To the Editor
With the advent of clustered, regularly interspaced, short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) technology1,2, researchers can construct gene drives that can bias the inheritance of edited alleles to alter entire populations3,4. As demonstrated with the mutagenic chain reaction in Drosophila4, the CRISPR-Cas9 system can propagate genomic modification together with the genome- editing machinery itself. Although gene drives might have the potential to control insect-borne diseases and agricultural pests5, substantial concerns have been raised over unanticipated ecological consequences as a result of drive use6. Here we report the development of a potential Cas9-based gene drive ‘brake’ that remains inert in a wild- type genome but is activated by Cas9 to both cleave the genomic cas9 sequence and to convert an incoming cas9 allele into a brake. This means that the propagation of the brake is favored in a cas9-carrying population.
We designed and synthesized a transgene system that we named Cas9-triggered chain ablation (CATCHA). The CATCHA transgene encodes a guide RNA (gRNA) that is expressed ubiquitously from a U6:2 promoter. The gRNA targets a site within the DNA sequence of cas9. The guide RNA is flanked by homology arms (of 1,042 bp and 1,003 bp) that match the cas9 sequences next to the gRNA-specified cleavage site (Fig. 1a). In the presence of both CATCHA and cas9, Cas9 proteins will be guided to cleave the cas9 genomic locus from which Cas9 proteins are expressed. Upon repair of the cleaved cas9 by homology-directed repair (HDR), the cas9 locus will be converted to CATCHA. Such conversion in heterozygous offspring favors amplification of CATCHA in the cas9- carrying population (Fig. 1a).
Figure 1.
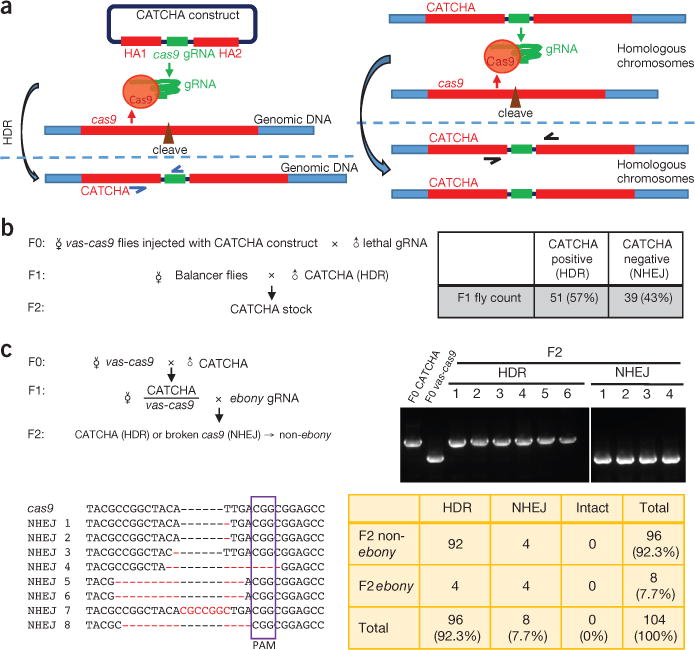
Schematics and proof of principle of CATCHA. (a) Left, the CATCHA construct encodes a gRNA targeting cas9, flanked by homology arms (HAI and HA2) corresponding to cas9 sequences next to the cleavage site specified by the gRNA (see Supplementary Methods for details). In the presence of genomically encoded Cas9, the CATCHA construct is integrated into the target locus via HDR. Successful HDR events can be identified by PCR with suitable primers (blue). Right, the genomically integrated CATCHA can further convert allelic cas9 sequences to CATCHA via HDR. Indels and converted alleles can be distinguished with PCR primers (black arrows). Primer information is available in Supplementary Methods. (b) Integrating CATCHA into the genome. vas-cas9 donor females injected with the CATCHA construct were crossed to males expressing gRNA targeting wg to select for ablated cas9 alleles. PCR was performed for each F1 survivor to identify CATCHA-positive progenies and establish stable stocks. NHEJ is presumed on the basis of PCR results. Balancers are fly chromosomes with multiple inversions that impede recombination and thereby maintain transgenes and mutations in stable stocks. (c) Converting genomic cas9 using genomic CATCHA. All 104 F2 offspring from one CATCHA male crossed to vas-cas9 females were genotyped using PCR. Representative DNA gel lanes are shown (top right). F0 CATCHA and F0 vas-cas9 are controls indicating the respective sizes of CATCHA (~1,200 bp) and NHEJ (~650 bp) bands. In sum, 96 F2 flies carried CATCHA, suggesting a conversion rate of 85%, estimated by the formula (CATCHA - total × 50%) / (total × 50%). All eight F2 flies with short bands (non-HDR) were sequenced (bottom left) and confirmed to carry NHEJ-mediated indels (nucleotides differing from cas9 sequence are shown in red). The four indels that maintain the reading frame (NHEJ 5–8) correspond to the four ebony flies. The purple box highlights the protospacer adjacent motif (PAM) sequence. See Supplementary Table 1 for the summary of all F2 phenotypes.
In a proof-of-principle experiment, we used CATCHA in vas-cas9 transgenic flies, which are commonly used for CRISPR- based transgenesis7, and in which Cas9 likely follows endogenous vas expression in the germline throughout development and is maternally deposited into embryos8,9. To introduce the CATCHA transgene into the genome, we injected vas-cas9 embryos with a plasmid carrying CATCHA (Fig. 1a, Supplementary Fig. 1 and Supplementary Methods). To enrich for converted alleles, the resultant F0 females were crossed to transgenic males that stably express gRNA that targets the essential gene wg10. This way, all surviving F1 progeny would carry ablated cas9 (Fig. 1b). Flies that still express functional Cas9 cannot survive owing to the engagement of the lethal gRNA. We found that the cas9 locus was converted to CATCHA in 57% (51 out of 90) of F1 progeny (Fig. 1b) as assayed by PCR (Fig. 1a), demonstrating that CATCHA can convert cas9, even with extragenomic delivery. The rest of the surviving F1 population (39 flies) are likely to carry cas9 loci with deletions or insertions (indels) resulted from nonhomologous end joining (NHEJ).
Next, we tested whether genomically encoded CATCHA can efficiently convert allelic vas-cas9 to CATCHA (Fig. 1a). We crossed five independent F0 CATCHA males to vas-cas9 females (Fig. 1c). To estimate the rate of cas9 ablation in one generation, we crossed F1 females to transgenic males stably expressing gRNA against the e (ebony) gene10, the disruption of which will result in a markedly darkened cuticle (Supplementary Fig. 2). The emergence of F2 flies with wild-type cuticle color (and thus, failed disruption of the ebony gene) is an indicator for the efficiency of CATCHA- mediated cas9 ablation. Overall, 93.4% of F2 cuticles had wild-type color, demonstrating successful impairment of cas9 function (Supplementary Table 1). To determine the nature of ablated cas9 alleles, we genotyped all F2 descendants of the first CATCHA male using PCR followed by sequencing (Fig. 1c). Of the 104 F2 flies, 96 carried either direct replication of the F0 CATCHA allele or HDR-mediated conversion of vas-cas9, indicating that 85% of vas-cas9 alleles were successfully converted to CATCHA (Fig. 1c). In addition, all eight non-CATCHA F2 flies carried NHEJ-mediated indels (Fig. 1c). Thus, genomic CATCHA is highly efficient in inactivating cas9 and converting most alleles to CATCHA.
In summary, we propose here a chain reaction named CATCHA that could potentially be used to inactivate genomic cas9 in insect populations and demonstrate that CATCHA can inactivate cas9 to near completion in a laboratory population of flies. However, it is important to note that further improvements and analyses will be required to establish whether CATCHA, or a similar approach, might be suitable for eventual use in either the laboratory or the field. First, it is worth testing whether cas9 can be inactivated using a nonallelic CATCHA. Given that gRNAs function in trans, we anticipate highly efficient cleavage regardless of CATCHA’s locus, as suggested by our own data (0 out of 104 F2 carried intact cas9 sequences, Fig. 1c) and previous work4,10. Although such a strategy would not provide an allelic template to convert the drive, it is much more convenient, as one premade CATCHA released at high frequency could be used to target different gene drives. Second, a small percentage of in-frame indels (Supplementary Table 1) might produce CATCHA-resistant alleles that encode functional Cas9 proteins; in that case, it might be possible to modify CATCHA to include two gRNAs to mediate double cleavage, deleting a longer segment of the cas9 coding sequence. Third, the interactions between a Cas9-based gene drive, CATCHA and wild-type alleles could be affected by the frequency of each of them in the population and the carrier insects’ fitness. To understand the efficacy of CATCHA under different scenarios, it will be necessary to estimate population dynamics using mathematical modeling. Lastly, field experiments would clearly need to be performed before deployment of CATCHA in any ecosystem is contemplated.
We envision that an improved version of CATCHA might be useful in selected situations. In a facility that is used to house cas9-based gene drive animals, it might be beneficial to maintain CATCHA animals in the same space; the latter might be able to neutralize the former in the event of mass escape, for example, that is caused by a catastrophe, such as an earthquake. Alternatively, if a cas9-based gene drive is already present in a wild population, CATCHA animals might be released into the wild to potentially function as a ‘cordon’ to contain cas9 propagation, followed by other sophisticated gene drives5 that could be added to remedy the damage. Scientists must be alert to the ethical implications of research6, but policies and regulations are not immune to human malice, negligence or natural disasters. Technical advancements based on this set of proof-of-principle experiments might offer a mechanism to reduce the spread of a Cas9-based gene drive through a population and thereby function as a potential safeguard against the unwanted consequences of such drives.
Supplementary Material
Acknowledgments
We thank D. Luginbuhl for generating transgenic flies, F. Port for providing gRNA flies, and J. Luo, T. Mosca, X. Wang, J. Li and J. Lui for advice and critical comments. This study was supported by an NIH grant (R01-DC005982) to L.L. X.J.G. was supported by an Enlight Foundation Interdisciplinary Fellowship. L.L. is an HHMI investigator.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper (doi:10.1038/nbt.3444).
Accession codes. GenBank: The plasmid sequence of CATCHA has been deposited under accession number KU212289. The sequencing results of eight NHEJ alleles have been deposited under accession numbers KU212290, KU212291, KU221292, KU221293, KU221294, KU221295, KU221296 and KU212297.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Hsu PD, Lander ES, Zhang F. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doudna JA, Charpentier E. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 3.DiCarlo JE, et al. bioRxiv. 2015 doi: 10.1101/013896. [DOI] [Google Scholar]
- 4.Gantz VM, Bier E. Science. 2015;348:442–444. doi: 10.1126/science.aaa5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esvelt KM, Smidler AL, Catteruccia F, Church GM. Elife. 2014 Jul 17; doi: 10.7554/eLife.03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbari OS, et al. Science. 2015;349:927–929. doi: 10.1126/science.aac7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gratz SJ, et al. Genetics. 2014;196:961–971. doi: 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay B, Jan LY, Jan YN. Cell. 1988;55:577–587. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- 9.Lasko PF, Ashburner M. Genes Dev. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- 10.Port F, Chin HM, Lee T, Bullock SL. Proc Natl Acad Sci USA. 2014;111:E2967–E2976. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


