Abstract
BACKGROUND: Intraventricular hemorrhage (IVH) is the most frequent, severe neurological complication of prematurity and is associated with posthemorrhagic hydrocephalus (PHH) in up to half of cases. PHH requires lifelong neurosurgical care and is associated with significant cognitive and psychomotor disability. Cerebrospinal fluid (CSF) biomarkers may provide both diagnostic information for PHH and novel insights into its pathophysiology.
OBJECTIVE: To explore the diagnostic ability of candidate CSF biomarkers for PHH.
METHODS: Concentrations of amyloid precursor protein (APP), soluble APPα (sAPPα), soluble APPβ, neural cell adhesion molecule-1 (NCAM-1), L1 cell adhesion molecule (L1CAM), tau, phosphorylated tau, and total protein (TP) were measured in lumbar CSF from neonates in 6 groups: (1) no known neurological disease (n = 33); (2) IVH grades I to II (n = 13); (3) IVH grades III to IV (n = 12); (4) PHH (n = 12); (5) ventricular enlargement without hydrocephalus (n = 10); and (6) hypoxic ischemic encephalopathy (n = 13). CSF protein levels were compared using analysis of variance, and logistic regression was performed to examine the predictive ability of each marker for PHH.
RESULTS: Lumbar CSF levels of APP, sAPPα, L1CAM, and TP were selectively increased in PHH compared with all other conditions (all P < .001). The sensitivity, specificity, and odds ratios of candidate CSF biomarkers for PHH were determined for APP, sAPPα, and L1CAM; cut points of 699, 514, and 113 ng/mL yielded odds ratios for PHH of 80.0, 200.0, and 68.75, respectively.
CONCLUSION: Lumbar CSF APP, sAPPα, L1CAM, and TP were selectively increased in PHH. These proteins, and sAPPα, in particular, hold promise as biomarkers of PHH and provide novel insight into PHH-associated neural injury and repair.
Keywords: Amyloid precursor protein, Biomarker, Cerebrospinal fluid, Intraventricular hemorrhage, L1CAM, Neurosurgery, Posthemorrhagic hydrocephalus, Preterm, sAPPα
ABBREVIATIONS
- APP
amyloid precursor protein
- ELISA
enzyme-linked immunosorbent assay
- HIE
hypoxic ischemic encephalopathy
- IVH
intraventricular hemorrhage
- L1CAM
L1 cell adhesion molecule
- LP
lumbar puncture
- NCAM-1
neural cell adhesion molecule-1
- NICU
neonatal intensive care unit
- p-tau
phosphorylated tau
- PHH
posthemorrhagic hydrocephalus
- PMA
postmenstrual age
- RCG
rostrocaudal gradient
- sAPPα
soluble amyloid precursor protein α
- sAPPβ
soluble amyloid precursor protein β
- TP
total protein
- VAD
ventricular access device
- VEWOH
ventricular enlargement without hydrocephalus
Intraventricular hemorrhage (IVH) is a common, severe neurological complication of prematurity, occurring in one-third of infants born ≤28 weeks postmenstrual age (PMA).1,2 Up to one-half of preterm infants with IVH develop posthemorrhagic hydrocephalus (PHH),3-5 a condition associated with substantial neurological disability and complex, lifelong neurosurgical care.1,6 Despite the morbidity associated with PHH and its treatment, there is no consensus regarding the diagnosis or treatment of PHH, largely because of a dearth of quantifiable PHH metrics.
At present, clinical assessment of infants with PHH includes physical parameters such as head circumference, tenseness of the anterior fontanel, and splaying of cranial sutures, as well as changes in vital signs, which occur late in the disease course. As a result, clinicians rely in large part on semiquantitative imaging-based measurements of ventricular size for clinical decision making. However, ventricular size is an imperfect metric for the treatment of neurological disease in preterm infants. Neurological injury (eg, IVH, infarct, hypoxia-ischemia), white matter volume loss, and impaired brain development are all common among preterm infants and impact ventricular size, irrespective of PHH. New tools are needed to complement existing measures of ventricular size and inform clinical research aimed at improving the diagnosis, treatment, and outcomes of infants with PHH.
Cerebrospinal fluid (CSF) proteins have been investigated as candidate biomarkers of PHH by our group and others.7-13 In particular, CSF levels of amyloid precursor protein (APP) isoforms, L1 cell adhesion molecule (L1CAM), and neural cell adhesion molecule-1 (NCAM-1) have been shown to be elevated in PHH and to normalize after initiation of clinical treatment.7 Furthermore, CSF APP levels have been shown to be associated with ventricular size and possibly intracranial pressure throughout the active PHH treatment interval.14 Thus, CSF levels of APP and related proteins represent promising candidate biomarkers of PHH. The potential role of CSF biomarkers in PHH should not be limited to diagnosis of the condition, however, nor even monitoring therapeutic efficacy, but may extend to the prediction of long-term neurological outcome and developmental impairment.
Prior investigations into CSF biomarkers of PHH and other types of pediatric hydrocephalus, including those from our group, share the limitation of using CSF acquired via lumbar puncture (LP) (in subjects without hydrocephalus) as the comparator for ventricular CSF obtained from children with hydrocephalus.7,8 Comparing ventricular with lumbar CSF introduces the potential for sampling artifact, because rostrocaudal protein gradients (RCGs) exist between ventricular and lumbar CSF and may vary both by protein and by neurological disease.15,16 For hydrocephalus, RCGs may also vary by hydrocephalus etiology.16
In the current study, we account for RCGs in PHH by comparing the levels of candidate biomarkers (APP, soluble APP-α, soluble APP-β, Aβ1-42, NCAM-1, L1CAM, tau, and phosphorylated tau [p-tau]) in CSF obtained from human infants exclusively via LP. Using this approach, candidate CSF biomarkers were compared not just between control and PHH but across a number of newborn neurological conditions, including IVH alone, hypoxic ischemic encephalopathy (HIE), and ventricular enlargement without hydrocephalus (VEWOH).
METHODS
Research Subjects
Approval from the Washington University Human Research Protection Office was acquired prior to initiation of this study (WU HRPO numbers 201101887 and 201203126). As part of routine clinical care, all preterm infants ≤34 weeks estimated PMA admitted to the St. Louis Children's Hospital Neonatal Intensive Care Unit (NICU) underwent routine head ultrasound examinations between 0 and 5 days of age to screen for IVH or other neurological injuries. IVH was graded according to the Papile criteria,17 and, if present, ultrasounds were repeated weekly to monitor for progressive ventricular dilatation. PHH was diagnosed in infants who demonstrated a frontal-occipital ratio ≥0.55 on ultrasound or magnetic resonance imaging (MRI),18 progressive increase in occipitofrontal circumference, splaying of the sagittal suture ≥2 mm in the midparietal region, and palpation of the anterior fontanel above the level of the surrounding bone.19 The current study includes subjects who were diagnosed with PHH and underwent LP before the neurosurgical implantation of a ventricular access device (VAD) for either diagnostic purposes (eg, to rule out infection before VAD implantation) or therapeutic purposes (eg, to enable ventricular decompression while awaiting VAD) (Table 1). None of the 12 infants with PHH in this study had loculated hydrocephalus or a trapped fourth ventricle at the time of acquisition of the CSF sample, and none went on to later develop a trapped fourth ventricle or require treatment for loculated hydrocephalus (shunt system with >1 ventricular catheter or fenestration procedure) at a median follow-up 27.2 ± 15.4 months. Additional subjects with clinical findings and radiological evidence (ultrasound and/or MRI) consistent with HIE or VEWOH were recruited for the study. MRIs of HIE subjects involved injury to the deep gray nuclei, cerebral cortex, and subcortical white matter. Those with VEWOH showed ventriculomegaly from cerebral malformation, “arrested hydrocephalus,” or other unidentifiable causes but did not develop macrocephaly (occipitofrontal circumference >97th centile) or require neurosurgical treatment. A cohort of infants diagnosed with IVH who did not go on to develop PHH were also included in the study. Infants without any known neurological insult or injury who underwent LP for routine sepsis evaluation (where all cultures were deemed negative and sepsis was ruled out) were recruited and termed “controls” (Table 1).
TABLE 1.
| Sample Size (n) | PMA Birth (wk) | Birthweight (g) | PMA Sample (wk) | Age of Sample (wk) | FOR | |
|---|---|---|---|---|---|---|
| Control | 33 | 29.7 ± 3.8 | 1539 ± 809.6 | 33.7 ± 4.3 | 4.0 ± 3.5 | 0.41 ± 0.04 |
| IVH I-II | 13 | 30.9 ± 4.5 | 1714 ± 795.2 | 33.0 ± 3.6 | 2.1 ± 2.4 | 0.41 ± 0.03 |
| IVH III-IV | 12 | 28.8 ± 5.9 | 1448 ± 921.1 | 34.8 ± 6.7 | 6.1 ± 5.0 | 0.50 ± 0.04 |
| PHH | 12 | 27.0 ± 3.2 | 1031 ± 430.5 | 29.5 ± 2.8 | 2.6 ± 1.4 | 0.64 ± 0.03 |
| VEWOH | 10 | 35.3 ± 4.6 | 2397 ± 1274.7 | 39.7 ± 5.0 | 4.4 ± 4.1 | 0.49 ± 0.08 |
| HIE | 13 | 37.6 ± 2.4 | 3023 ± 587.7 | 39.3 ± 2.9 | 1.8 ± 2.2 | 0.36 ± 0.03 |
aPMA, postmenstrual age; FOR, frontal-occipital ratio, a measure of ventricular size; IVH, intraventricular hemorrhage; PHH, posthemorrhagic hydrocephalus; VEWOH, ventricular enlargement without hydrocephalus; HIE, hypoxic-ischemic encephalopathy.
bValues are shown as mean ± standard deviation.
Acquisition of Biospecimens
Lumbar CSF samples were collected from February 1, 2012 to March 1, 2015. During this same time interval, the rate of posthemorrhagic ventricular dilatation at our institution was 5 to 7 cases/year (total of 17 cases). Twelve of these patients (65%) were recruited and included in this study. Lumbar punctures were performed by the clinical team in the NICU under sterile conditions and for clinical purposes only. Immediately after LP, the samples were transported directly to the St. Louis Children's Hospital clinical laboratory where they were frozen at −20°C for a maximum of 2 days. They were then transported on ice to the Washington University Neonatal CSF Repository, where they were stored at −80°C. Before experimental analysis, samples were thawed and centrifuged at 2500 rpm for 6 minutes, and the supernatant was analyzed for protein levels.
In all cases, CSF microbiological cultures sent at the time of the LP remained sterile (cultures were monitored for 3.68 ± 0.13 days); per our NICU practice, anaerobic CSF cultures were not routinely performed. Data for CSF cell count and differential analysis were recorded from the electronic medical record. Using Bonadio et al's20 proposed threshold red blood cell count of >1000/mm3, “traumatic” LPs were noted in 15 of 56 (26.8%) of the CSF samples (IVH and PHH samples excluded), which is consistent with or lower than previously reported data for LPs in neonates.21,22 For neonatal plasma samples, blood was collected from newborn infants in evacuated tubes with anticoagulant. The plasma was separated within 2 hours, aliquoted, and stored at −80°C until experimental analysis.
Measurement of Protein Concentrations
Commercially available enzyme-linked immunosorbent assays (ELISAs) were used to measure the concentration of APP, soluble APP α and β (sAPPα and sAPPβ), Aβ42, L1CAM, NCAM-1, tau, and p-tau. Sandwich Duoset ELISA development systems (R&D Systems, catalog #DY-850 and DY-2408 respectively; Minneapolis, Minnesota) were used to measure APP and NCAM-1 as previously described.14 L1CAM levels were measured using a commercially available kit (DRG, catalog #EIA5074; Mountainside, New Jersey) and have also been described previously.14 sAPPα and sAPPβ were measured using ELISAs (IBL-International, catalog #27734 and 27732, respectively; Toronto, Ontario, Canada). The levels of tau, p-tau, and Aβ42 were also determined by ELISAs (Fujirebio, Ghent, Belgium; catalog #80226, 80062, 80177). In each instance, ELISAs were run according to the manufacturer's protocol. In brief, plates were coated with a primary capture antibody and blocked before incubation with CSF or plasma samples. After washing, the secondary antibody was added, followed by streptavidin-horseradish peroxidase and tetramethylbenzidine. The reaction was then stopped with sulfuric acid. The plates were washed between each of the previous steps, with the exception of the final one. All ELISAs were run in duplicates and the 96-well plates were read at 450 nm on a Versamax microplate reader (Molecular Devices; Sunnyvale, California). Protein concentrations were then determined using a 4-parameter logistic standard curve as detailed by the manufacturers.
Measurement of Total CSF Protein
The Pierce Bicinchoninic Acid protein assay kit (Thermo Scientific; Waltham, Massachusetts) was used to estimate the total protein (TP) level in each sample. In brief, samples and bovine serum albumin standards were placed into microplate wells in duplicate. The working reagent was then added and the plate was incubated at 37°C for 30 minutes. The plate was then cooled to room temperature, and the absorbance was measured at 562 nm on a plate reader. TP levels were determined using a 4-parameter logistic standard curve.
Statistical Analysis
One-way analysis of variance was used to test for association of CSF proteins with PHH and alternate outcome categories (IVH, VEWOH, HIE, and control). A Bonferroni corrected threshold (0.05/9 = 0.0055) was calculated for multiple comparisons. Proteins APP, sAPPα, and L1CAM showed association and were used in simple logistic regressions of PHH on candidate biomarker concentration to examine their predictive ability. Sensitivity, specificity, and odds ratios were used to evaluate candidate cut point concentrations. Significance was set at a P value of < .05. SAS 9.4 was used for all analyses.
RESULTS
Clinical Characteristics and Subject Groupings
Six groups of research subjects were identified based on clinical history and events and radiologic findings (ultrasound and/or MRI): (1) control subjects (no known neurological insult or injury, n = 33); (2) IVH grades I to II (n = 13); (3) IVH grades III to IV (n = 12); (4) PHH (n = 12); (5) HIE (n = 13); and (6) VEWOH (n = 10). Please refer to Table 1 for summary characteristics of subject groupings. There was no difference among the first 4 groups for birth weight, PMA at the time of birth, or PMA at the time of acquisition of the CSF sample; however, compared with PHH subjects, subjects with HIE and VEWOH tended to have higher birth weights (P < .001 and P = .007, respectively) and be older at birth (P < .001 and P = .002, respectively).
Candidate CSF Biomarkers of PHH
Lumbar CSF levels of APP, sAPPα, sAPPβ, Aβ42, NCAM-1, L1CAM, tau, p-tau, and TP were measured as detailed in Methods (Table 2). Among APP and its derivatives, CSF APP and sAPPα were increased in PHH compared with all other conditions (P < .001 and P < .001, respectively; Figure 1A, B). CSF sAPPβ was different between PHH and control (P = .044), and Aβ42 was not significantly different among any groups. CSF L1CAM was elevated in PHH relative to all other conditions (P < .001; Figure 1C). NCAM-1 was elevated in PHH relative to the other conditions with the exception of IVH III to IV (P = .137). Total tau was not different among groups (P = .095), but p-tau was variable, greater in PHH than control (P =.015), IVH I to II (P = .022), HIE (P < .007), and VEWOH (P < .006). To account for multiple comparisons, the Bonferroni threshold was calculated as 0.05/9 or 0.0055. Three candidate CSF biomarkers, APP, sAPPα, and L1CAM, met the corrected threshold for significance (P < .001 for each) and were selective for PHH.
TABLE 2.
Lumbar CSF Levels of APP, sAPPα, sAPPβ, Aβ1-42, NCAM-1, L1CAM, Tau, P-tau, and TP Measured Across the Six Primary Groups and Plasmaa,b
| APP | sAPPα | sAPPβ | Aβ1-42 | NCAM-1 | L1CAM | Tau | P-tau | TP (μg/mL) | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 406.7 ± 290.8 | 136.2 ± 81.7 | 1139 ± 942.7 | 0.792 ± 0.3 | 140.5 ± 68.5 | 74.43 ± 71.7 | 6.09 ± 4.7 | 1.14 ± 0.6 | 1077 ± 435.1 |
| IVH I-II | 313.1 ± 237.3 | 163.1 ± 130.9 | 848.9 ± 460.5 | 0.861 ± 0.5 | 104.9 ± 51.9 | 63.36 ± 33.1 | 3.46 ± 3.1 | 0.99 ± 0.5 | 1365 ± 1098 |
| IVH III-IV | 523.1 ± 278.6 | 377.9 ± 376.5 | 1085 ± 698.6 | 1.274 ± 0.7 | 258.9 ± 129.3 | 76.00 ± 51.1 | 9.55 ± 12.9 | 1.47 ± 0.8 | 1963 ± 952.1 |
| PHH | 1416 ± 806.3 | 1667 ± 1227 | 16 678 ± 36 068 | 0.953 ± 0.7 | 356.4 ± 152.4 | 191.8 ± 93.5 | 20.28 ± 33.9 | 1.98 ± 1.2 | 3748 ± 2403 |
| VEWOH | 330.7 ± 267.9 | 97.4 ± 60.9 | 659.5 ± 760.1 | 0.821 ± 0.7 | 119.4 ± 77.1 | 28.68 ± 22.3 | 5.68 ± 6.4 | 0.71 ± 0.6 | 772.2 ± 371.3 |
| HIE | 371.3 ± 209.5 | 168.9 ± 171.6 | 1002 ± 1605 | 0.335 ± 0.2 | 184.9 ± 72.9 | 24.82 ± 22.0 | 9.20 ± 9.6 | 0.85 ± 0.4 | 1060 ± 448.7 |
| Plasma | 139.87 ± 21.4 | 54.02 ± 31.9 | 21.43 ± 3.2 | 0.555 ± 0.2 | 482.33 ± 63.1 | 15.75 ± 4.1 | 0.242 ± 0.1 | 0.109 ± 0.001 | 49139 ± 6187 |
aAPP, amyloid precursor protein; HIE, hypoxic-ischemic encephalopathy; IVH, intraventricular hemorrhage; L1CAM, L1 cell adhesion molecule; NCAM-1, neural cell adhesion molecule-1; PHH, posthemorrhagic hydrocephalus; sAPPα, soluble amyloid precursor protein α; sAPPβ, soluble amyloid precursor protein β; TP, total protein; VEWOH, ventricular enlargement without hydrocephalus.
bProtein levels are shown as mean ± standard deviation and all are in ng/mL, except TP, which is in μg/mL.
FIGURE 1.
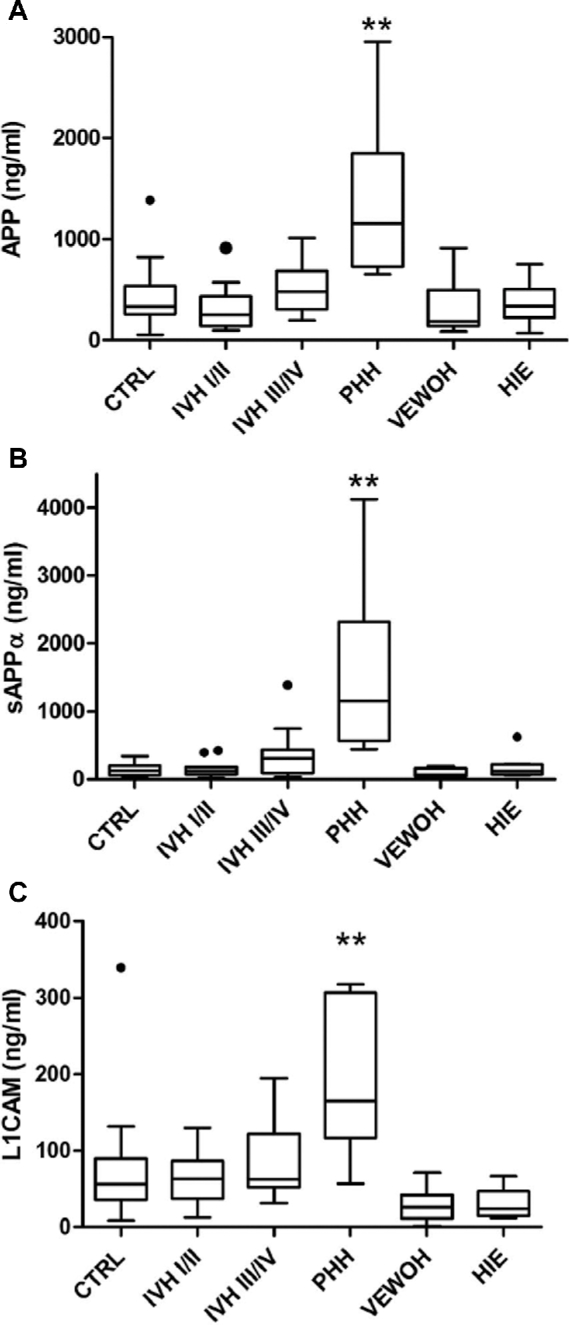
Comparison of lumbar CSF APP A, sAPPα B, and L1CAM C levels across 6 neonatal comparison groups: (1) control subjects (CTRL, no known neurological insult or injury); (2) IVH grades I to II; (3) IVH grades III to IV; (4) PHH; (5) HIE; and (6) VEWOH. Boxes represent the median with 25th and 75th percentiles and the whiskers depict the interquartile range multiplied by 1.5. The PHH levels of all 3 proteins were significantly different than all other groups (P < .050; denoted by **). APP, amyloid precursor protein; HIE, hypoxic ischemic encephalopathy; IVH, intraventricular hemorrhage; L1CAM, L1 cell adhesion molecule; PHH, posthemorrhagic hydrocephalus; sAPPα, soluble amyloid precursor protein α; VEWOH, ventricular enlargement without hydrocephalus.
Total CSF protein was also elevated in PHH (P < .001; Table 2), consistent with findings from other groups studying various forms of hydrocephalus.9 Although it is possible that blood or blood breakdown products contributed to this difference, this is unclear because TP was significantly lower in IVH III to IV compared with PHH (P = .005). To examine whether blood or blood breakdown products could be contributing to the increases in CSF APP, sAPPα, L1CAM, and NCAM-1, the levels of these proteins were measured in age-matched neonatal plasma samples. With the exception of NCAM-1, which trended toward higher levels in the plasma samples compared with PHH CSF (P = .139), APP, sAPPα, and L1CAM were significantly lower in plasma than in PHH CSF (P = .009, P = .003, and P = .003, respectively). These relationships extended to plasma vs control CSF; NCAM-1 was higher in plasma (P < .001), while APP and L1CAM trended toward lower levels in plasma (P = .085 and P = .122, respectively); sAPPα was significantly lower in plasma (P = .015). Thus, it is unlikely that blood or blood breakdown products are responsible for the observed PHH-associated increases in CSF APP, sAPPα, and L1CAM.
Logistic regression was used to evaluate the relationship between each of the candidate CSF biomarkers (APP, sAPPα, sAPPβ, Aβ42, NCAM-1, L1CAM, tau, and p-tau) and CSF cell count parameters (total cells, nucleated cells, red blood cells, neutrophils, lymphocytes, monocytes, eosinophils, and macrophages). None of the candidate biomarkers demonstrated a significant relationship with any of the cell count parameters, with the exception of CSF L1CAM with CSF neutrophils (P = .022) and CSF monocytes (P = .022).
CSF APP, sAPPα, and L1CAM as Diagnostic Biomarkers of PHH
The predictive ability of lumbar CSF APP, sAPPα, and L1CAM levels for PHH were examined by first generating a logistic plot for each candidate biomarker using the measurements for CSF samples from infants with PHH and those without PHH (control, IVH grades I-II, IVH grades III-IV, HIE, VEWOH) (Figures 2A-2C). Simple logistic regression was performed on each biomarker to determine the strength of prediction for PHH and cut points were chosen based on combinations of sensitivity and specificity (Tables 3-4). CSF APP (cut point = 699.67 ng/mL, sensitivity = 0.91, specificity = 0.89), sAPPα (cutpoint = 514.43 ng/mL, sensitivity = 0.91, specificity = 0.95), and L1CAM (cut point = 113.33 ng/mL, sensitivity = 0.91, specificity = 0.89), yielded odds ratios for PHH of 80.00, 200.00, and 68.75, respectively.
FIGURE 2.
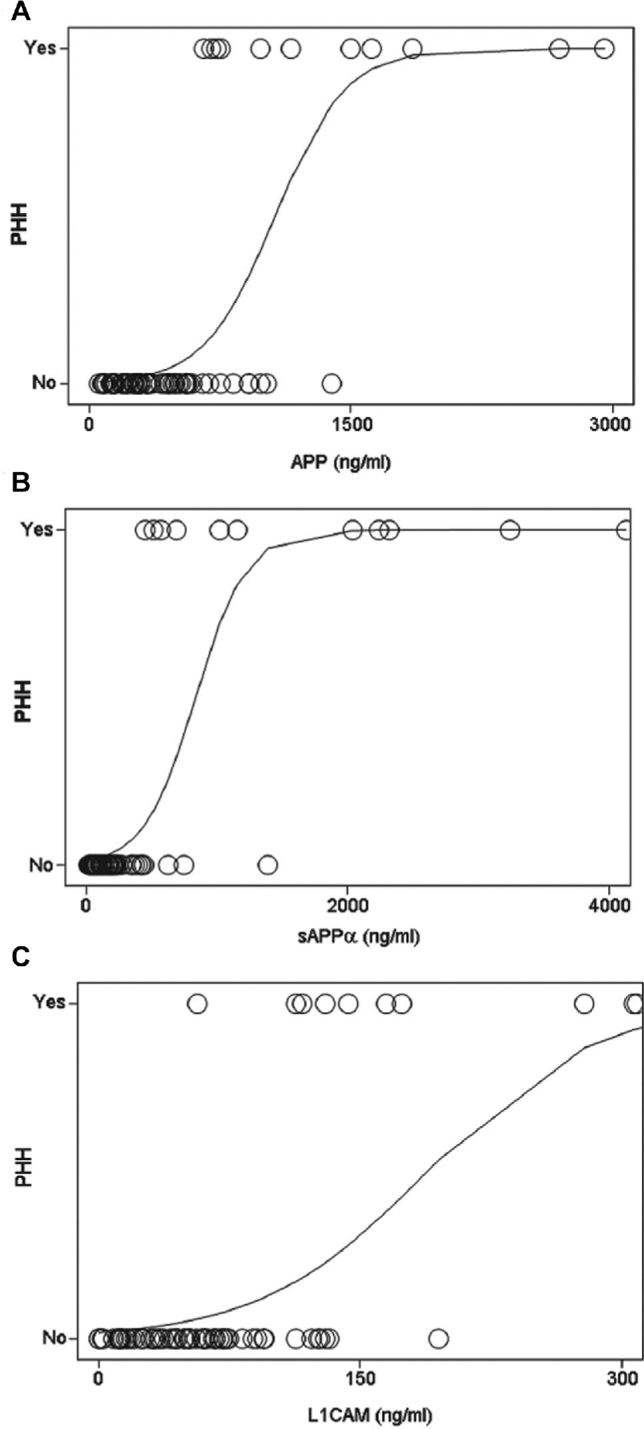
Logistic curve for APP A, sAPPα B, and L1CAM C showing the relationship between protein concentration and the presence or absence of PHH. For the purposes of this analysis, PHH samples were assigned a value of 1.0 and samples from all other conditions were assigned 0.0. APP, amyloid precursor protein; L1CAM, L1 cell adhesion molecule; PHH, posthemorrhagic hydrocephalus; sAPPα, soluble amyloid precursor protein α.
TABLE 3.
Sensitivity and Specificity of Candidate Biomarkers for PHH for Throughout a Range of Concentrationsa
| APP | sAPPα | L1CAM | ||||||
|---|---|---|---|---|---|---|---|---|
| Conc. | Sens. | Spec. | Conc. | Sens. | Spec. | Conc. | Sens. | Spec. |
| 653.62 | 1.0 | 0.87 | 441.94 | 1.0 | 0.94 | 56.76 | 1.0 | 0.57 |
| 699.67 | 0.91 | 0.89 | 514.43 | 0.91 | 0.95 | 113.33 | 0.91 | 0.89 |
| 823.55 | 0.64 | 0.90 | 688.65 | 0.73 | 0.97 | 125.62 | 0.73 | 0.91 |
aAPP, amyloid precursor protein; Conc., candidate biomarker concentration (in ng/mL); L1CAM, L1 cell adhesion molecule; sAPPα, soluble amyloid precursor protein α; sens., sensitivity; spec., specificity.
TABLE 4.
| APP | sAPPα | L1CAM | |
|---|---|---|---|
| P | 0.0006 | 0.0019 | 0.0006 |
| ROC AUC | 0.954 | 0.981 | 0.921 |
| Cut point (ng/mL) | 699.67 | 514.43 | 113.33 |
| Odds ratio (95% CI) | 80.00 (8.9-722.5) | 200.00 (18.9-2118.4) | 68.75 (7.7-611.4) |
| Relative risk (95% CI) | 33.5 (4.6-243.6) | 46.92 (6.6-335.3) | 31.1 (4.3-226.6) |
aAPP, amyloid precursor protein; CI, confidence interval; ROC AUC, receiver operating characteristics area under the curve; CI, confidence interval; L1CAM, L1 cell adhesion molecule; sAPPα soluble amyloid precursor protein α.
bBonferroni corrected threshold (0.0055).
DISCUSSION
In recent years, biomarkers have been sought to facilitate the diagnosis of PHH and improve clinical management of this devastating condition (reviewed in Mehrar8). Previous work from our group has shown that ventricular CSF levels of APP, L1CAM, and other protein mediators of neurodevelopment are increased in PHH and resolve after initiation of neurosurgical treatment.7 CSF APP, in particular, also appears to be associated with ventricular size, and possibly intracranial pressure, in infants undergoing treatment for PHH.14 Along with much of the work in this field, these studies share the limitation of using lumbar CSF as the comparator for ventricular CSF obtained from children with PHH. Such a comparison introduces the potential for sampling artifact due to RCGs, which have been observed for specific proteins in a number of diseases.15,16,23,24 The objective of the current study was to eliminate the potential bias of RCGs and examine the levels of APP, L1CAM, and related proteins using lumbar CSF from infants with PHH and other neurological conditions relevant to the neonate.
Studies into RCGs suggest that the levels of brain-derived proteins (eg, neuron-specific enolase and S100B) remain consistent regardless of access site, whereas blood-derived proteins (eg, albumin and β-trace) tend to be higher in lumbar CSF.25 These findings are supported by recent proteomic analysis.26 However, at least 1 study found differential abundance of amyloid isoforms (lumbar levels higher) and tau isoforms (ventricular levels higher) in normal-pressure hydrocephalus, with albumin levels highest in lumbar samples.16 The etiology of RCGs remains uncertain, although investigators have speculated that the gradients reflect diffusion or transport of serum proteins into the CSF, or CSF proteins into the blood, as a function of distance from the brain (the primary site of brain-derived proteins).16,23
The findings presented in this study provide convincing evidence that lumbar CSF levels of APP, sAPPα, and L1CAM are elevated in untreated PHH compared with neonates without neurological injury and those with IVH alone (grades I-II and grades III-IV), HIE, and VEWOH. Related APP derivatives sAPPβ and Aβ42, as well as tau and p-tau, did not demonstrate the same degree of selectivity for PHH. NCAM-1 was increased in both PHH and grade III to IV IVH, possibly because of its high concentration in plasma. Thus, in this study of relatively small sample size, CSF APP, sAPPα, and L1CAM appeared the most appropriate CSF biomarkers of PHH. The strong predictive ability of APP, L1CAM, and, in particular, sAPPα, for PHH is an important and entirely novel finding.
The high levels of TP observed in PHH in our lumbar CSF samples will not surprise clinicians who treat PHH regularly. In many centers, serial LPs are used as a temporizing measure for PHH until the condition resolves or the infant undergoes a more definitive neurosurgical procedure, and large increases in TP are expected in this setting. To our knowledge, a comparison of TP in lumbar CSF of infants with IVH alone, PHH (or other neurological conditions), or adults with hydrocephalus, has not been reported previously.
In addition to any potential role in PHH diagnosis or clinical management, CSF biomarkers of PHH give important insight into the pathophysiology of this condition. PHH afflicts preterm infants during the critical developmental period corresponding to 23 weeks PMA to term-equivalent age and beyond. Seminal neurodevelopmental events, including precursor migration and differentiation, synaptogenesis, and the generation of neural networks occur during this interval and are almost certainly affected by PHH. As noted previously, many of the CSF biomarkers involved in PHH are protein mediators of neurodevelopment.7 For example, L1CAM is involved in neuronal migration, axonal growth, and synaptogenesis.27-32 Indeed, mutations in L1CAM have been linked to the debilitating conditions of mental retardation, aphasia, spastic paraplegia, adducted thumbs (MASA syndrome), X-linked hydrocephalus, and corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraplegia, and hydrocephalus (CRASH syndrome).33-37
APP has long been known to be increased in the setting of axonal injury or stretch,38 but accumulating evidence also strongly supports that APP is an important trophic factor, with roles in neural stem cell development, neurite outgrowth and synaptogenesis, and neural repair.39 Posttranslational APP processing is complex and may occur at a number of stages, each of which affects the ultimate fate and biological activity of APP and its derivatives. Perhaps the most critical processing point is the cleavage of cell surface APP by α- or β-secretase. If cleaved by α-secretase (a disintegrin and metalloproteinase), sAPPα is generated; if cleaved by β-secretase (β-site APP-cleaving enzyme 1), sAPPβ results.40 Although β-site cleavage and sAPPβ are associated with downstream production of amyloid-β1-42, the primary component of neuritic plaques in Alzheimer disease, sAPPα appears to function as a trophic factor for neural precursor cell development, neurite growth and synapse formation, and even neuronal growth, survival, and repair (reviewed in Dawkins and Small39). The results from this study clearly demonstrated specific increases in CSF sAPPα in PHH, and, although it is possible that sAPPα may simply result from the neurological injury itself, it is intriguing to consider that sAPPα may be serving a neuronal survival or repair function.
It is worth noting that in adults, sAPPα and sAPPβ, but not tau and p-tau, are also diagnostic biomarkers for distinguishing between idiopathic normal-pressure hydrocephalus and Alzheimer disease.41 Specifically, the cutoff value for predicting cognitive outcomes in Miyajima et al's41 study was 198 ng/mL, which is considerably lower than our value of 514 ng/mL for sAPPα. In a related study, Pyykko et al42 concluded that increases in APP isoforms were independent of neuroinflammation mechanisms in patients with idiopathic normal-pressure hydrocephalus. This finding lends further support to our contention that the trophic effects of APP are important for functional outcome.
The role of neuroinflammation in PHH is an active area of study for our laboratory and others.43-48 Intraventricular hemorrhage with or without PHH may incite inflammatory processes within the CSF and within the central nervous system more broadly, potentially creating a chemical meningitis. To explore the influence of neuroinflammation in PHH, we analyzed the relationship between our candidate CSF biomarker levels and the CSF cell count and differential analysis results (including total cells, nucleated cells, red blood cells, neutrophils, lymphocytes, monocytes, eosinophils, and macrophages) in the CSF samples tested. In this relatively small series, no significant relationships existed between CSF APP, sAPPα, sAPPβ, Aβ42, NCAM-1, L1CAM, tau, p-tau, and differential cell counts in CSF, with the exception of CSF L1CAM and neutrophils (P = .022) and monocytes (P = .025). This is not entirely surprising, because the biomarkers investigated in this study are primarily associated with neurodevelopment and neurological injury, rather than inflammation. One relevant exception to this generalization is L1CAM, which has recently been shown to be induced by transforming growth factor-β149 and have a signaling role in inflammation via nuclear factor-κB.50
Limitations
A number of limitations in this study must be acknowledged. In this study of human newborn infant lumbar CSF samples, there were no true “control” infants; even those subjects with no known neurological injury were born prematurely and subject to the influence of myriad clinical factors that could potentially confound the results (eg, clinical status concerning for sepsis, long periods of ventilation). Additionally, in utero or developmental events and pre- and perinatal complications (preterm labor, rupture of membranes, breech presentation, placental abruption) could potentially influence CSF protein levels in these very preterm infants. In the analysis of our specimens, spectrum bias may have attenuated the comparison between conditions, particularly between IVH III to IV and PHH. Similarly, the degree of ventricular enlargement in our VEWOH samples was modest, again introducing the possibility of spectrum bias and limiting the ability to rigorously test the effect of ventricular enlargement alone (no hydrocephalus) on CSF biomarker levels. Of note, VEWOH and HIE infants had greater PMA at birth and higher birth weights than the other groups, limiting stringent comparisons between groups. Finally, although it would be ideal to examine biomarker levels in both ventricular and lumbar CSF simultaneously, clinical and ethical challenges prevent this from being performed routinely in these preterm infants.
Future studies will focus on validating the results of this study in a larger collection of CSF samples and examining the levels of these candidate biomarkers of PHH (APP, sAPPα, and L1CAM) in parallel blood samples. We will also explore the relationship of these biomarkers to neurosurgical (shunt malfunction or infection, need for surgical revision, association with multiloculated hydrocephalus) and neurodevelopmental outcomes. Similarly, we will also investigate the levels of these and related biomarkers in hydrocephalus of other etiologies.
CONCLUSION
In the current study, we report the novel observation that the lumbar CSF levels of APP, sAPPα, and L1CAM are specifically and significantly elevated in untreated PHH. In addition to their potential role as diagnostic biomarkers, these proteins provide novel insights into the pathophysiology of PHH and possible mechanisms of neural injury and neural repair.
Disclosures
Drs Limbrick and McAllister receive research funds and/or research equipment for unrelated research projects from Medtronic, Inc. (Minneapolis, Minnesota), Karl Storz, Inc. (Tuttlingen, Delaware), andAesculap, Inc. (Center Valley, Pennsylvania). Dr Limbrick has received philanthropic equipment contributions for humanitarian relief work from Karl Storz, Inc. and Aesculap, Inc. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
This project was supported by a career development award to Dr Limbrick (NIH/NINDS K23 NS075151), the Washington University Intellectual and Developmental Disabilities Research Center (NIH/NICHD P30 HD0627171), and the Washington University Institute for Clinical and Translational Research (NIH CTSA award UL1 RR024992). Additional support was provided through the Children's Surgical Sciences Institute, St. Louis Children's Hospital (to Dr Limbrick) and the Washington University Dean's Fund (S. A. Silver). Dr Morgan was supported through a Howard Hughes Medical Institute fellowship and Dr Rao was supported by the Women's and Infant's Health Specimen Consortium.
Acknowledgments
We would like to express our profound gratitude to the patients and families who graciously participated in this study and the teams of physicians and nurses that cared for them.
REFERENCES
- 1. Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121(5):e1167-e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stoll BJ, Hansen NI, Bell EF et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10): 1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Limbrick DD Jr, Mathur A, Johnston JM et al. Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr. 2010;6(3):224-230. [DOI] [PubMed] [Google Scholar]
- 4. Murphy BP, Inder TE, Rooks V et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87(1):F37-F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vassilyadi M, Tataryn Z, Shamji MF, Ventureyra EC. Functional outcomes among premature infants with intraventricular hemorrhage. Pediatr Neurosurg. 2009;45(4):247-255. [DOI] [PubMed] [Google Scholar]
- 6. Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 2012;9 (3):242-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morales DM, Townsend RR, Malone JP et al. Alterations in protein regulators of neurodevelopment in the cerebrospinal fluid of infants with posthemorrhagic hydrocephalus of prematurity. Mol Cell Proteomics. 2012; 11(6):M111 011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merhar S. Biomarkers in neonatal posthemorrhagic hydrocephalus. Neonatology. 2012;101(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naureen I, Waheed Kh A, Rathore AW et al. Fingerprint changes in CSF composition associated with different aetiologies in human neonatal hydrocephalus: inflammatory cytokines. Childs Nerv Syst. 2014;30(7):1155-1164. [DOI] [PubMed] [Google Scholar]
- 10. Okamoto T, Takahashi S, Nakamura E et al. Increased expression of matrix metalloproteinase-9 and hepatocyte growth factor in the cerebrospinal fluid of infants with posthemorrhagic hydrocephalus. Early Hum Dev. 2010;86(4):251-254. [DOI] [PubMed] [Google Scholar]
- 11. Schmitz T, Heep A, Groenendaal F et al. Interleukin-1beta, interleukin-18, and interferon-gamma expression in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus–markers of white matter damage? Pediatr Res. 2007;61(6):722-726. [DOI] [PubMed] [Google Scholar]
- 12. Felderhoff-Mueser U, Buhrer C, Groneck P, Obladen M, Bartmann P, Heep A. Soluble Fas (CD95/Apo-1), soluble Fas ligand, and activated caspase 3 in the cerebrospinal fluid of infants with posthemorrhagic and nonhemorrhagic hydrocephalus. Pediatr Res. 2003;54(5):659-664. [DOI] [PubMed] [Google Scholar]
- 13. Heep A, Stoffel-Wagner B, Bartmann P et al. Vascular endothelial growth factor and transforming growth factor-beta1 are highly expressed in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus. Pediatr Res. 2004; 56(5):768-774. [DOI] [PubMed] [Google Scholar]
- 14. Morales DM, Holubkov R, Inder TE et al. Cerebrospinal fluid levels of amyloid precursor protein are associated with ventricular size in post-hemorrhagic hydrocephalus of prematurity. PLoS One. 2015;10(3):e0115045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tarnaris A, Toma AK, Chapman MD et al. Rostrocaudal dynamics of CSF biomarkers. Neurochem Res. 2011;36(3):528-532. [DOI] [PubMed] [Google Scholar]
- 16. Brandner S, Thaler C, Lelental N et al. Ventricular and lumbar cerebrospinal fluid concentrations of Alzheimer's disease biomarkers in patients with normal pressure hydrocephalus and posttraumatic hydrocephalus. J Alzheimers dis. 2014;41(4): 1057-1062. [DOI] [PubMed] [Google Scholar]
- 17. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatrics. 1978;92(4):529-534. [DOI] [PubMed] [Google Scholar]
- 18. O'Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29(5):245-249. [DOI] [PubMed] [Google Scholar]
- 19. Wellons JC III, Holubkov R, Browd SR et al. The assessment of bulging fontanel and splitting of sutures in premature infants: an interrater reliability study by the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 2013;11(1):12-14. [DOI] [PubMed] [Google Scholar]
- 20. Bonadio WA, Smith DS, Goddard S, Burroughs J, Khaja G. Distinguishing cerebrospinal fluid abnormalities in children with bacterial meningitis and traumatic lumbar puncture. J Infect Dis. 1990;162(1):251-254. [DOI] [PubMed] [Google Scholar]
- 21. Glatstein MM, Zucker-Toledano M, Arik A, Scolnik D, Oren A, Reif S. Incidence of traumatic lumbar puncture: experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila). 2011;50(11):1005-1009. [DOI] [PubMed] [Google Scholar]
- 22. Nigrovic LE, Kuppermann N, Neuman MI. Risk factors for traumatic or unsuccessful lumbar punctures in children. Ann Emerg Med. 2007;49(6): 762-771. [DOI] [PubMed] [Google Scholar]
- 23. Huhmer AF, Biringer RG, Amato H, Fonteh AN, Harrington MG. Protein analysis in human cerebrospinal fluid: physiological aspects, current progress and future challenges. Dis Markers. 2006;22(1-2):3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310(2):173-186. [DOI] [PubMed] [Google Scholar]
- 25. Brandner S, Thaler C, Lewczuk P, Lelental N, Buchfelder M, Kleindienst A. Neuroprotein dynamics in the cerebrospinal fluid: intraindividual concomitant ventricular and lumbar measurements. Eur Neurol. 2013;70(3-4):189-194. [DOI] [PubMed] [Google Scholar]
- 26. Aasebo E, Opsahl JA, Bjorlykke Y, Myhr KM, Kroksveen AC, Berven FS. Effects of blood contamination and the rostro-caudal gradient on the human cerebrospinal fluid proteome. PLoS One. 2014;9(3):e90429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10(1):19-26. [DOI] [PubMed] [Google Scholar]
- 28. Panicker AK, Buhusi M, Thelen K, Maness PF. Cellular signalling mechanisms of neural cell adhesion molecules. Front Biosci. 2003;8:d900–911. [DOI] [PubMed] [Google Scholar]
- 29. Schmid RS, Maness PF. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol. 2008;18(3):245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schafer MK, Nam YC, Moumen A et al. L1 syndrome mutations impair neuronal L1 function at different levels by divergent mechanisms. Neurobiol Dis. 2010;40(1):222-237. [DOI] [PubMed] [Google Scholar]
- 31. Kamiguchi H. The mechanism of axon growth: what we have learned from the cell adhesion molecule L1. Mol Neurobiol. 2003;28(3):219-228. [DOI] [PubMed] [Google Scholar]
- 32. Dityatev A, Bukalo O, Schachner M. Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron Glia Biol. 2008;4(3): 197-209. [DOI] [PubMed] [Google Scholar]
- 33. Fransen E, Lemmon V, Van Camp G, Vits L, Coucke P, Willems PJ. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur J Hum Genet. 1995;3(5):273-284. [DOI] [PubMed] [Google Scholar]
- 34. Fransen E, Van Camp G, Vits L, Willems PJ. L1-associated diseases: clinical geneticists divide, molecular geneticists unite. Hum Mol Genet. 1997;6(10): 1625-1632. [DOI] [PubMed] [Google Scholar]
- 35. Kanemura Y, Okamoto N, Sakamoto H, Shofuda T, Kamiguchi H, Yamasaki M. Molecular mechanisms and neuroimaging criteria for severe L1 syndrome with X-linked hydrocephalus. J Neurosurg. 2006;105(5 suppl):403-412. [DOI] [PubMed] [Google Scholar]
- 36. Weller S, Gartner J. Genetic and clinical aspects of X-linked hydrocephalus (L1 disease): mutations in the L1CAM gene. Hum Mutat. 2001;18(1):1-12. [DOI] [PubMed] [Google Scholar]
- 37. Jouet M, Kenwrick S. Gene analysis of L1 neural cell adhesion molecule in prenatal diagnosis of hydrocephalus. Lancet. 1995;345(8943):161-162. [DOI] [PubMed] [Google Scholar]
- 38. Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160(2):139-144. [DOI] [PubMed] [Google Scholar]
- 39. Dawkins E, Small DH. Insights into the physiological function of the beta-amyloid precursor protein: beyond Alzheimer's disease. J Neurochem. 2014;129(5): 756-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci. 2011;34:185-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyajima M, Nakajima M, Ogino I, Miyata H, Motoi Y, Arai H. Soluble amyloid precursor protein alpha in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. Eur J Neurol. 2013;20(2):236-242. [DOI] [PubMed] [Google Scholar]
- 42. Pyykko OT, Lumela M, Rummukainen J et al. Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS One. 2014;9(3):e91974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lattke M, Magnutzki A, Walther P, Wirth T, Baumann B. Nuclear factor kappaB activation impairs ependymal ciliogenesis and links neuroinflammation to hydrocephalus formation. J Neurosci. 2012;32(34):11511–11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McAllister JP., II Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med. 2012;17(5):285–294. [DOI] [PubMed] [Google Scholar]
- 45. Deren KE, Packer M, Forsyth J et al. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol. 2010;226(1):110-119. [DOI] [PubMed] [Google Scholar]
- 46. Ryckman KK, Dagle JM, Kelsey K, Momany AM, Murray JC. Replication of genetic associations in the inflammation, complement, and coagulation pathways with intraventricular hemorrhage in LBW preterm neonates. Pediatr Res. 2011;70(1):90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gram M, Sveinsdottir S, Cinthio M et al. Extracellular hemoglobin—mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J Neuroinflammation. 2014;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gram AS, Skov J, Thorkil P, Sidelmann JJ, Stallknecht BM, Bladbjerg EM. Biomarkers of coagulation, fibrinolysis, endothelial function, and inflammation in arterialized venous blood. Blood Coagul Fibrinolysis. 2014;25(4):349-352. [DOI] [PubMed] [Google Scholar]
- 49. Schafer H, Struck B, Feldmann EM et al. TGF-beta1-dependent L1CAM expression has an essential role in macrophage-induced apoptosis resistance and cell migration of human intestinal epithelial cells. Oncogene. 2013;32(2): 180–189. [DOI] [PubMed] [Google Scholar]
- 50. Kiefel H, Pfeifer M, Bondong S, Hazin J, Altevogt P. Linking L1CAM-mediated signaling to NF-kappaB activation. Trends Mol Med. 2011;17(4):178-187. [DOI] [PubMed] [Google Scholar]


