-PET has been regarded as a gold standard for directly imaging the oxygen metabolic rate in the living brain. However, two major drawbacks associated with 15O-PET imaging have seriously limited its availability and applicability: one is the very short half-life of 15O (2.04 min), which requires expensive equipment such as a cyclotron to produce the 15O-tracers on site; and the other is the inability of the 15O-PET in distinguishing the 15O signals of the oxygen substrate from that of metabolically produced water, so additional measurements and complicated mathematical models are needed to calculate the CMRO2 values [8, 9]. Magnetic resonance technology is a widely used neuroimaging modality in present-day medical research and clinical practice. 17O MRS (or imaging) and 1H MRI-based approaches have been developed for assessing CMRO2. Even though the 17O-MR approach is technically much simpler than 15O-PET since it only detects the metabolically generated water signal (see details below) and does not require the injection of large radiation doses to the subject, its initial utilization was slow, with only a small number of publications in the first decade after inception that mainly focused on feasibility assessment [10–16]. The major challenge in advancing this technology can be attributed to the extremely low intrinsic sensitivity of 17O as compared to that of 1H (~1×105 fold differences) at natural abundance [17]. On the other hand, 1H MRI detects abundant water signals with great sensitivity, although the water signals in general cannot be directly related to oxygen metabolism. To specifically assess cerebral oxygenation, several methods based on 1H MRI have been introduced [18–23]. They include (i) the blood oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) in combination with hypercapnic and hyperoxic respiratory challenges to calibrate the fMRI signals [19, 21]; (ii) susceptometry-based or T2-based methods to determine the cerebral venous oxygen saturation (Yv) [22, 23]; and (iii) a quantitative BOLD approach to extract the venous cerebral blood volume (CBV) and deoxyhemoglobin concentration from the transverse relaxation time (T2 or T2*) [18, 20]. However, these indirect methods have their own technical challenges and limitations; and various assumptions, predetermined parameters and different models must be applied in estimating CMRO2.
High/ultrahigh field MR scanner technology emerged in the 1990s and the extraordinary sensitivity gain of 17O detection at higher field observed in 2001 have stimulated new interests and efforts in the field of in vivo 17O MRS research [24, 25]. After more than ten years of technology development and validation, the in vivo 17O MRS/MRI methodology has now been established as a valuable tool for studying cerebral oxygen metabolism in preclinical animal models and in human brains under various physiopathological conditions [25–39]. Details of the technology development can be found in several earlier review articles [40–43]. In the present review, we will briefly describe the methodology and its utility for non-invasive investigation and quantitative understanding of the roles of oxygen metabolism in health and disease. The main scope of this review will be on the new advancements and recent applications in this research field.
Methodology Overview
Among the three stable isotopes of oxygen (i.e., 16O, 17O and 18O), 16O represents over 99.7% of the composition, but unfortunately does not possess a nuclear spin; only 17O with a spin number of I=5/2 can be detected by MR. In this section, we will introduce different in vivo 17O MR methods according to their signal sources and/or related uses with either a spectroscopic or imaging approach,.
Steady-state detection of the natural abundance 17O signal
The relative amount of 17O in oxygen in nature is very rare (only 0.037% of total oxygen). Because the concentration of major metabolites is in the range of few millimolar to tens millimolar, long 17O MR acquisition times are required to accumulate reasonable signal for meaningful detection or adequate signal-to-noise ratio (SNR) of oxygen-containing metabolites in tissue samples. Figure 1 illustrates natural abundance in vivo 17O MR spectra obtained in live and postmortem rat brains with a 11.7T scanner that required several million scans over 6 and 14 hours, respectively, in which a number of broad resonances from oxygen in phosphate, sulfonic and carbonyl groups covering a wide chemical shift range are detected [44]. It is clear that the SNR of these natural abundance 17O signals is insufficient for practical applications aiming to monitor oxygen-containing metabolites. The only molecule that can be easily detected by in vivo 17O MRS techniques is water, a major component of the tissue. However, with known water content and molar concentration in different tissues, the natural abundance water signal in 17O MR spectra can serve as an internal reference for quantification purposes. This is an important advantage of the in vivo 17O MR methodology and is crucial for its application.
Figure 1.
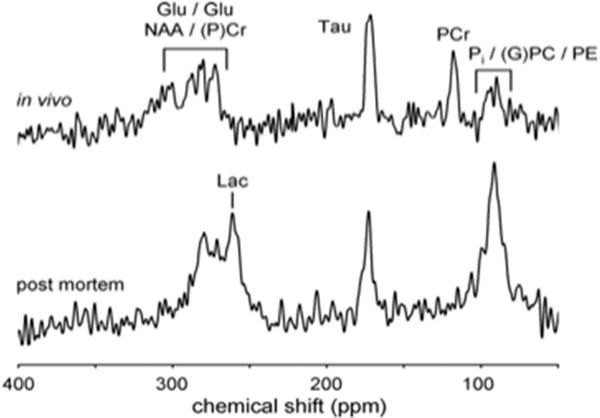
Pulse-acquired natural abundance 17O MR spectra of rat brain: live (top, 1.5 million averages) and post mortem (bottom, 3.5 million averages) brains obtained with 6 and 14 hours data acquisition, respectively (chemical shifts are referenced to the water signal at 0 ppm). Adapted from reference [44]. Copyright © 2008 Elsevier Inc.
Dynamic monitoring of the metabolism of 17O-labled oxygen gas
A unique feature of the in vivo 17O MR technique is its ability to directly and quantitatively monitor the production of metabolic water after the introduction of 17O-labeled oxygen (17O2) gas to an animal or human. It is well known that when oxygen 17O2 molecules enter the blood stream, they are brought to the organ or tissue of interest via effective blood circulation and perfusion. At the cellular level, the oxygen consumption occurs in the mitochondria through cellular respiration that generate 17O-labeled water (H217O); the rate of H217O production reflects the oxygen consumption rate. Since the 17O2 molecules that are either bound to hemoglobin or dissolved in blood are invisible to 17O MR detection, only H217O molecules, the final product of the oxygen metabolism, has an 17O signal; therefore, dynamic in vivo 17O MR measurements can be used to monitor the production of H217O and determine the metabolic rate of oxygen in the targeted tissue or organ. The principle of this technique, the quantification model and corresponding mass balance equation, as well as the simplified method for determining CMRO2 and cerebral blood flow (CBF) in small animal brains via a brief (few minutes) inhalation of 17O2 gas, are schematically illustrated in Figure 2; the detailed methodology and its validity, which has been experimentally verified in rat brains, can be found elsewhere [26, 29, 31, 34, 38–42].
Figure 2.
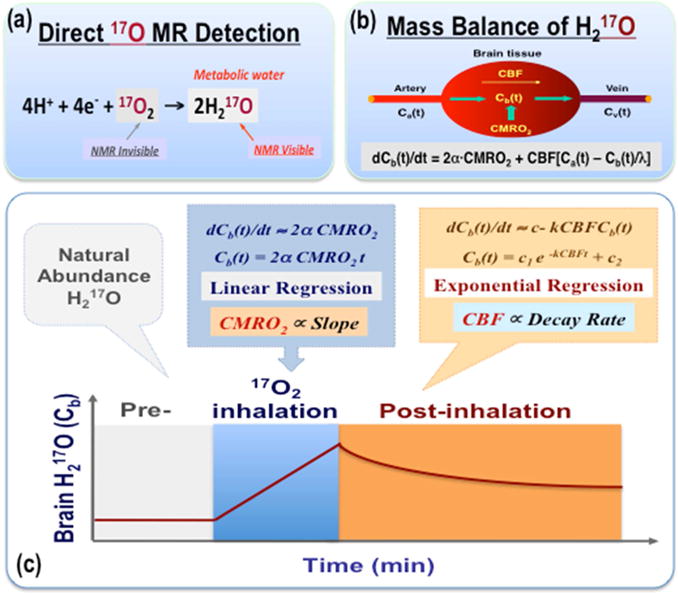
Schematic illustration of in vivo 17O MR technique for quantifying CMRO2 and CBF in rodents via a brief inhalation of 17O-labeled oxygen gas: the principle (a), quantification model and mass balance equation (b), as well as the simplified method for calculating CMRO2 and CBF based on the brain H217O time course (c) are displayed. Detailed description and explanation of the method can be found in references [38–39 & 40–43].
Blood flow measurement with exogenous tracer of H217O
Introducing 17O-labeled water as an exogenous tracer and using dynamic in vivo 17O MR technique to monitor the evolution of the H217O signal in targeted tissue or organ enables the determination of the washout rate of the H217O tracer, which represents the blood flow or perfusion rate [26, 31]. This approach is the same as conventional tracer techniques used in biomedical research or in the clinic. The ideal method of introducing H217O tracer is via a bolus injection to a feeding artery (e.g. the internal carotid artery) as shown in Figure 3 for CBF measurement, which is straightforward for CBF quantification but requires invasive procedures to gain access to the desired artery [26, 31]. Alternatively, intravenous injection is less invasive and more common for human application, but the CBF quantification is more complicated and requires the tracer input function.
Figure 3.
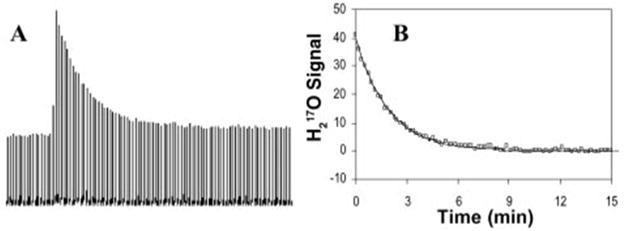
17O-MR based CBF measurement: (A) stacked plots of the cerebral H217O spectra after a bolus injection of H217O into an internal carotid artery of a rat; (B) exponential decay fitting of the H217O washout curve for calculating CBF. Adapted from reference [26]. Copyright © 2002, National Academy of Sciences, U.S.A.
The use of metabolic H217O as an endogenous tracer for CBF measurement
The 17O MR signal of the metabolic water generated in the mitochondria following a 17O2 inhalation can serve as a perfusion tracer, and its decay rate (k) can be used to determine the CBF value with an experimentally derived correction factor (i.e., CBF ≈ 1.86 × k). This empirical approach was demonstrated in rat brains across a wide range of conditions [39]. This finding suggests that the dynamic in vivo 17O MR technique is capable of simultaneously and noninvasively determining both CMRO2 and CBF as schematically shown in Figure 2. Thus, 17O MR provides a new method for studying the cerebral oxygen metabolism and perfusion underlying brain function and disease. In addition, the oxygen extraction fraction (OEF) that reflects the balance between oxygen supply and usage can be calculated from the corresponding CMRO2 and CBF values using the relationship of OEF = CMRO2/(Ca,O2×CBF), where Ca,O2 is a constant representing the arterial oxygen concentration of the brain [38]. Therefore, by combining the dynamic in vivo 17O MR technique with a short 17O2 gas inhalation, it is possible to quantify three important physiological parameters, namely CMRO2, CBF and OEF in a completely non-invasive manner. A similar approach and quantification model in human brain has recently been developed [45].
In vivo 17O MRS imaging (or MRI)
Another important feature of the in vivo 17O MR technology is its capability of detecting not only the dynamic change of the 17O signal but also the signal distribution in space via three-dimensional (3D) 17O MRS imaging [41, 42] or MRI approaches, although conventional MRI sequences are not applicable due to the extremely short T2 relaxation time of the H217O water (in the range of few milliseconds) [25, 28, 46, 47]. Thus, with this technique, it is possible to assess regional oxygen consumption and perfusion rates in the living brain or in other organs. Furthermore, after introducing an exogenous 17O-tracer, the 17O signal can reach a new steady state within a reasonable time window, e.g. ~10–20 minutes in the brain, which permits repeated measurements with subsequent tracer administration under different conditions [34]. This approach enables the quantification of the same parameter with absolute values at different states, as well as the changes due to altered physiological conditions or brain states [34, 35, 48].
The aforementioned methodological aspects of the in vivo 17O MR technology reveal its unique utility for biomedical research in the area of oxygen metabolism, energetics and vascular-metabolic relationships in a living organ. It should be noted that the spatial and temporal resolution of dynamic 17O MR imaging are dependent upon the available 17O signal; thus, 17O sensitivity is the key limiting factor that determines the spatial/temporal resolution and the reliability of the 17O MR imaging.
Advantage of high/ultrahigh fields for in vivo 17O MR imaging
17O has a roughly 7 times lower gyromagnetic ratio and 2700 times lower natural abundance than 1H, which leads to 105 times lower sensitivity compared to 1H [17, 42]. The advantage of the high/ultrahigh magnetic field strength for improving the sensitivity of the 17O MR imaging is essential for its potential applications. Such an advantage stems from the quadrupolar relaxation mechanism of the 17O-water. The nearly field-independent relaxation times of the 17O-water eliminates the potential signal loss commonly occurring in the 1H MRS or MRI at higher field because increasing field strength will not prolong the longitudinal relaxation time (T1) and/or shorten the T2 (or T2*) of the 17O-water. Thus, the acquisition efficiency of the 17O MR signal will not be compromised [25, 28, 46, 47]. The extremely short T1 of the 17O-water permits fast acquisition and more signal averaging in unit sample time, thus enhancing the apparent 17O SNR. Also, the effect of the magnetic field (B0) inhomogeneity on the 17O-water linewidth, which is inversely proportional to the T2*, is very small (negligible) compared to the effect of the quadrupolar relaxation [25, 28, 46, 47]. Therefore, in vivo 17O MR measurement is virtually insensitive to the B0 shimming, a challenging procedure faced by many other in vivo MRS methodologies.
The field-dependent relationship between the apparent 17O-SNR (normalized to that of 9.4T) and the field strength across a wide B0 range from zero up to 16.4T that was obtained from rat brains is displayed in Figure 4. The reported SNR increase with field strength is based on the empirical relationship of SNR ≈ C × B0β with C = 0.015 and β =1.9. This value of β is slightly higher than the theoretical prediction (β =1.75) based on the work of Hoult et al. [49]; this could be due to imperfect control of the experimental variables (e.g. the Q factor (quality factor) of the radio frequency (RF) coils is not identical at different fields) used in the two studies. The finding of field-dependent 17O-SNR enhancement suggests that compared to in vivo 1H, 31P and 13C MRS methodologies, the in vivo 17O MRS can benefit the most from the sensitivity gain at high/ultrahigh field strengths [46].
Figure 4.
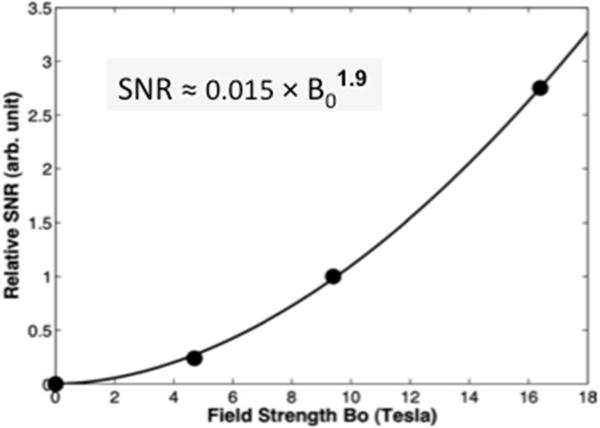
Field dependence of the relative 17O SNR in the rat brains (filled circles) normalized to the SNR value measured at 9.4. The equation represents the fitting result (solid line) of the experimental data covering the B0 fields of zero to 16.4 T. Adapt from reference [46]. Copyright © 2012 Wiley Periodicals, Inc.
Recent development and applications of in vivo 17O MRS and imaging
Preclinical study in animal brain
As noted above, currently, the in vivo 17O MR methodology has been established for the simultaneous dynamic imaging of three important physiological parameters, namely CMRO2, CBF and OEF in preclinical animal brain. An example of such a study is mouse brain, in which the animals have undergone transit middle cerebral arterial occlusion (tMCAo) (Figure 5). The figure displays anatomic regions of post-stroke brains with voxels located in ischemic and intact hemispheres, corresponding to dynamic H217O signals before, during and after 2-min 17O2 inhalation; reproducibility between two repeated imaging measurements in the same animal; and the results of CMRO2, CBF and OEF imaging indicate significant changes in the ischemia affected brain region [38]. This study demonstrates a promising 17O-MR based imaging technique with the merits of robustness, simplicity, noninvasiveness and reliability, features that are essential for imaging abnormal oxygen metabolism and perfusion in diseased brains.
Figure 5.
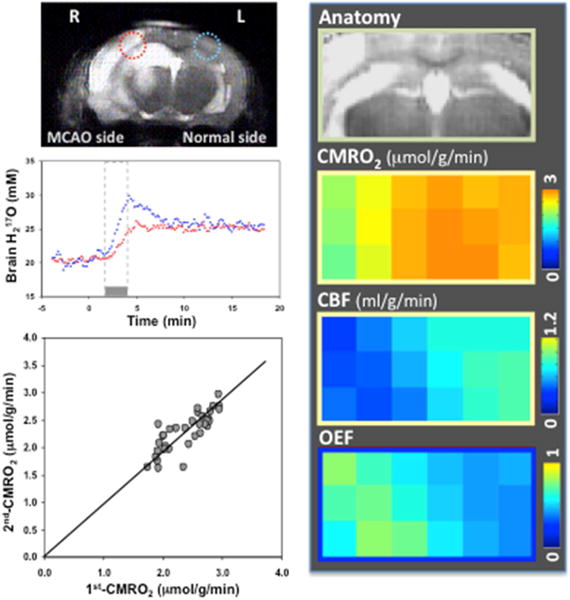
In vivo 17O MR application in stroke mice: anatomic image with ischemic and intact voxels identified; corresponding dynamic H217O signals before, during and after a 2-min 17O2 inhalation in a mouse model of tMCAo; reproducibility of repeated imaging measurements; and CMRO2, CBF and OEF maps indicating significant changes in ischemia affected brain region. Adapted from reference [38]. Copyright © 2012 Elsevier Inc.
Several animal species commonly used in preclinical studies have been tested, which include mouse, rat, cat and swine at field strengths from 3T up to 16.4T; the results confirm the feasibility of dynamic in vivo 17O MRS imaging for studying oxygen metabolism in these preclinical models [35, 38, 39, 48, 50–52]. This novel 17O-MR based CMRO2 imaging technique has been successfully applied to Huntington disease mouse brains and used as a metabolic imaging tool to assess the mitochondrial function in diseased brain in vivo by directly determining the cerebral oxygen consumption deficit under metabolic stress [51]. It is expected that more studies of this kind will be forthcoming once the research community recognizes the promise of this new technology.
Translational study in human brain
The dynamic in vivo 17O MRS imaging approach can be easily applied to the human brain to monitor the change of H217O signals during and after a short 17O2 inhalation [32]. However, to calculate the oxygen consumption rate from the dynamic H217O signals, the simplified model used in small animals is not suitable due to the slower exchange of the 17O-labeled oxygen gas in the human body.
A three-phase model has been proposed, in which the time-dependent function of the 17O-labeled oxygen gas fraction in arterial blood is modeled based on an estimated replacement rate of fresh 17O-oxygen in the blood. In addition, two rate constants of KL and KG representing the loss and gain of H217O within the imaging voxel, respectively, are included in the proposed quantification model, whereas the CBF is not presented in the model since its influence on the measured signal is accounted for by the rate constants KL and KG [36]. Figure 6 shows results of human brain CMRO2 mapping at 9.4T and two representative H217O time-courses in voxels with low and high metabolic rates. The model provides reasonable fitting to the 17O MR data yielding regional CMRO2 values with corresponding KG and KL constants [36].
Figure 6.
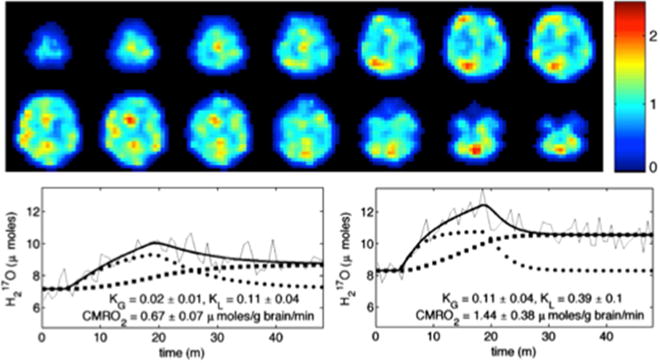
17O MR based CMRO2 imaging in human brain: CMRO2 maps in mol/g brain/min unit (top) and representative H217O time-courses for single voxels corresponding to low (bottom left, white matter) and high (bottom right, gray matter) metabolic rates. The three-phase metabolic model of water production (thick line) accurately describes the 17O MR data (thin line) to yield CMRO2 values with KG and KL constants shown. Adapted from reference [36]. Copyright © 2010 Elsevier Inc.
Recently, a modified quantification model was proposed for human application. In this model, the exchange function of the 17O-labeled oxygen in the human lung is accounted for by its influence on the fractional change of 17O2 in the arterial blood. The 17O2 gas exchange rate can be experimentally determined from a simple breathing test using non-labeled oxygen gas [45, 53]. This additional step is necessary for short 17O2 inhalation because the transition of the oxygen gas in human lung takes ~2 min or longer to reach a steady state. More importantly, not only is it possible to obtain the CMRO2 value, but the CBF value can also be obtained from the model fitting of the dynamic H217O signals in the human brain tissue. Consequently, all three parameters of CMRO2, CBF and OEF can be simultaneously determined from the non-invasive 17O MRS imaging measurement in humans with a 17O2 inhalation as short as 2–3 min [45, 53].
Feasibility assessments in normal human brain at resting or functional stimulated state, and in diseased human brains were performed at a field strength of 3T up to 9.4T; the results are encouraging, indicating that the in vivo 17O MRS imaging technique has the potential to become a promising neuroimaging modality for the translational study of oxygen metabolism in humans [45, 53–56]. It is worth pointing out, however, that future prospects of this methodology will be largely affected by the challenges encountered in expanding this research field for routine applications at clinical settings (see more discussion later).
Study of oxygen metabolism in extracerebral tissues
Besides the brain, the heart is another highly aerobic organ consuming a large amount of oxygen since the oxygen metabolism is essential for supporting the mechanical work of myocyte contractions. The feasibility of applying the in vivo 17O MR technique to hearts has been tested in rats at 9.4T [37, 57] and in human hearts at 3T [54]. The preliminary results as displayed in Figure 7 suggest that it is feasible to establish a noninvasive imaging modality with in vivo 17O MR detection and appropriate quantification modeling for simultaneously imaging the myocardial oxygen metabolic rate (MVO2) and myocardial perfusion [37].
Figure 7.
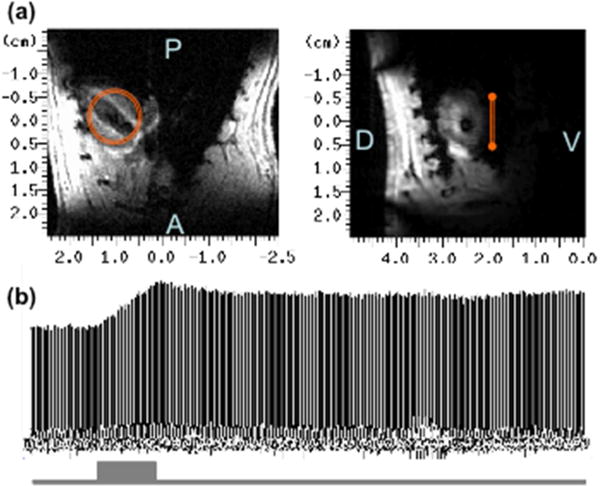
In vivo 17O MR application in heart: (a) 1H MRI of rat heart in axial (left panel) and sagittal (right panel) orientation showing the 17O surface coil location; (b) stack plot of the heart 17O-water signals acquired before, during (grey bar) and after the inhalation of 17O2 gas.
Challenges and the potential of the in vivo 17O MRS and imaging
The most important application of the in vivo 17O MRS is to non-invasively study the oxygen metabolism in living animals or humans with introduction of 17O2 gas. A major obstacle in this regard is the relatively high cost due to the limited supply of the 17O-enriched oxygen gas. Currently, only few vendors can provide 17O-isotope-labeled oxygen gas in large quantities with high enrichment, while the demand for this oxygen isotope is also limited. Increasing the demand may stimulate 17O-oxygen production and lower its cost; but at present, saving the cost by reducing or improving the efficiency of the 17O-oxygen gas usage seems to be the main focus, which is particularly crucial for human studies. Rebreathing pure 17O2 with the CO2 removed from the expired gas [36], and using a demand oxygen delivery system to reduce the total 17O2 gas supply [55, 56] and/or recycling the expired 17O2 gas for animal studies [32, 45, 53] are a few approaches being explored for this purpose. An alternative approach that administers 17O2-enriched blood substitutes instead of 17O2 inhalation has been suggested with the assumption that the high oxygen affinity of the artificial blood would improve its delivery efficiency [43, 58]; although the effectiveness and ability of this approach for accurately quantifying the metabolic rate of oxygen is yet to be determined. On the other hand, minimizing the 17O2 gas inhalation time is a more effective way to lower the cost, although it requires a higher temporal resolution of the dynamic 17O measurement.
Limited detection sensitivity is another major challenge encountered in the in vivo 17O MR study. This is particularly critical for dynamic 17O MR imaging since both spatial and temporal resolutions are key factors demanding high 17O sensitivity. High/ultrahigh field scanners are employed in the 17O MRS imaging studies due to the advantages in gain of sensitivity. It has been shown that a few millimeter spatial resolution in preclinical studies [26, 34, 35, 37–39, 48, 50, 51] and close to a centimeter in human studies [32, 45, 53] with ~10–15 sec temporal resolution can be reached with B0 ≥ 7T. Relatively lower resolutions are expected with lower field magnets, e.g., a 3T clinical scanner, or when large volume coils are employed [30, 36, 54, 56]. It is worth mentioning that while 17O MR benefits from the sensitivity gain at a higher field, it also requires a larger RF power to achieve the same RF pulse flip angle because of its low γ-ratio. Interestingly, a recent study has found that the high RF power demand in the 17O MR measurement is significantly compensated by its high B1 efficiency as compared to that of 1H MR at high/ultrahigh fields [59]. Of course, for human application, the specific absorption rate (SAR) has to be closely monitored to ensure it is below the FDA guideline, which could limit our ability to optimize the 17O MR acquisition parameters in the human studies.
Despite the limitation in the 17O detection sensitivity, the apparent SNR (i.e., the SNR in unit acquisition time) of the 17O MRS is still better than that of 1H, 13C and 31P MRS, if the identical coil performance, acquisition scheme (e.g. at fully relaxed condition) and metabolite concentrations are used in comparison. Such unexpected superiority in sensitivity may be attributed to the relaxation times of the 17O nucleus, which are extremely short (in the order of few milliseconds), thus allowing efficient signal averaging during acquisition. To further improve the spatial resolution of the 17O-MR based CMRO2 mapping, novel MR imaging techniques with efficient signal acquisition scheme and/or denoising capability are worthy of exploration [60].
In summary, after nearly three decades, in vivo 17O MRS and imaging is slowly emerging as a promising MR technique for noninvasively studying oxygen metabolism and perfusion in aerobic organs with the capability of imaging regional metabolic rates of oxygen and its change; thus providing a valuable tool for biomedical research of oxygen metabolism, mitochondrial function and tissue energetics in health and disease.
Acknowledgments
The authors thank Drs. Byeong-Yuel Lee, Xiao Liu, Ming Lu, Kamil Ugurbil, Nanyin Zhang, Xiaoliang Zhang, Yi Zhang and Mr. John Strupp and Hannes Wiesner for their technical assistance and support. The reviewed work was partially funded by the NIH grants of NS041262, NS057560, NS070839, R24 MH106049, R24 MH106049-S1, P30 NS076408, P41 EB015894 and S10 RR026783; and the W.M. Keck Foundation.
Abbreviations
- α
17O enrichment fraction of inhaled 17O2 gas
- B0
Magnetic field strength
- BOLD
Blood oxygenation level dependent
- Ca(t)
Time-dependent H217O concentration in excess of the natural abundance level in the arterial blood
- Cb(t)
Time-dependent H217O concentration in excess of the natural abundance level in the brain tissue
- Cv(t)
Time-dependent H217O concentration in excess of the natural abundance level in the venous blood
- CBF
Cerebral blood flow
- CBV
Cerebral blood volume
- CMRO2
Cerebral metabolic rate of oxygen
- FDA
Food and Drug Administration (U.S.)
- fMRI
Functional magnetic resonance imaging
- H217O
Oxygen-17 labeled water
- k
Decay rate of the endogenous H217O tracer
- KL
Rate constants reflecting the loss of H217O within the imaging voxel
- KG
Rate constants reflecting the gain of H217O within the imaging voxel
- λ
Brain-blood partition coefficient
- MR
Magnetic resonance
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- MVO2
Myocardial oxygen metabolic rate
- OEF
Oxygen extraction fraction
- 17O2
Oxygen-17 labeled oxygen gas
- PET
Positron emission tomography
- Q factor
Quality factor of the radio frequency coil
- RF
Radio frequency
- SAR
Specific absorption rate
- SNR
Signal-to-noise ratio
- T1
Longitudinal relaxation time
- T2 (or T2*)
Transverse relaxation time (or apparent T2)
- tMCAo
Transit middle cerebral arterial occlusion
- Yv
Cerebral venous oxygen saturation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shulman RG, Brown TR, Ugurbil K, Ogawa S, Cohen SM, den Hollander JA. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979;205:160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- 2.Mateescu GD, Yvars GM, Dular T. Oxygen-17 Magnetic Resonance Imaging. Proc Inter Soc Magn Reson Med. 1987;6:929. [Google Scholar]
- 3.Mateescu GD, Yvars GM, Dular T. Water, Ions and O-17 Magnetic Resonance Imaging. In: Lauger P, Packer L, Vasilescu V, editors. Water and Ions in Biological Systems. Birkhauser Verlag, Basel-Boston-Berlin; 1988. pp. 239–250. [Google Scholar]
- 4.Mateescu GD, Yvars GM, Maylish-Kogovsek L, LaManna JC, Lust WD, Sudilovsky D. Oxygen-17 MRI and MRS of the brain, the heart and coronary arteries. Proc Inter Soc Magn Reson Med. 1989;8:659. [Google Scholar]
- 5.Arai T, Nakao S, Mori K, Ishimori K, Morishima I, Miyazawa T, Fritz-Zieroth B. Cerebral oxygen utilization analyzed by the use of oxygen-17 and its nuclear magnetic resonance. Biochem Biophys Res Commun. 1990;169:153–158. doi: 10.1016/0006-291x(90)91447-z. [DOI] [PubMed] [Google Scholar]
- 6.Arai T, Mori K, Nakao S, Watanabe K, Kito K, Aoki M, Mori H, Morikawa S, Inubushi T. In vivo oxygen-17 nuclear magnetic resonance for the estimation of cerebral blood flow and oxygen consumption. Biochem Biophys Res Commun. 1991;179:954–961. doi: 10.1016/0006-291x(91)91911-u. [DOI] [PubMed] [Google Scholar]
- 7.Pekar J, Ligeti L, Ruttner Z, Lyon RC, Sinnwell TM, van Gelderen P, Fiat D, Moonen CT, McLaughlin AC. In vivo measurement of cerebral oxygen consumption and blood flow using 17O magnetic resonance imaging. Magn Reson Med. 1991;21:313–319. doi: 10.1002/mrm.1910210217. [DOI] [PubMed] [Google Scholar]
- 8.Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- 9.Ter-Pogossian MM, Eichling JO, Davis DO, Welch MJ. The measure in vivo of regional cerebral oxygen utilization by means of oxyhemoglobin labeled with radioactive oxygen-15. J Clin Invest. 1970;49:381–391. doi: 10.1172/JCI106247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiat D, Kang S. Determination of the rate of cerebral oxygen consumption and regional cerebral blood flow by non-invasive 17O in vivo NMR spectroscopy and magnetic resonance imaging: Part 1. Theory and data analysis methods. Neurol Res. 1992;14:303–311. doi: 10.1080/01616412.1992.11740074. [DOI] [PubMed] [Google Scholar]
- 11.Fiat D, Ligeti L, Lyon RC, Ruttner Z, Pekar J, Moonen CT, McLaughlin AC. In vivo 17O NMR study of rat brain during 17O2 inhalation. Magn Reson Med. 1992;24:370–374. doi: 10.1002/mrm.1910240218. [DOI] [PubMed] [Google Scholar]
- 12.Fiat D, Dolinsek J, Hankiewicz J, Dujovny M, Ausman J. Determination of regional cerebral oxygen consumption in the human: 17O natural abundance cerebral magnetic resonance imaging and spectroscopy in a whole body system. Neurol Res. 1993;15:237–248. doi: 10.1080/01616412.1993.11740143. [DOI] [PubMed] [Google Scholar]
- 13.Fiat D, Kang S. Determination of the rate of cerebral oxygen consumption and regional cerebral blood flow by non-invasive 17O in vivo NMR spectroscopy and magnetic resonance imaging. Part 2. Determination of CMRO2 for the rat by 17O NMR, and CMRO2, rCBF and the partition coefficient for the cat by 17O MRI. Neurol Res. 1993;15:7–22. doi: 10.1080/01616412.1993.11740100. [DOI] [PubMed] [Google Scholar]
- 14.Mateescu GD, Fercu D. Concerted 17O/31P magnetic resonance spectroscopy: a novel approach for in vivo correlation of oxygen consumption and phosphate metabolism. Adv Exp Biol. 1994;361:234. [Google Scholar]
- 15.Pekar J, Sinnwell T, Ligeti L, Chesnick AS, Frank JA, McLaughlin AC. Simultaneous measurement of cerebral oxygen consumption and blood flow using 17O and 19F magnetic resonance imaging. J Cereb Blood Flow Metab. 1995;15:312–320. doi: 10.1038/jcbfm.1995.36. [DOI] [PubMed] [Google Scholar]
- 16.Mateescu GD, Cabrera ME. In vivo 17O magnetic resonance spectroscopy. Determination of temperature effects on metabolic rates (Q10 factor) Adv Exp Med Biol. 1997;411:585–590. [PubMed] [Google Scholar]
- 17.Gerothanassis IP. Oxygen-17 NMR spectroscopy: Basic principles and applications (Part I) Progress in NMR Spectroscopy. 2010;56:95–197. doi: 10.1016/j.pnmrs.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 18.An H, Lin W, Celik A, Lee YZ. Quantitative measurements of cerebral metabolic rate of oxygen utilization using MRI: a volunteer study. NMR Biomed. 2001;14:441–447. doi: 10.1002/nbm.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulte DP, Kelly M, Germuska M, Xie J, Chappell MA, Okell TW, Bright MG, Jezzard P. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage. 2012;60:582–591. doi: 10.1016/j.neuroimage.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christen T, Bolar DS, Zaharchuk G. Imaging brain oxygenation with MRI using blood oxygenation approaches: methods, validation, and clinical applications, AJNR. American journal of neuroradiology. 2013;34:1113–1123. doi: 10.3174/ajnr.A3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauthier CJ, Hoge RD. Magnetic resonance imaging of resting OEF and CMRO2 using a generalized calibration model for hypercapnia and hyperoxia. Neuroimage. 2012;60:1212–1225. doi: 10.1016/j.neuroimage.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab. 2010;30:1598–1607. doi: 10.1038/jcbfm.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med. 2008;60:357–363. doi: 10.1002/mrm.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ugurbil K, Adriany G, Andersen P, Chen W, Garwood M, Gruetter R, Henry PG, Kim SG, Lieu H, Tkac I, Vaughan T, Van De Moortele PF, Yacoub E, Zhu XH. Ultrahigh field magnetic resonance imaging and spectroscopy. Magnetic resonance imaging. 2003;21:1263–1281. doi: 10.1016/j.mri.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Zhu XH, Merkle H, Kwag JH, Ugurbil K, Chen W. 17O relaxation time and NMR sensitivity of cerebral water and their field dependence. Magn Reson Med. 2001;45:543–549. doi: 10.1002/mrm.1073. [DOI] [PubMed] [Google Scholar]
- 26.Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of 17O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A. 2002;99:13194–13199. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mateescu GD. Functional oxygen-17 magnetic resonance imaging and localized spectroscopy. Adv Exp Med Biol. 2003;510:213–218. doi: 10.1007/978-1-4615-0205-0_35. [DOI] [PubMed] [Google Scholar]
- 28.Thelwall PE, Blackband SJ, Chen W. Field dependence of 17O T1, T2 and SNR – in vitro and in vivo studies at 4.7, 11 and 17.6 Tesla. Proc Intl Soc Mag Reson Med. 2003;11:504. [Google Scholar]
- 29.Zhang X, Zhu XH, Tian R, Zhang Y, Merkle H, Chen W. Measurement of arterial input function of 17O water tracer in rat carotid artery by using a region-defined (REDE) implanted vascular RF coil. Magma. 2003;16:77–85. doi: 10.1007/s10334-003-0013-9. [DOI] [PubMed] [Google Scholar]
- 30.Fiat D, Hankiewicz J, Liu S, Trbovic S, Brint S. 17O magnetic resonance imaging of the human brain. Neurol Res. 2004;26:803–808. doi: 10.1179/016164104X5156. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Zhu XH, Lei H, Ugurbil K, Chen W. Simplified methods for calculating cerebral metabolic rate of oxygen based on 17O magnetic resonance spectroscopic imaging measurement during a short 17O2 inhalation. J Cereb Blood Flow Metab. 2004;24:840–848. doi: 10.1097/01.WCB.0000125885.54676.82. [DOI] [PubMed] [Google Scholar]
- 32.Zhu XH, Zhang X, Zhang N, Zhang Y, Strupp J, Ugurbil K, Chen W. High-field 17O Study of 3D CMRO2 Imaging in human visual cortex. Proc Intl Soc Mag Reson Med. 2006;14:409. [Google Scholar]
- 33.Zhu XH, Zhang Y, Chen W. Simultaneous and Ultrafast Monitoring of CMRO2, CBF and pO2 Changes in Response to Acute Global Ischemia in Rat Brain Proc. Intl Soc Mag Reson Med. 2007;15:2393. [Google Scholar]
- 34.Zhu XH, Zhang Y, Zhang N, Ugurbil K, Chen W. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/hypothermia comparison study. J Cereb Blood Flow Metab. 2007;27:1225–1234. doi: 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]
- 35.Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson IC, Thulborn KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage. 2010;51:723–733. doi: 10.1016/j.neuroimage.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Zhu XH, Zhang Y, Chen W. In Vivo 17O MRS Imaging for Assessing Myocardial Oxygen Metabolism in Rat Heart at 9.4T. Proc Intl Soc Mag Reson Med. 2010;18:172. [Google Scholar]
- 38.Zhu XH, Chen JM, Tu TW, Chen W, Song SK. Simultaneous and noninvasive imaging of cerebral oxygen metabolic rate, blood flow and oxygen extraction fraction in stroke mice. Neuroimage. 2013;64:437–447. doi: 10.1016/j.neuroimage.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu XH, Zhang Y, Wiesner HM, Ugurbil K, Chen W. In vivo measurement of CBF using 17O NMR signal of metabolically produced H217O as a perfusion tracer. Magn Reson Med. 2013;70:309–314. doi: 10.1002/mrm.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Zhu XH, Ugurbil K. Imaging Cerebral Metabolic Rate of Oxygen Consumption (CMRO2) using 17O NMR Approach at Ultra-high Field. In: Shulman RG, Rothman DL, editors. Brain Energetics and Neuronal Activity. John Wiley & Sons Ltd; New York: 2004. pp. 125–146. [Google Scholar]
- 41.Zhu XH, Chen W. In vivo oxygen-17 NMR for imaging brain oxygen metabolism at high field. Progress in nuclear magnetic resonance spectroscopy. 2011;59:319–335. doi: 10.1016/j.pnmrs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo 17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- 43.Gordji-Nejad A, Mollenhoff K, Oros-Peusquens AM, Pillai DR, Shah NJ. Characterizing cerebral oxygen metabolism employing oxygen-17 MRI/MRS at high fields. MAGMA. 2014;27:81–93. doi: 10.1007/s10334-013-0413-4. [DOI] [PubMed] [Google Scholar]
- 44.de Graaf RA, Brown PB, Rothman DL, Behar KL. Natural abundance 17O NMR spectroscopy of rat brain in vivo. J Magn Reson. 2008;193:63–67. doi: 10.1016/j.jmr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu XH, Liu X, Lu M, Wiesner HM, Ugurbil K, Chen W. In Vivo 17O MR Imaging and Quantification of CMRO2, CBF and OEF in Human Visual Cortex at Rest and during Activation. Proc Intl Soc Mag Reson Med. 2014;22:3763. [Google Scholar]
- 46.Lu M, Zhang Y, Ugurbil K, Chen W, Zhu XH. In vitro and in vivo studies of 17O NMR sensitivity at 9.4 and 16.4 T. Magn Reson Med. 2013;69:1523–1527. doi: 10.1002/mrm.24386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiesner HM, Balla DZ, Shajan G, Scheffler K, Ugurbil K, Chen W, Uludag K, Pohmann R. 17O relaxation times in the rat brain at 16.4 tesla. Magn Reson Med. 2015 doi: 10.1002/mrm.25814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiesner HM, Pohmann R, Balla DZ, Chen W, Ugurbil K, Uludag K. Measurement of CMRO2 changes by somatosensory stimulation in rat using oxygen-17 at 16.4 T. Proc Intl Soc Mag Reson Med. 2011;19:1500. [Google Scholar]
- 49.Hoult DI, Richards RE. The signal-to-noise ratio of the nuclear magnetic resonance experiment. 1976. J Magn Reson. 2011;213:329–343. doi: 10.1016/j.jmr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Cui W, Zhu XH, Vollmers ML, Colonna ET, Adriany G, Tramm B, Dubinsky JM, Oz G. Non-invasive measurement of cerebral oxygen metabolism in the mouse brain by ultra-high field 17O MR spectroscopy. J Cereb Blood Flow Metab. 2013;33:1846–1849. doi: 10.1038/jcbfm.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou S, Lepak VC, Eberly LE, Roth B, Cui W, Zhu XH, Oz G, Dubinsky JM. Oxygen consumption deficit in Huntington disease mouse brain under metabolic stress. Human molecular genetics. 2016 doi: 10.1093/hmg/ddw138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mellon EA, Beesam RS, Baumgardner JE, Borthakur A, Witschey WR, 2nd, Reddy R. Estimation of the regional cerebral metabolic rate of oxygen consumption with proton detected 17O MRI during precision 17O2 inhalation in swine. Journal of neuroscience methods. 2009;179:29–39. doi: 10.1016/j.jneumeth.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu XH, Wiesner HM, Lee BY, Lu M, Chen W. Quantitative and Simultaneous Imaging of CMRO2, CBF and OEF in Resting Human Brain. Proc Intl Soc Mag Reson Med. 2015;23:895. [Google Scholar]
- 54.Borowiak R, Groebner J, Haas M, Hennig J, Bock M. Direct cerebral and cardiac 17O-MRI at 3 Tesla: initial results at natural abundance. MAGMA. 2014;27:95–99. doi: 10.1007/s10334-013-0409-0. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann SH, Begovatz P, Nagel AM, Umathum R, Schommer K, Bachert P, Bock M. A measurement setup for direct 17O MRI at 7 T. Magn Reson Med. 2011;66:1109–1115. doi: 10.1002/mrm.22871. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann SH, Radbruch A, Bock M, Semmler W, Nagel AM. Direct 17O MRI with partial volume correction: first experiences in a glioblastoma patient. MAGMA. 2014;27:579–587. doi: 10.1007/s10334-014-0441-8. [DOI] [PubMed] [Google Scholar]
- 57.Lu M, Atthe B, Mateescu GD, Flask CA, Yu X. Assessing mitochondrial respiration in isolated hearts using 17O MRS. NMR Biomed. 2012;25:883–889. doi: 10.1002/nbm.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delapaz R, Gupte P. Potential application of 17O MRI to human ischemic stroke. Adv Exp Med Biol. 2011;701:215–222. doi: 10.1007/978-1-4419-7756-4_29. [DOI] [PubMed] [Google Scholar]
- 59.Wiesner HM, Luo W, Yang QX, Zhu XH, Schillak S, Chen W. Quantitative Study of TX/RX-efficiency of X-Nuclear MRS/MRI at High/Ultrahigh Field. Proc Intl Soc Mag Reson Med. 2014;22:810. [Google Scholar]
- 60.Qian Y, Stenger VA, Boada FE. Parallel imaging with 3D TPI trajectory: SNR and acceleration benefits. Magnetic resonance imaging. 2009;27:656–663. doi: 10.1016/j.mri.2008.10.008. [DOI] [PubMed] [Google Scholar]


