Abstract
We provide a systematic review to support the European Palliative Care Research Collaboration development of clinical guidelines for cancer patients suffering from cachexia. CENTRAL, MEDLINE, PsycINFO, ClinicalTrials.gov, and a selection of cancer journals have been searched up until 15 April 2016. The systematic literature research yielded 4214 publications with 21 of these included in the final evaluation. Regarding minerals, our search identified only one study examining the use of magnesium with no effect on weight loss. As far as vitamins are concerned, vitamin E in combination with omega‐3 fatty acids displayed an effect on survival in a single study, vitamin D showed improvement of muscle weakness in prostate cancer patients, and vitamin C supplementation led to an improvement of various quality of life aspects in a sample with a variety of cancer diagnoses. For proteins, a combination therapy of β‐hydroxy‐β‐methylbutyrate (HMB), arginine, and glutamine showed an increase in lean body mass after 4 weeks in a study of advanced solid tumour patients, whereas the same combination did not show a benefit on lean body mass in a large sample of advanced lung and other cancer patients after 8 weeks. L‐carnitine led to an increase of body mass index and an increase in overall survival in advanced pancreatic cancer patients. Adverse effects of food supplementation were rare and showed mild intensity. There is not enough solid evidence for the use of minerals, vitamins, proteins, or other supplements in cancer. No serious adverse effects have been reported with dietary supplementation.
Keywords: Cancer cachexia, Minerals, Vitamins, Micronutrients, Dietary supplements, Systematic review, Guidelines
Introduction
Cachexia is often seen in cancer patients in advanced stages of the disease. The European Palliative Care Research Collaborative has defined cancer‐related cachexia as follows: ‘Cancer cachexia is a multi‐factorial syndrome defined by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. The pathophysiology is characterized by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism’.1 With regard to the underlying causes, there is an interplay between systemic inflammation and hypermetabolism due to neoplasma, nutritional and/or intake factors related to tumour or treatment induced anorexia, changes in physiological uptake and/or storage and biopsychosocial aspects of functional impairment.2
The aetiology of micronutrient deficiency is multifaceted. Cancer may impede the usual intake of micronutrients. In addition, inflammatory activity and gastrointestinal symptoms of the cancer itself or the catabolic effect of the anti‐neoplastic therapy may result in malnutrition, which also reduces micronutrient intake.3 Lack of dietary supplements4, 5 may also play a role in cancer aetiology, and supplementation with these elements has been put forward as a preventive measure. Against this backdrop, there is an ongoing discussion on the need for dietary supplementation with micronutrients such as vitamins, minerals, proteins, or certain trace elements.6 However, there is no clear indication for the importance of these substances for treatment of cachexia or cachexia‐related symptoms. Therefore, expert guidelines from the American Cancer Society, the World Cancer Research Fund, and the American Institute for Cancer Research advise patients with cancer against the use of food supplements and advocate obtaining nutrients from normal food intake whenever possible.7, 8 Nevertheless, the American Cancer Society guide for informed choices describes a probable benefit when taking a standardized food supplement containing multiple vitamins and minerals during and after cancer treatment in order to cover the daily demand, even though the daily requirement of micronutrients for a cancer patient is not known. The Cancer Society argues that this demand could not be covered because of loss of appetite, maldigestion, or malabsorption as a consequence of tumour or treatment side effects.9 To date this recommendation is based on weak evidence.
In our systematic review, the term ‘food supplements’ or the synonymously used term ‘dietary supplements’ is based on the definition of the European Food Safety Authority: ‘Food supplements are concentrated sources of nutrients or other substances with a nutritional or physiological effect, whose purpose is to supplement the normal diet. Food supplements are marketed ‘in dose’ form, for example as pills, tablets, capsules, or liquids in measured doses etc. Supplements may be used to correct nutritional deficiencies or maintain an adequate intake of certain nutrients’.10
In a large survey on food supplements, 73% of cancer patients had used supplements in the past month reporting a significant decrease in appetite loss;11 67 subjects (29.8%) had breast cancer, 40 (17.8%) had colorectal cancer, 32 (14.2%) had lung cancer, and 86 (38.2%) had other forms of cancer.
As part of the development of guidelines for the treatment of cachexia in cancer patients, the European Palliative Care Research Collaborative performed a Delphi procedure on a set of guideline statements.1 Two statements where no consensus was reached were used as starting points for a systematic review. Treatment of cachexia in advanced cancer patients using fish oil was subject of another systematic review prepared by Ries et al.2 The guideline on dietary supplements stated that there is not enough evidence for a general recommendation. Patients who are not able to consume the recommended daily amount of minerals, vitamins, and proteins may try to compensate this deficit with supplements. However, the proposal failed to reach an adequate level of consensus, and a systematic review was commissioned accordingly.
We aimed to evaluate the efficacy of vitamin, mineral, proteins, and dietary supplements for cachexia in cancer patients.
Methods
This review is part of the development of clinical practice guidelines of the European Palliative Care Research Centre (PRC) on the treatment of cachexia in patients with cancer.
Criteria for considering studies in this review
The review included studies comparing treatment with or without vitamin, mineral, proteins, or other dietary supplements in cancer patients suffering from cachexia or cachexia‐related symptoms. Studies comparing different supplements were also included. Publications were excluded if they reported on animals, children, or non‐cancer patients.
Perioperative treatment of cachectic patients for curative or palliative surgery with minerals, vitamins, or other supplements was not the primary focus of the review. These studies were included, but evaluated separately.
Studies were included if they included cancer patients with cachexia, indicated by weight loss >5% in 6 months, ongoing hypermetabolism and/or reduced food intake.
A spreadsheet was designed with data from each included trial. Information on study design, study size by means of patient number, setting, study limitations, patient characteristics, outcome measures, and results were entered and evaluated. A meta‐analysis was not possible as a variety of outcome measures were used, and study designs were not comparable.
A recommendation according to the GRADE methodology (positive or negative and strong or weak recommendation)12, 13 was drafted from the evidence of the reviewed literature.
Search methods for identification of studies
To identify studies, we developed a detailed search strategy (Appendix 1–3) for each electronic database and other resources. The search was restricted to publications in the English language. As a brief quality check for our search strategy, we selected two well‐known publications of high relevance for our review and checked whether these publications were covered by the search strategy.14, 15 Using this strategy, we could confirm the accuracy and validity of our literature search.
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) up until 15 April, 2016; search strategy as detailed in Appendix 1;
MEDLINE (OVID) from inception up until 15 April, 2016; search strategy as detailed in Appendix 2;
PsycINFO (OVID) from inception up until 15 April, 2016; search strategy as detailed in Appendix 3.
Searching other resources:
We screened the references of identified articles for additional studies. Published abstracts were also obtained through searches of ClinicalTrials.gov database and conference proceedings.
Data collection
Selection of studies
We retrieved in full all studies with an abstract referring to the subject of vitamins, minerals, proteins, or other dietary supplementations aimed at treating cachexia in cancer patients. Eligible studies had to define cachexia as an outcome measure.
Data extraction and management
Two authors (MM and M) extracted data (Figure 1) using a standard data extraction form and reviewed the data from the studies. Findings were cross‐checked in a second step by three autors (MM, RC, and CS). Four authors (LR, MMa, SK, and HC) cross‐checked a sub‐sample. We resolved disagreement by consensus.
Figure 1.
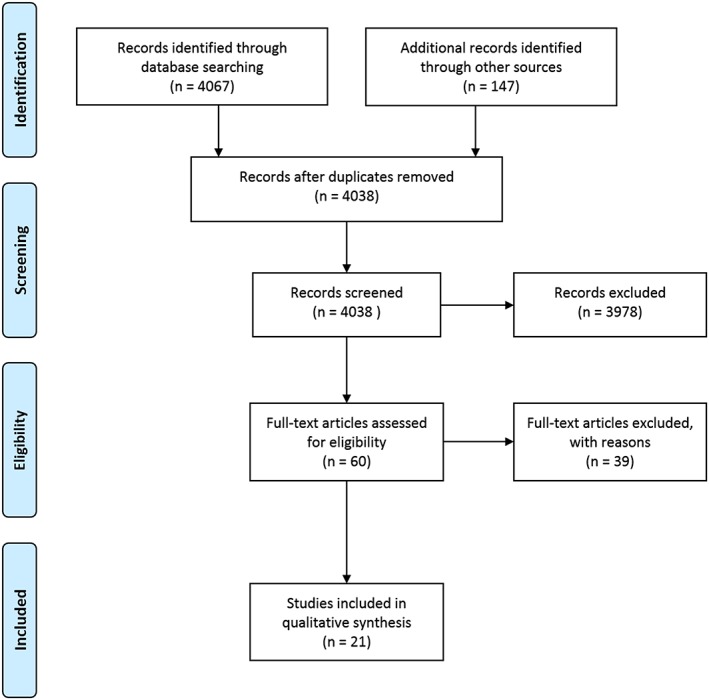
Study flow diagram.
Assessment of risk of bias in included studies
Two authors (MM and M) independently assessed risk of bias by the Cochrane risk of bias tool (Figures 2 and 3) for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions,16 with any disagreements resolved by discussion or by involving other review authors (LR, HC, and RC). We assessed the following for each study:
Figure 2.
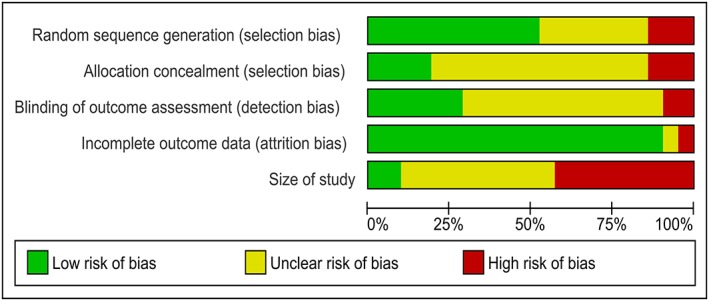
Risk of bias graph: review of authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.

Risk of bias summary: review of authors' judgements about risk of bias items for each included study.
Random sequence generation (checking for possible selection bias)
We assessed the method used to generate the allocation sequence as follows: low risk of bias (any truly random process, e.g. random number table; computer random number generator); and unclear risk of bias (method used to generate sequence not clearly stated).
Allocation concealment (checking for possible selection bias)
The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as follows: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, and opaque envelopes); and unclear risk of bias (method not clearly stated).
Blinding of outcome assessment (checking for possible detection bias)
We assessed the methods used to blind study participants and outcome assessors from the knowledge of which intervention a participant received. We assessed the methods as follows: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets, matched in appearance and smell); and unclear risk of bias (study states that it was blinded but does not provide an adequate description of how this was achieved).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcomedata)
We assessed the methods used to deal with incomplete data as follows: low risk (less than 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis); unclear risk of bias (used ‘last observation carried forward’ analysis); and high risk of bias (used ‘computer’ analysis).
Size of study (checking for possible biases confounded by small size)
We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50–199 participants per treatment arm); and high risk of bias (fewer than 50 participants per treatment arm).
Risk of bias in included studies
The findings are presented in the ‘Risk of bias’ graph (Figure 2), which reviews the authors' judgments about each risk of bias item shown as percentages across all included studies and the ‘Risk of bias’ summary (Figure 3), which reviews the authors' judgments about each risk of bias item for each included study.
Results
We screened 4214 publications. Twenty‐one papers were considered for final evaluation (Figure 1).
Trials of mineral supplements
The literature search identified one randomized controlled trial on the use of magnesium in 17 patients with advanced testicular cancer and weight loss but found no significant differences in weight loss between groups17 (Table 1).
Table 1.
Trials with minerals
| Study | Design | Supplement | Type of application | Number of patients | Cancer type | Setting | Assessed tissue | Outcome measure | Narrative summary of results | Adverse side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Willox et al. 198617 | RCT | Magnesium | i.v; tablet p.o. | 16 | Testicular; ovarian cancer | Magnesium supplementation (study group) vs. no supplementation (control group) in the course of treatment with cis‐diamminedichloroplatinum II (cis‐platin) over 14 months | Urine, blood | EORTC | After 14 months serum magnesium concentration was significantly higher in the study group (0.62 ± 0.009 vs. 0.50 ± 0.07; P < 0.01). Weight loss did not differ significantly between the groups; control group showed significantly greater renal tubular damage. | Discontinuation due to ‘metallic’ taste of magnesium; number of dropouts due to adverse effects not reported. |
EORTC, European Organization for Research and Treatment of Cancer; i.v., intravenous; p.o., per oral; RCT, Randomized controlled trial
Trials of vitamin supplements
Our literature search included one crossover study of 16 patients with advanced prostate cancer treated with vitamin D. Six patients reported improved muscle strength after vitamin supplementation.18
Vitamin C supplementation was tested in a sample of 39 patients with stomach (10), lung (7), liver (1), breast (4), cervix (1), colorectal (9), biliary (2), and other (5) cancer sites in terminal stage. Vitamin C was substituted intravenously and orally, and patients improved on different subscales of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30) including physical and cognitive function, appetite loss, fatigue, and nausea/vomiting.19
Treatment of 60 patients with generalized solid tumours (breast, gastrointestinal, lung, liver, and pancreas) with a combination of omega‐3 fatty acids and vitamin E did not have any effect on body weight compared with placebo.20 The combination showed significant increase in survival for all patients compared with the placebo group. However, the authors did not differentiate between the specific impact of vitamin E supplementation compared with omega‐3 fatty acids (Table 2).
Table 2.
Trials with vitamins
| Study | Design | Supplement | Type of application | Number of patients | Cancer type | Setting | Assessed tissue | Outcome measure | Narrative summary of results | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Yeom et al. 200719 | Prospective study | Vitamin C | i.v.; tablet p.o. | 39 | Various primary neoplasms | All patients received 10 g of vitamin C i.v. twice in 3 days and 4 g of vitamin C p.o. per day for a week. | Blood | EORTC | Pre‐treatment versus post‐treatment scores after 1 week supplementation showed improved global health (36 ± 18 vs. 55 ± 16; P = 0.001); patients reported significantly higher scores for physical (66 ± 20 vs. 72 ± 15, P = 0.037), role (59 ± 31 vs. 73 ± 22, P = 0.002), emotional (68 ± 24 vs. 78 ± 19, P = 0.001), and cognitive (69 ± 23 vs. 80 ± 16, P = 0.002) function and significantly lower scores for fatigue (52 ± 24 vs. 40 ± 19, P = 0.001), nausea/vomiting (24 ± 25 vs. 11 ± 15, P = 0.001), pain (30 ± 32 vs. 21 ± 25, P = 0.013), and appetite loss (50 ± 43 vs. 31 ± 29, P = 0.005). | None |
| Van Veldhuizen et al. 200018 | Phase‐II‐crossover‐study | Vitamin D | Liquid p.o. | 16 | Prostate cancer | All patients received Vitamin D 2000 units daily for 12 weeks following a 4 week placebo period | Blood | Muscle strength at enrollment and every 4 weeks; serum calcium and vitamin D measured at each visit | 12 weeks scores to 4 weeks scores (placebo period) showed no significant pre‐post‐treatment difference; reduced pain scores in four patients (25%) and improved muscle strength in six patients (37%). | None |
| Gogos et al. 199820 | RCT | Omega‐3 fatty acids plus vitamin E | Capsule p.o. | 60 | Various primary neoplasms | 18 g fish oil + 200 mg vitamin E (study group) vs. placebo (control group) daily until death | Blood | T‐cell‐subsets, cytokine production, nutritional response, Karnofsky index, survival | After 40 days, study group showed a significant increase in TNF‐α levels (369 ± 32 vs.784 ± 207, P < 0.05), Karnofsky index (51 ± 3 vs. 72 ± 4, P = 0.01) and a significant prolonged survival (no exact numbers presented; P = 0.025), while there was no effect on IL‐1, IL‐6, and body weight. | Mild abdominal discomfort; transient diarrhoea; number of dropouts due to adverse effects not reported. |
EORTC, European Organization for Research and Treatment of Cancer; IL, Interleukin; i.v., intravenous; p.o., per oral; RCT, Randomized controlled trial; TNF, Tumour Necrosis Factor
Trials with proteins and other dietary supplements
In a randomized controlled study of 32 cachectic advanced solid tumours (stage IV) patients from several types of cancer such as colon, ovarian, lung, pancreatic, and other cancer, May et al. tested a combination of β‐hydroxy‐β‐methylbutyrate (HMB), arginine, and glutamine and showed an overall benefit with an increase in lean body mass (LBM), improved mood, less weakness, and improved haematological parameters after 4 weeks compared with placebo21 (Table 3). A mixture of HMB, glutamine, and arginine or an isonitrogenous, isocaloric control was supplemented in 472 advanced lung and other cancer patients. However, there was no statistically significant difference in the 8 week LBM between the two arms.22
Table 3.
Trials with other dietary supplements or combinations
| Study | Design | Supplement | Type of application | Number of patients | Cancer type | Setting | Assessed tissue | Outcome measure | Narrative summary of results | Adverse effect |
|---|---|---|---|---|---|---|---|---|---|---|
| May et al. 200221 | RCT | HMB, arginine, and glutamine | Liquid p.o. | 32 | Various primary neoplasms | Treatment with HMB (3 g/day), l‐arginine 14 g/day), l‐glutamine (14 g/day [HMB/Arg/Gln]) (study group) vs. isonitrogenous mixture of nonessential amino acids (control group) over a 24 weeks period | Blood | Body weight; FFM | After 24 weeks of supplementation study group showed significant increase in body weight (2.27 ± 1.17 vs. 0.27 ± 1.39, P = 0.06) and FFM (1.6 ± 0.94 kg vs. 0.48 ± 1.08; P < 0.05). | None |
| Berk et al. 200822 | RCT | HMB, arginine | Liquid p.o. | 472 | Various primary neoplasms | Mixture of HMB, glutamine, arginine (study group) vs. an isonitrogenous, isocaloric mixture (control group) twice a day for 8 weeks | LBM | Post‐treatment measurement after 8 weeks supplementation showed no significant difference in lean body mass. | Nausea, constipation, and/or diarrhoea; 30 patients dropped out due to side effects | |
| Mantovani et al. 201014 | RCT | Megesterol, eicosapentaenoic acid, carnitine and thalidomide, plus polyphenol, lipoic acid, carbocysteine, vitamin E, vitamin A, and vitamin C orally | Tablet; liquid p.o. | 332 | Various primary neoplasms | 5 groups: (1) Megesterol, (2) eicosapentaenoic acid, (3) carnitine, (4) thalidomide, and (5) mixture of (1)–(4); additionally in all groups polyphenol, lipoic acid, carbocysteine, vitamin E, vitamin A, and vitamin C | Blood | LBM, REE, MFSI‐SF, IL‐6, TNF‐α, ECOG PS, Appetite VAS, EORTC QLQ‐C30, Euro QoL EQ‐5D | Post‐treatment measurement after 4 months supplementation showed that group 5 was superior to all other groups concerning increase in LBM (DEXA) (43.8 ± 9.4 vs. 44.9 ± 7.7; P = 0.015) and appetite (P = 0.0003). | Diarrhoea (2 patients) |
| Kraft et al. 201215 | RCT | l‐carnitine | Liquid p.o. | 72 | Pancreatic cancer | Oral l‐carnitine (4 g) (study group) vs. placebo (control group) for 12 weeks | Blood | BMI, EORTC‐QLQ‐C30, BFI | Post‐treatment measurement after 12 weeks supplementation showed increase of BMI in study group (3.4 ± 1.4% vs. −1.5 ± 1.4%, P < 0.05); trend towards increased overall survival in the study group (median 519 ± 50 d vs. 399 ± 43 d, P = n.s.), and reduced hospital‐stay (36 ± 4 days vs. 41 ± 9 days, P = n.s.). | Nausea (8 patients), diarrhoea (2 patients), which may have been caused by concomitant chemotherapy |
| Mantovani et al. 200623 | Phase II study with Simon two‐stage design | Polyphenol, antioxidant, pharmaco‐nutritional support enriched | Tablet; liquid p.o. | 39 | Various primary neoplasms | All patients received integrated treatment over 4 months with high polyphenols content, antioxidants (A‐lipoic acid, carbocysteine lysine salt, vitamin E, vitamin A, vitamin C), and pharmaco‐nutritional support enriched with two cans per day omega‐3 fatty acids, medroxyprogesterone acetate, and selective cyclooxygenase‐2 inhibitor celecoxib | Blood | Weight, LBM, Appetite, REE, Grip strength, laboratory, ECOG, EORTC QLQ‐C30, Euro QL‐5D, MFSI‐SF | Post‐treatment measurement after 4 months supplementation showed increase of body weight (55.1 ± 10 vs. 57 ± 9.8 kg, P = 0.031) as did LBM (38 ± 9 vs. 39.7 ± 8.7; P = 0.024), and appetite (5.5 ± 2.5 vs.7.0 ± 1.6; P = 0.004). | None |
| Hunter et al. 198924 | Prospective randomized trial | BCAA | i.v. | 9 | Intra‐abdominal carcinoma | All patients received both conventional TPN containing 19% BCAA (AA) and isocaloric, isonitrogenous TPN containing 50% BCAA (BCAA‐TPN) in random order for a minimum of 24 h | Blood, urine, breath sample | CO2, albumin, leucine, tyrosine | After a minimum supplementation of 24 h study group showed increased flux of leucine (158.0 ± 37.2 vs. 243.5 ± 75.8 µmol/kg h; P < 0.025) and tyrosine (35.0 ± 84 vs. 42.6 ± 11.0 µmol/kg h; P < 0.05) | None |
| Tayek et al.198625 | RCT | BCAA | i.v. | 10 | Intra‐abdominal carcinoma | All participants were given isonitrogenous amounts of both a conventional total parenteral nutrition (TPN) formula containing 19% BCAA a BCAA‐enriched TPN formula containing 50% of the amino acids as BCAA in a random order over 2–5 days. | Blood, urine | Protein kinetic, albumin synthesis | After 2–5 days, BCAA‐enriched formula group showed significant increases in whole body protein synthesis (2.2 ± 0.2 g protein/kg BW/day vs.3.9 ± 0.3; P < 0.005) and leucine balance (2.5 ± 0.4 g leucine/day vs. 6.5 ± 0.6; P < 0.001). | None |
| Yeh et al. 201326 | RCT | EE and isocal. Ethanwell contains several ingredients, including omega‐3 fatty acids, glutamine, selenium, and CoQ10. Ethanzyme is an enzyme product composed of multiple probiotics and vitamins. | Liquid p.o. | 68 | Head and neck cancer | Patients were randomly assigned to receive either EE supplement (study group) or Isocal supplement (control group) for a 3 month period | Blood | Body weight, serum albumin, prealbumin | After 8 weeks, EE regimen significantly improved body weight compared with controls (9.0 ± 1.8 vs. −7.3 ± 3.3; P < 0.05) as well as serum albumin (24.7 ± 9.5 vs. 2.8 ± 6.5; P < 0.05) and prealbumin levels (23.6 ± 7.8 vs. 6.1 ± 14.4; P < 0.05). | Some patients suffered from accumulating treatment‐related side effects (oral mucositis, emesis). Number of dropouts due to adverse effects not reported. |
AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; BCAA, Branched‐chain amino acid; BFI, Brief Pain Inventory; CO2, Carbon dioxide; ECOG, Eastern Cooperative Oncology Group performance status; EORTC, European Organization for Research and Treatment of Cancer; EORTC QLQ C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30; FAACT, Functional Assessment of Cancer Therapy; FFM, Fat free mass; HMB, ß‐hydroxy‐ß‐methylbutyrate; IL, Interleukin; i.v., intravenous; LBI, Lean body mass; MFSI‐SF, Multidimensional Fatigue Symptom Inventory–Short Form; p.o., per oral; REE, Resting Energy Expenditure; NAI, Neutrophil Adhesivity Index; RCT, Randomized Controlled Trial; TNF, Tumour Necrosis Factor; TPN, Total parenteral nutrition
Seventy‐two participants with advanced pancreatic cancer taking L‐carnitine showed an increase in body mass index (BMI) by 3.4 ± 1.4%; a decrease in BMI was observed in the control group. There was also a trend towards an increased overall survival in the L‐carnitine group and reduced hospital‐stay.15 In another controlled trial, 332 patients were randomized into five treatment arms, comparing megesterol, eicosapentaenoic acid, carnitine, and thalidomide with a combination of all four substances in the fifth arm.14 An analysis of pre‐treatment to post‐treatment changes showed that LBM significantly increased, while the resting energy expenditure decreased in the combination arm. Thus, study findings revealed that the combined supplementation was superior. Carnitine alone did not show any benefits.
In a small study of nine malnourished participants with intra‐abdominal cancer, participants received both conventional total parenteral nutrition (TPN) containing 19% branched‐chain amino acids (BCAA) and isocaloric, isonitrogenous TPN containing 50% BCAA (BCAA‐TPN).24 The trial showed that the fractional albumin synthesis rate increased significantly on daily BCAA‐TPN. Another study from Tayek et al. investigated the effect of a BCAA‐enriched solution in 10 malnourished patients with intra‐abdominal metastatic adenocarcinoma.25 The participants were given isonitrogenous amounts of both a conventional (TPN) formula containing 19% BCAA and a BCAA‐enriched TPN formula containing 50% of the amino acids as BCAA in a random order. BCAA‐enriched formulae group showed significant increases in whole body protein synthesis and leucine balance. Both studies demonstrated potential clinical benefits associated with BCAA‐enriched TPN in cancer cachexia patients.
Supplementation with combinations of antioxidants, vitamins, omega‐3 fatty acids, medroxyprogesterone acetate, and celecoxib23 was used in a study of 39 cancer patients. The study reported positive effects stabilizing or increasing weight, LBM, and appetite.
In another study, an Ethanwell/Ethanzyme (EE) regimen was investigated in 68 malnourished patients with head and neck cancer.26 Ethanwell is a protein‐dense and energy‐dense oral nutritional supplement that contains several ingredients including omega‐3 fatty acids, glutamine, selenium, and CoQ10. Ethanzyme is an enzyme product composed of multiple probiotics and vitamins. The result showed that an EE regimen improved body weight as well as serum albumin and prealbumin levels in head and neck cancer patients with a BMI <19. However, methodology in both abovementioned studies did not allow to differentiate the beneficial effects of the individual substances in the combination therapies.
Perioperative supplementation
Nine studies on the use of different combinations of arginine, glutamine, alanine, glycine, BCAA, omega‐3 fatty acids, and RNA in a perioperative setting were identified including a total of 791 cancer patients27, 28, 29, 30, 31, 32, 33, 34, 35 (Table 4). Two of these studies investigated patients with major weight loss at the time of admission.27, 30 In five studies,27, 30, 32, 34, 35 arginine was supplemented in different mixtures. Supplementation showed beneficial effects with regard to length of hospital stay,27, 34 postoperative infections,27 increase in BMI,35 and albumin, prealbumin, and lymphocyte levels.35 One study32 in 32 head and neck cancer patients also reported an overall long‐term survival (34.8 months vs. 20.7 months). In two studies, glutamine supplementation was investigated.29, 33 Improved nitrogene balance and intracellular glutamine concentration29 and shortened hospital stay33 were relevant clinical effects.
Table 4.
Trials with other dietary supplements or combinations in the perioperative setting
| Study | Design | Supplement | Type of application | Number of patients | Cancer type | Setting | Assessed tissue | Outcome measure | Narrative summary of results | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|
| Gianotti et al. 200227 | RCT | Arginine, omega‐3 fatty acids, and RNA | Liquid p.o. (preoperative); per jejunal feeding (postoperative) | 305 | Gastrointestinal Cancer | (1) Oral supplementation for 5 days before surgery with 1 L/day of a formula enriched with arginine, omega‐3 fatty acids, and RNA, with no nutritional support given after surgery; (2) Same preoperative treatment plus postoperative jejunal infusion with the same enriched formula; (3) Control group without supplementation | Blood | Incidence of postoperative infections, length of hospital stay | Supplementation significantly shortened length of hospital stay in group 1 vs. controls (11.6 ± 4.7 days vs. 14.0 ± 7.7; P = 0.008) and group 2 vs. controls (12.2 ± 4.1 days vs. 14.0 ± 7.7, P = 0.03) and less postoperative infections in the period up to 30 days after hospital discharge as measured by incidence (group 1 vs. controls 14 vs. 31, P = 0.006; group 2 vs. controls 16 vs. 31, P = 0.02) | Abdominal cramping/bloating (72 patients), diarrhoea (13 patients), vomiting (5 patients) |
| Stehle et al. 198929 | RCT | Glutamine and glycine | i.v. | 12 | Colon, rectum cancer | Study group was supplemented with a synthetic glutamine‐containing dipeptide, l‐alanyl‐l‐glutamine, alanine‐N. Control group received glycine‐N supplementation. | Urine, blood, biopsy of quadriceps femoris | Nitrogen balance and glutamine concentration | Cumulative nitrogen balance was significantly better in the study group on 5th post operative day (−7.1 ± 2.2 vs. −18.1 ± 1.7 g N/day; P < 0.001); muscle intracellular glutamine concentration was maintained in the study group, whereas it decreased in the control group (17.5 ± 1.0 vs. 12.0 ± 0.6 mmol/L, P < 0.001). | None |
| Snyderman et al. 199928 | RCT | Immune‐enhancing nutritional supplement from Novartis product (Impact, Replete) | Liquid p.o.; enteral | 136 | Squamous cell carcinoma of the oral cavity, pharynx, larynx | Patients were divided into 4 groups: (1) supplemented diet pre‐operative and post‐operative, (2) supplemented diet post‐operative, (3) standard diet pre‐operative and post‐operative, and (4) standard diet post‐operative | Blood | Dietary intake, changes in weight, laboratory evaluations of nutritional status, tolerance of tube feedings, infectious and wound healing complications, and duration of hospitalization | Significant decrease in the incidence of post‐operative infectious complications during hospitalization in intention to treat analysis in supplementation groups 1 + 2 vs. standard diet control groups 3 + 4 (23% vs. 45% incidence, P = 0.04) | None |
| van Bokhorst‐De Van Der Schueren et al. 200130 | RCT | Arginine | Liquid per tube feeding | 49 | Head and neck cancer | Patients were divided into 3 groups: (1) standard pre‐operative and post‐operative tube feeding, (2) pre‐operative enteral nutrition in which 41% of the casein was replaced by arginine and standard post‐operative tube feeding, (3) no pre‐operative and standard post‐operative tube feeding, study period up to 7 days post‐operatively | Blood | Body weight, body composition, upper midarm circumference, skinfold thickness, muscle function, albumin at recruitment, 1, 4, and 7 days as well as on the day of discharge | No significant changes in nutritional status on all outcome measurements at 1, 4 and 7 days post‐operatively | None |
| Yamanaka et al. 199031 | RCT | BCAA | i.v. | 34 | Gastric cancer | Total parenteral nutrition solution supplemented with 31% BCAA vs. 21% BCAA was administered | Blood | Plasma amino acid levels were measured after administration | Administration of TPN solution supplemented with 31% BCAA was more effective to improve protein metabolism than 21% BCAA‐enriched TPN | None |
| Buijs et al. 201032 | RCT | Arginine vs. standard perioperative enteral nutrition | Liquid per tube feeding | 32 | Head and neck cancer | Participants were divided into 2 groups: (1) arginine‐supplemented perioperative enteral nutrition (study group) and (2) standard perioperative enteral nutrition (control group), outcome over a 10 year period | — | The primary outcome was long‐term (≥10 years) survival. Secondary outcomes included the long‐term appearance of loco‐regional recurrence, distant metastases, and second primary tumours | Study group had a significantly better overall survival (34.8 months vs. 20.7 months; P = 0.019) and a better disease‐specific survival (94.4 months vs. 20.8 months; P = 0.022) | None |
| Aliyazicioglu et al. 201333 | RCT | Standard and/or glutamine dipeptide and/or omega‐3 fatty acids supplemented TPN | i.v. | 36 | Colorectal cancer | Patients were randomly divided into four groups: (1) standard TPN (control group), (2) TPN with glutamine solution (S‐D), (3) TPN with omega‐3 fatty acid solution (S‐O), and (4) TPN with omega‐3 fatty acids solution and glutamine (S‐d‐O). Treatments were given for 7 days after the operation | Blood | Albumin, AST, ALT, NAI, IL‐8, length of stay | The length of hospital stay in supplemented groups was significantly shorter compared with control group (7.37 ± 1.77 in S‐D; 7.13 ± 1.73 in S‐O; 8.2 ± 1.14 in S‐d‐O vs. 12.48 ± 5.43; P < 0.05). All supplemented groups also showed significant increase (7 day postoperative) in NAI compared with control group (P < 0.05) | None |
| Braga et al. 200234 | RCT | Arginine, omega‐3 fatty acids, and RNA | Liquid p.o. (preoperative); per feeding tube (postoperative) | 150 | Gastrointestinal cancer | (1) Post‐operative standard diet; (2) For 7 days orally 1 L/day liquid diet enriched arginine, omega‐3 fatty acids, and RNA for pre‐operative and same as control for post‐operative; (3) enriched diet pre‐operative and post‐operative | Blood | Postoperative complication and length of stay | Administration of supplemented diet before and after surgery shortened the pre‐operative (13.2 days) and perioperative (12.0 days) length of stay compared with controls (15.3 days) (P = 0.01 and P = 0.001, respectively) | Abdominal cramps or distention (29 patients), diarrhoea (13 patients), vomiting (4 patients) |
| de Luis et al. 201335 | RCT | Arginine, omega‐3 fatty acids | Liquid p.o. | 37 | Head and neck cancer | Oral consume of two (Group I) or three cans (Group II) per day of a specially designed omega‐3 fatty acids and arginine enhanced supplement for a 12 week period | Blood | Albumin, prealbumin, transferrin, lymphocytes, BMI, fat mass, FFM | After a 12 week period Group II showed significant increases in weight (69.4 ± 9.4–74.6 ± 8.9; P < 0.05), FFM (50.4 ± 11–53.0 ± 8.4; P < 0.05). Albumin, prealbumin, transferrin, and lymphocytes increased in both groups (P < 0.05). | Nausea (2 patients) |
AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; BCAA, Branched‐chain amino acid; BMI, Body mass index; CO2, Carbon dioxide; ECOG, Eastern Cooperative Oncology Group performance status; EORTC, European Organization for Research and Treatment of Cancer; EORTC QLQ C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30; FAACT, Functional Assessment of Cancer Therapy; FFM, Fat free mass; IL, Interleukin; i.v., intravenous; MFSI‐SF, Multidimensional Fatigue Symptom Inventory–Short Form; p.o., per oral; REE, Resting Energy Expenditure; NAI, Neutrophil Adhesivity Index; RCT, Randomized Controlled Trial; TNF, Tumour Necrosis Factors; TPN, Total parenteral nutrition
Adverse effects with dietary supplements
Adverse effects were metallic taste after magnesium supplementation,17 diarrhoea,14, 15 and nausea15 after L‐carnitine supplementation, or mild abdominal discomfort and transient diarrhoea after a mixture of omega‐3 polyunsaturated fatty acids plus vitamin E.20 HMB in combination with arginine was associated with nausea, constipation, and diarrhoea.22 EE regimen26 led to oral mucositis and emesis. Arginine in combination with omega‐3 fatty acids and/or RNA27, 34, 35 was associated with abdominal cramping, bloating, diarrhoea, nausea, and vomiting.
Discussion
A number of studies on the use of minerals, vitamins, proteins, and other supplements for the treatment of cancer cachexia were found. However, only 21 studies fulfilled the inclusion criteria and were part of this systematic review (Figure 1).
The search terms were formulated broadly (Appendix 1–3), to cover all relevant studies. The number of participants varied across studies. Most of them investigated supplementation with a combination of substances and did not provide any information on effects of single ingredients. Outcome measures across all studies included body mass, pain, muscle strength, appetite, grip strength, quality of life, and serum levels of IL‐6 and TNF‐α. None of the studies reported effect sizes.
The paucity of data from high quality studies on food supplements became evident in the evaluation process, even though deficiency concerning minerals,36, 37 vitamins,38 and proteins39 was found in several studies on cancer patients.
Minerals such as selenium or magnesium have been discussed not only for nutrition but also for immuno‐function and cancer prevention; their effect on cachexia has not been investigated in detail. Thus, our literature search identified only one study on mineral supplementation examining the use of magnesium in a randomized controlled trial.17 There was no effect of this intervention on weight loss (Table 1), so that a recommendation of magnesium to prevent weight loss in cancer is not justified.
Studies with vitamin supplements18, 19, 20 were slightly more promising (Table 2). In one study, vitamin D supplementation showed improvement of muscle weakness in prostate cancer patients;18 however, as measurement of muscle strength was the only outcome measure, no conclusion can be drawn concerning weight loss. With regard to vitamin C, oral and intravenous supplementation in terminal cancer patients led to improvement of several domains of quality of life such as physical and cognitive function, fatigue and appetite loss, as well as nausea.19 A single study on vitamin E in combination with omega‐3 fatty acids displayed an effect on survival.20 Altogether, additional research on vitamin D, vitamin C, and vitamin E supplementation is recommended to give a clearer picture of possible advantages of vitamin supplementations in cancer.
Looking at studies with proteins and other dietary supplements the combination of HMB, arginine, and glutamine showed interesting results (Table 3). In one study, 32 patients gained an average of about 2 kg of body weight.21 This study was one of three studies confirming the positive effects of this combination in a variety of diagnoses/conditions such as HIV/AIDS patients and healthy adults.40 Another study, on a far larger sample base of around 470 cancer patients, found no significant difference with regard to LBM after 8 weeks however a strong trend in the direction of an increase in LBM as measured by both bio‐impedance and skin‐fold measurements.22 In summary, the effect of the combination of HMB, arginine, and glutamine on weight gain should be investigated in further studies on cancer patients investigating time periods of several months.
With regard to perioperative supplementation arginine27, 30, 32, 34, 35 and glutamine29, 33 in combination with other supplements displayed interesting clinical effects (length of hospital stay, infections, and overall survival) and improved protein levels (albumin and glutamine). On the other hand, mixtures containing arginine were associated with gastrointestinal side effects such as abdominal cramping, nausea, and vomiting. To date, there is not enough evidence to answer the question, whether clinical benefits of arginine supplementation in the perioperative setting justify abovementioned adverse side effects.
Carnitine deficits have been identified in 78% of patients with advanced cancer, with resurgent levels in most of these patients after carnitine supplementation.39 One study confirmed carnitine as a promising food supplement in pancreatic cancer patients.15 Patients with carnitine supplementation significantly gained weight with a BMI increase of over 3% on average and improved overall survival. However, of 72 enrolled patients, only 26 completed the study so that external validity of study findings is limited. In a further study,14 results showed that L‐carnitine in combination with medroxyprogesteron acetate/megestrol acetate, eicosapentaenoic acid, and thalidomide had a positive effect on LBM, fatigue, and appetite. However, L‐carnitine supplementation alone did not have the same positive effect; therefore, further investigation on the influence of L‐carnitine on cachexia is needed.
Two studies analysing supplementation with BCCA showed clinical benefits as measured by the albumin synthesis rate and leucine flux; however, the very small sample size did not allow for valid conclusions to be drawn. A study using a mixture of minerals, vitamins, proteins, and probiotics (EE regimen) showed improvement in body weight as well as serum albumin and prealbumin levels in head and neck cancer patients with a BMI <19. However, the study design does not allow for differentiation of the contribution of each of the ingredients to weight gain.
Adverse effects were reported in studies supplementing minerals,17 vitamins,20 and proteins.14, 15, 22, 26, 27, 34, 35 In most cases, gastrointestinal side effects were reported. These effects showed mild intensity and seldom led to discontinuation or change of treatment. However, it should be noted that the dosage of supplements was controlled in abovementioned studies. As many supplements can be purchased without prescription or at the local supermarket excessive supplementation may be seen in cancer patients who are concerned about micronutrient deficiencies and there may be a risk of potentially harmful self‐medication. A survey among breast cancer patients showed the potential for excessive vitamin/mineral use among one‐third of respondents.41 Even though in studies on humans to date no major adverse events due to food supplementation have been reported, in animal studies, supplementation with N‐acetylcysteine and vitamin E accelerated lung cancer progression in mice.42
Regarding limitations of our systematic review, expanding the search to additional databases or to non‐English literature might have resulted in more hits. However, it seems improbably that there is a significantly larger body of evidence not identified by our search strategy.
In summary, studies with a greater number of participants are urgently needed, although problems with recruitment and high attrition have been identified in many other reviews in advanced cancer or palliative care. Similarly, the positive effects of some studies with combination therapies lend support to the necessity of additional research on the individual components. In order to prioritize research ambitions and provide useful guidance for cancer and palliative care, studies should focus on the effect of food supplements on nutritional status and cachexia‐related symptoms in patients and cancer types most affected by cachexia.
Conclusions
Following the GRADE methodology, no positive recommendation could be expressed for the use of minerals, vitamins, proteins, or other supplements in cancer patients. On the other hand, no serious adverse effects have been associated with dietary supplementation. Further research is needed to identify the efficacy and safety of these supplements to be able to give clear evidence‐based recommendations.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.43
The main sources of funding of the PRC are The Norwegian Cancer Society, The Norwegian University of Science and Technology, and Trondheim University Hospital. Additional support has been awarded by the Open Society Institute (USA), the Floriani Foundation (Italy), and by an unrestricted grant from Nycomed.
Conflict of interest
None declared.
Supporting information
Appendix 1. CENTRAL search strategy.
Appendix 2. MEDLINE search strategy.
Appendix 3. PsycINFO search strategy.
Supporting info item
Supporting info item
Supporting info item
Mochamat, , Cuhls, H. , Marinova, M. , Kaasa, S. , Stieber, C. , Conrad, R. , Radbruch, L. , and Mücke, M. (2017) A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: a European Palliative Care Research Centre cachexia project. Journal of Cachexia, Sarcopenia and Muscle, 8: 25–39. doi: 10.1002/jcsm.12127.
References
- 1. Radbruch L, Elsner F, Trottenberg P, Strasser F, Baracos V, Fearon K. Clinical practice guidelines on cancer cachexia in advanced cancer patients [Internet]. Eur Palliat Care Res Collab 2010. [cited 2016 Apr 23]. Available from: http://www.epcrc.org/guidelines.php?p=cachexia [Google Scholar]
- 2. Ries A, Trottenberg P, Elsner F, Stiel S, Haugen D, Kaasa S, et al. A systematic review on the role of fish oil for the treatment of cachexia in advanced cancer: an EPCRC cachexia guidelines project. Palliat Med 2012;26:294–304. [DOI] [PubMed] [Google Scholar]
- 3. Hoffman FA. Micronutrient requirements of cancer patients. Cancer 1985;55:295–300. [DOI] [PubMed] [Google Scholar]
- 4. Brinkman MT, Buntinx F, Kellen E, Dagnelie PC, Van Dongen MCJM, Muls E, et al. Dietary intake of micronutrients and the risk of developing bladder cancer: results from the Belgian case‐control study on bladder cancer risk. Cancer Causes Control CCC 2011;22:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Westin T, Ahlbom E, Johansson E, Sandström B, Karlberg I, Edström S. Circulating levels of selenium and zinc in relation to nutritional status in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 1989;115:1079–1082. [DOI] [PubMed] [Google Scholar]
- 6. Balstad TR, Solheim TS, Strasser F, Kaasa S, Bye A. Dietary treatment of weight loss in patients with advanced cancer and cachexia: a systematic literature review. Crit Rev Oncol Hematol 2014;91:210–221. [DOI] [PubMed] [Google Scholar]
- 7. Rock CL, Doyle C, Demark‐Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:242–274. [DOI] [PubMed] [Google Scholar]
- 8. World Cancer Research Fund and American Institute for Cancer Research . Cancer Surviv [Internet]. [cited 2015 Aug 6]; Available from: http://www.dietandcancerreport.org/cancer_prevention_recommendations/recommendation_cancer_survivors.php [Google Scholar]
- 9. Doyle C, Kushi LH, Byers T, Courneya KS, Demark‐Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin 2006;56:323–353. [DOI] [PubMed] [Google Scholar]
- 10. Food Supplements | European Food Safety Authority [Internet]. [cited 2016 Apr 12]. Available from: http://www.efsa.europa.eu/en/topics/topic/supplements
- 11. Lis CG, Cambron JA, Grutsch JF, Granick J, Gupta D. Self‐reported quality of life in users and nonusers of dietary supplements in cancer. Support Care Cancer 2006;14:193–199. [DOI] [PubMed] [Google Scholar]
- 12. Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atkins D, Briss PA, Eccles M, Flottorp S, Guyatt GH, Harbour RT, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res 2005;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantovani G, Macciò A, Madeddu C, Serpe R, Massa E, Dessì M, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010;15:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kraft M, Kraft K, Gärtner S, Mayerle J, Simon P, Weber E, et al. L‐Carnitine‐supplementation in advanced pancreatic cancer (CARPAN) ‐ a randomized multicentre trial. Nutr J 2012;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Altman DG, Sterne JAC. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Internet]. The Cochrane Collaboration; 2011. Available from: www.cochrane‐handbook.org.
- 17. Willox JC, McAllister EJ, Sangster G, Kaye SB. Effects of magnesium supplementation in testicular cancer patients receiving cis‐platin: a randomised trial. Br J Cancer 1986;54:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Veldhuizen PJ, Taylor SA, Williamson S, Drees BM. Treatment of vitamin D deficiency in patients with metastatic prostate cancer may improve bone pain and muscle strength. J Urol 2000;163:187–190. [DOI] [PubMed] [Google Scholar]
- 19. Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients' health‐related quality of life after high dose vitamin C administration. J Korean Med Sci 2007;22:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega‐3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer 1998;82:395–402. [DOI] [PubMed] [Google Scholar]
- 21. May PE, Barber A, D'Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer‐related wasting using oral supplementation with a combination of beta‐hydroxy‐beta‐methylbutyrate, arginine, and glutamine. Am J Surg 2002;183:471–479. [DOI] [PubMed] [Google Scholar]
- 22. Berk L, James J, Schwartz A, Hug E, Mahadevan A, Samuels M, et al. A randomized, double‐blind, placebo‐controlled trial of a beta‐hydroxyl beta‐methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer 2008;16:1179–1188. [DOI] [PubMed] [Google Scholar]
- 23. Mantovani G, Macciò A, Madeddu C, Gramignano G, Lusso MR, Serpe R, et al. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti‐cyclooxygenase‐2 showing efficacy and safety in patients with cancer‐related anorexia/cachexia and oxidative stress. Cancer Epidemiol Biomark Prev 2006;15:1030–1034. [DOI] [PubMed] [Google Scholar]
- 24. Hunter DC, Weintraub M, Blackburn GL, Bistrian BR. Branched chain amino acids as the protein component of parenteral nutrition in cancer cachexia. Br J Surg 1989;76:149–153. [DOI] [PubMed] [Google Scholar]
- 25. Tayek JA, Bistrian BR, Hehir DJ, Martin R, Moldawer LL, Blackburn GL. Improved protein kinetics and albumin synthesis by branched chain amino acid‐enriched total parenteral nutrition in cancer cachexia: A prospective randomized crossover trial. Cancer 1986;58:147–157. [DOI] [PubMed] [Google Scholar]
- 26. Yeh K‐Y, Wang H‐M, Chang JW‐C, Huang J‐S, Lai C‐H, Lan Y‐J, et al. Omega‐3 fatty acid‐, micronutrient‐, and probiotic‐enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:41–48. [DOI] [PubMed] [Google Scholar]
- 27. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002;122:1763–1770. [DOI] [PubMed] [Google Scholar]
- 28. Snyderman CH, Kachman K, Molseed L, Wagner R, D'Amico F, Bumpous J, et al. Reduced postoperative infections with an immune‐enhancing nutritional supplement. Laryngoscope 1999;109:915–921. [DOI] [PubMed] [Google Scholar]
- 29. Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lawin P, et al. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet Lond Engl 1989;1:231–233. [DOI] [PubMed] [Google Scholar]
- 30. van Bokhorst‐De Van Der Schueren MA, Quak JJ, von Blomberg‐van der Flier BM, Kuik DJ, Langendoen SI, Snow GB, et al. Effect of perioperative nutrition, with and without arginine supplementation, on nutritional status, immune function, postoperative morbidity, and survival in severely malnourished head and neck cancer patients. Am J Clin Nutr 2001;73:323–332. [DOI] [PubMed] [Google Scholar]
- 31. Yamanaka H, Kanemaki T, Tsuji M, Kise Y, Hatano T, Hioki K, et al. Branched‐chain amino acid‐supplemented nutritional support after gastrectomy for gastric cancer with special reference to plasma amino acid profiles. Nutr Burbank Los Angel Cty Calif 1990;6:241–245. [PubMed] [Google Scholar]
- 32. Buijs N, van B van der Schueren MA, Langius JA, Leemans CR, Kuik DJ, Vermeulen MA, et al. Perioperative arginine‐supplemented nutrition in malnourished patients with head and neck cancer improves long‐term survival. Am J Clin Nutr 2010;92:1151–1156. [DOI] [PubMed] [Google Scholar]
- 33. Aliyazicioglu T, Cantürk NZ, Simsek T, Kolayli F, Çekmen M. Effects of standard and/or glutamine dipeptide and/or omega‐3 fatty acid‐supplemented parenteral nutrition on neutrophil functions, interleukin‐8 level and length of stay‐ A double blind, controlled, randomised study. East Afr Med J 2013;90:59–66. [PubMed] [Google Scholar]
- 34. Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg 2002;137:174–180. [DOI] [PubMed] [Google Scholar]
- 35. de Luis DA, Izaola O, Cuellar L, Terroba MC, de la Fuente B, Cabezas G. A randomized clinical trial with two doses of a omega 3 fatty acids oral and arginine enhanced formula in clinical and biochemical parameters of head and neck cancer ambulatory patients. Eur Rev Med Pharmacol Sci 2013;17:1090–1094. [PubMed] [Google Scholar]
- 36. Dopfel RP, Schulmeister K, Schernhammer ES. Nutritional and lifestyle correlates of the cancer‐protective hormone melatonin. Cancer Detect Prev 2007;31:140–148. [DOI] [PubMed] [Google Scholar]
- 37. MacFie J, Burkinshaw L. Body composition in malignant disease. Metabolism 1987;36:290–294. [DOI] [PubMed] [Google Scholar]
- 38. Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev J Clin Ther 2008;13:6–20. [PubMed] [Google Scholar]
- 39. Cruciani RA, Dvorkin E, Homel P, Malamud S, Culliney B, Lapin J, et al. Safety, tolerability and symptom outcomes associated with L‐carnitine supplementation in patients with cancer, fatigue, and carnitine deficiency: a phase I/II study. J Pain Symptom Manage 2006;32:551–559. [DOI] [PubMed] [Google Scholar]
- 40. Rathmacher JA, Nissen S, Panton L, Clark RH, Eubanks May P, Barber AE, et al. Supplementation with a combination of beta‐hydroxy‐beta‐methylbutyrate (HMB), arginine, and glutamine is safe and could improve hematological parameters. JPEN J Parenter Enteral Nutr 2004;28:65–75. [DOI] [PubMed] [Google Scholar]
- 41. Monnin S, Schiller MR, Sachs L, Smith AM. Nutritional concerns of women with breast cancer. J Cancer Educ 1993;8:63–69. [DOI] [PubMed] [Google Scholar]
- 42. Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 2014;6:221ra15. [DOI] [PubMed] [Google Scholar]
- 43. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. CENTRAL search strategy.
Appendix 2. MEDLINE search strategy.
Appendix 3. PsycINFO search strategy.
Supporting info item
Supporting info item
Supporting info item


