Abstract
Background
There is no consensus on how best to define low muscle mass in patients with end‐stage renal disease. Use of muscle mass normalized to height‐squared has been suggested by geriatric societies but may underestimate sarcopenia, particularly in the setting of excess adiposity. We compared four definitions of low muscle mass in a prevalent hemodialysis cohort.
Methods
ACTIVE/ADIPOSE enrolled prevalent patients receiving hemodialysis from the San Francisco and Atlanta areas from June 2009 to August 2011. Whole‐body muscle mass was estimated using bioelectrical impedance spectroscopy, performed before a midweek dialysis session (n = 645; age 56.7 ± 14.5 years, 41% women). We defined low muscle mass as muscle mass of 2SD or more below sex‐specific bioelectrical impedance spectroscopy‐derived means for young adults (18–49 years) from National Health and Nutrition Examination Survey and indexed to height2, body weight (percentage), body surface area (BSA) by the DuBois formula, or Quételet's body mass index (BMI). We compared prevalence of low muscle mass among the four methods and assessed their correlation with strength and physical performance.
Results
The prevalence of low muscle mass ranged from 8 to 32%. Muscle mass indexed to height2 classified the smallest percentage of patients as having low muscle mass, particularly among women, whereas indexing by BSA classified the largest percentage. Low muscle mass/height2 was present almost exclusively among normal or underweight patients, whereas indexing to body weight and BMI classified more overweight and obese patients as having low muscle mass. Handgrip strength was lower among those with low muscle mass by all methods except height2. Handgrip strength was directly and modestly correlated with muscle mass normalized by percentage of body weight, BSA, and BMI (ρ = 0.43, 0.56, and, 0.64, respectively) and less so with muscle/height2 (ρ = 0.31, P < 0.001). The difference in grip strength among patients with low vs. normal muscle mass was largest according to muscle/BMI (−6.84 kg, 95% CI −8.66 to −5.02, P < 0.001). There were significant direct correlations of gait speed with muscle mass indexed to percentage of body weight, BSA, and BMI but not with muscle mass indexed to height2.
Conclusions
Skeletal muscle mass normalized to height2 may underestimate the prevalence of low muscle mass, particularly among overweight and obese patients on hemodialysis. Valid detection of sarcopenia among obese patients receiving hemodialysis requires adjustment for body size.
Keywords: Low muscle mass, Sarcopenia, Handgrip strength, Gait speed, Hemodialysis
Introduction
Reduction in skeletal muscle mass with declining muscle strength or function occurs gradually with increasing age and has been called ‘sarcopenia’.1 This process may be more severe among vulnerable populations, such as those with end‐stage renal disease (ESRD). In recognition that chronic conditions can contribute to sarcopenia and that loss of strength or function often accompanies muscle wasting, sarcopenia has more recently been defined by both low muscle mass and reduced muscle function.2 This syndrome is associated with impaired functional capacity, low physical performance, and higher risk of death among community‐dwelling elderly individuals.3, 4, 5 Lower muscle mass, either measured by serum creatinine as a surrogate or by body composition analysis, was independently associated with worse survival in maintenance hemodialysis patients.6, 7 Additionally, higher lean body mass has been associated with lower mortality risk in a large multi‐ethnic ESRD cohort.8
Expert panels from around the world are in agreement about the importance of sarcopenia and about its general definition,2, 9, 10, 11 but there is no consensus regarding operational criteria for sarcopenia. The cut‐points to define low muscle mass and the metrics used to normalize muscle mass have varied across studies depending upon the measurement techniques used to assess muscle (whole body vs. appendicular muscle mass) and the data available from reference populations.12, 13 We and others recently found similar variation across studies among patients with ESRD receiving maintenance hemodialysis.14, 15, 16
Although skeletal muscle mass indexed to height‐squared is a commonly employed metric of relative muscle mass,17, 18 it has been recognized that normalization by this method may underestimate the prevalence of low muscle mass, particularly in the setting of excess adiposity.19 Overweight or obese individuals whose muscle mass is low relative to their body size may not be classified as sarcopenic if muscle mass is adjusted only for height. New data in elderly populations show that muscle mass adjusted more generally for body size rather than height alone is more strongly correlated with physical function than muscle mass indexed to height‐squared, and some experts have recommend alternative approaches to indexing.3, 12, 20
Study of the biology of sarcopenia among patients on dialysis and the associations of sarcopenia with disability and death could lead to better treatment options, but different methods of defining low muscle mass have been employed in the dialysis population and have yielded different estimates of the prevalence.16 Furthermore, previous studies have indicated that the associations between muscle mass and strength among patients on dialysis may differ from associations among healthy individuals.21, 22 Thus, there is a need to evaluate associations between muscle size and strength in this population in order to determine which measures of muscle size are most closely associated with strength.
In the present study, we used data from a cohort of patients receiving hemodialysis that includes measurements of height, weight, and estimation of muscle mass using bioelectrical impedance spectroscopy (BIS) to compare the prevalence of low muscle mass using muscle mass indexed with height2, body weight, body surface area (BSA), and body mass index (BMI), and to examine the relation between low muscle mass according to these distinct methods of normalization and muscle strength and physical performance. We hypothesized that muscle mass normalized to height2 would classify fewer patients as having low muscle mass, particularly among overweight or obese patients, and would be less strongly correlated with handgrip strength or walking speed in comparison to normalization by body weight and two commonly employed methods of assessing body size.
Methods
Study design and participants
ACTIVE/ADIPOSE (A Cohort To Investigate the Value of Exercise/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD) was a United States Renal Data System (USRDS) Special Study conducted by the Nutrition and Rehabilitation/Quality of Life Special Studies Centers23 that enrolled 771 prevalent adult hemodialysis patients from seven dialysis centres in the San Francisco Bay Area and seven centres from the Atlanta metropolitan area from June 2009 and August 2011. Patients were eligible to participate if they were over 18 years of age, receiving maintenance hemodialysis for at least 3 months, English or Spanish‐speaking, and able to provide informed consent. The study was approved by the Institutional Review Boards at the University of California San Francisco and Emory University, and all participants provided written informed consent.
Study coordinators interviewed participants before or during a dialysis session, abstracted recent clinical and laboratory data from medical records, and measured body composition by BIS on the same day prior to the start of the dialysis session. Patients' data were also linked to data from the ESRD Medical Evidence Report (Center for Medicare & Medicaid Services Form 2728) available in the USRDS. ACTIVE/ADIPOSE participants who had data for body composition, muscle strength, and physical performance available (n = 645, 84%) were included in these analyses.
Measurements of body composition, muscle mass, strength and physical performance
Study coordinators measured height using a stadiometer and recorded weight to the nearest 0.1 kg as the mean of the last three post‐dialysis weight measurements in kilogrammes. BSA was derived from the equation 0.007184 × weight (kg)0.425 × height (cm)0.725 according to the Du Bois formula.24 BMI was calculated as weight divided by height in metres squared. The total‐body muscle mass was evaluated by multifrequency whole‐body BIS, performed before a midweek dialysis session, using a portable device that scans 256 frequencies between 4 and 1000 kHz (SFB7; ImpediMed, San Diego, CA). BIS was measured before dialysis for practical reasons. Predialysis BIS is being used increasingly in clinical practice to guide dialysis fluid removal.25 Because extracellular water and intracellular water (ICW) can be determined with acceptable precision with multifrequency BIS26, 27 and our equations to estimate muscle mass distinguish between extracellular water and intracellular water28, we felt that predialysis measurements would have acceptable accuracy and would have greater clinical relevance than post‐dialysis measures because many dialysis facilities already perform predialysis BIS. Patients were placed in a supine position at least 10 min before measurement. Electrodes were placed in a tetrapolar configuration using the wrist and ankle on the side opposite the dialysis vascular access with proximal and distal electrodes 5 cm apart. Ten consecutive measures were performed within a 1 min period. Total body water was estimated using the resistance extrapolated to infinite frequency. Extracellular water was estimated from resistance extrapolated to zero frequency. The equation for calculation of total‐body muscle mass (kg) was 9.52 + 0.331 × whole body BIS‐derived intracellular volume (L) + 0.180 × pre‐dialysis weight (kg) + 2.77 (if man) –0.113 × age (years). The results of this equation gave a value of R 2 = 0.937 (P < 0.0001) compared with muscle mass from whole‐body MRI in a cohort of patients on hemodialysis.28
Weakness was based on measurement of handgrip strength and assessed on the non‐fistula hand before a dialysis session using a hydraulic hand dynamometer (BASELINE®; Fabrication Enterprise, Inc., Irvington, NY, USA). For participants with an indwelling dialysis catheter, we used the dominant hand to test handgrip strength first then repeat the same protocol in the other. Participants were seated at a table with a proper chair height to ensure that their arms could comfortably rest on the table at a right angle with the elbow bent at a 90° angle and shoulder, forearm, and wrist in a neutral position. Forearm and dynamometer were supported by the table top. Participants were instructed to apply as much force as possible to obtain the best performance. Three trials were performed with a 15 s rest period between each trial. The first trial was discarded as a warm up session, and the highest force exerted in the latter two trials was recorded. Participants were asked to walk a marked 15 ft course at a usual pace. Two trials were conducted, and the faster of the two walks was used for analysis. Gait speed was calculated for each participant using distance in metres divided by time to walk the course in seconds.
Definitions of sarcopenia, low muscle strength and low physical performance
The BIS‐derived total‐body muscle mass was indexed to height2, body weight (percentage), BSA, and BMI. Low muscle mass was defined as muscle mass of 2SD or more below sex‐specific means of healthy young adults (18–49 years) for each indexing strategy. Reference populations and cutoff points of BIS‐derived whole body muscle mass were obtained from the National Health and Nutrition Examination Survey (NHANES) 2003–200429 using a Stata specific survey command that accounts for the stratified, multistage, probability sampling survey design of NHANES data. Low muscle strength was defined as handgrip strength of less than 26 kg in men and 16 kg in women, and low physical performance was defined as gait speed of less than or equal to 0.8 m/s according to the Foundation for the National Institutes of Health (FNIH) criteria.30, 31
Statistical analysis
Patient characteristics were described using mean ± SD for normally distributed and median (interquartile range) for non‐normally distributed variables. We assessed the correlations between measures using Pearson's correlation (ρ). The prevalence of low muscle mass by different criteria and characteristics of patients included in the analysis were compared between each metric using Student's t‐test, Wilcoxon rank sum, or chi‐squared tests as appropriate. We used univariable linear regression analyses to study associations among muscle mass and strength or gait speed and logistic regression to assess the extent to which low muscle mass was associated with weak grip and slow gait. We also performed multivariable regression analysis using age, sex, race, and diabetes as covariates. Interactions between low muscle mass and sex, race, and diabetes were tested. Analyses were performed in Stata 13 (StataCorp LP, College Station, TX), and P values less than 0.05 were considered statistically significant.
Results
Patient characteristics and prevalence of low muscle mass
Six hundred forty‐five of the 771 ACTIVE/ADIPOSE participants (84%) had data on body composition and were included in these analyses. Patients included in the analysis had shorter dialysis vintage, higher serum creatinine and albumin concentrations, and a lower prevalence of diabetes mellitus (Table S1). The average age of participants in the analysed cohort was 56.7 ± 14.5 years; 41.4% of participants were women. Sixty‐two percent of participants were black, 23.9% white and 43.9% had diabetes (Table 1). Median dialysis vintage was 2.8 (1.3–5.4) years. Women had significantly higher BMI than men (29.2 ± 7.6 and 27.4 ± 6.4 kg/m2, P = 0.001, respectively). Average percent body fat was 25.5 ± 9.3% in men and 35.9 ± 8.3% in women. The total prevalence of low muscle mass ranged from 8 to 32% depending on the criterion used to standardize total‐body muscle mass. Muscle mass indexed to height2 classified the smallest percentage of patients as having low muscle mass and sarcopenia (low muscle mass combined with low muscle strength), particularly among women, whereas indexing by BSA classified the largest percentage (Table 1). Men had a statistically significantly higher prevalence of low muscle mass than women when muscle mass was normalized to height2 and BSA but not body weight or BMI.
Table 1.
Patient characteristics
| Parametres | Total (n = 645) | Men (n = 378) | Women (n = 267) | P value |
|---|---|---|---|---|
| Age, years | 56.7 (14.5) | 55.5 (14.3) | 58.5 (14.5) | 0.01 |
| Black, % | 61.5 | 59.0 | 65.2 | 0.11 |
| Diabetes, % | 43.9 | 39.4 | 50.2 | 0.01 |
| BMI, kg/m2 | 28.1 (6.9) | 27.4 (6.4) | 29.2 (7.6) | 0.001 |
| BSA, m2 | 1.9 (0.3) | 1.9 (0.2) | 1.8 (0.2) | <0.001 |
| Dialysis vintage, years | 2.8 (1.3–5.4) | 2.6 (1.2–5.2) | 2.9 (1.4–5.9) | 0.36 |
| Serum creatinine, mg/dL | 8.4 (2.7) | 8.9 (2.9) | 7.6 (2.3) | <0.001 |
| Serum albumin, g/dL | 4.0 (0.4) | 4.0 (0.4) | 4.0 (0.3) | 0.08 |
| Prevalence of low muscle mass* by each index, % | ||||
| Muscle mass/height2 | 8.1 | 12.2 | 2.3 | <0.001 |
| Muscle mass/body weight (×100) | 25.3 | 27.8 | 21.7 | 0.08 |
| Muscle mass/BSA | 32.4 | 37.3 | 25.5 | 0.002 |
| Muscle mass/BMI | 25.0 | 24.9 | 25.1 | 0.95 |
| Prevalence of low muscle mass and low muscle strength** by each index, % | ||||
| Muscle mass/height2 | 3.9 | 5.6 | 1.5 | 0.01 |
| Muscle mass/body weight (×100) | 11.4 | 12.0 | 10.5 | 0.56 |
| Muscle mass/BSA | 15.9 | 18.6 | 12.0 | 0.02 |
| Muscle mass/BMI | 14.0 | 14.1 | 13.9 | 0.93 |
BMI, body mass index; BSA, body surface area
Data are presented as mean ± SD and median (25th to 75th).
P < 0.05 consider significantly different between men and women.
Presence of low muscle mass defined as muscle mass ≥2SD below normal mean of young adults. The mean‐2SD values for men and women are 7.89 and 6.05 kg/m2 for muscle mass/height2, 32.68 and 27.85% for muscle mass/body weight (%), 14.31 and 11.64 kg/m2 for muscle mass/BSA, 0.97 and 0.72 m2 for muscle mass/BMI, respectively.
Presence of low muscle strength defined as handgrip strength <26 and <16 kg in men and women, respectively.
Association of low muscle mass with body composition
The association of low muscle mass with BMI and body fat varied according to the definition of low muscle mass (Table 2 and Figure 1). Patients with low muscle mass indexed to height2 and to BSA had significantly lower BMI than those in the normal muscle mass groups by these criteria (21.1 ± 2.4 vs. 28.8 ± 6.9 kg/m2, P < 0.001 for height2 and 24.3 ± 3.8 vs. 30.0 ± 7.3 kg/m2, P < 0.001 for BSA). In contrast, patients who were classified as having low muscle mass by percentage of body weight and muscle mass indexed to BMI had significantly higher BMI than those with normal muscle mass (Table 2).
Table 2.
Characteristics of patients with normal and low muscle mass groups according to each normalization method
| Variables | Muscle/height2 (kg/m2) | Muscle/body weight (%) | Muscle/BSA (kg/m2) | Muscle/BMI (m2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal (n = 593) | Low (n = 52) | P | Normal (n = 482) | Low (n = 163) | P | Normal (n = 436) | Low (n = 209) | P | Normal (n = 484) | Low (n = 161) | P | |
| Muscle metric (units vary) | 9.6 ± 1.8 | 7.2 ± 0.7 | <0.001 | 34.4 ± 4.0 | 29.4 ± 2.3 | <0.001 | 14.8 ± 1.6 | 12.5 ± 1.3 | <0.001 | 1.0 ± 0.2 | 0.8 ± 0.1 | <0.001 |
| BMI (kg/m2) | 28.8 ± 6.9 | 21.1 ± 2.4 | <0.001 | 26.8 ± 6.3 | 32.1 ± 7.2 | <0.001 | 30.0 ± 7.3 | 24.3 ± 3.8 | <0.001 | 27.3 ± 6.6 | 30.6 ± 7.3 | <0.001 |
| Percent fat (%) | 30.4 ± 4.2 | 24.0 ± 8.9 | <0.001 | 27.4 ± 9.9 | 37.1 ± 7.1 | <0.001 | 30.0 ± 10.9 | 29.5 ± 8.7 | 0.53 | 28.2 ± 10.3 | 34.8 ± 8.4 | <0.001 |
| Handgrip strength (kg) | 26.5 ± 10.6 | 25.2 ± 10.3 | 0.40 | 27.1 ± 10.7 | 24.3 ± 10.1 | 0.004 | 27.8 ± 11.2 | 23.3 ± 8.5 | <0.001 | 28.1 ± 10.7 | 21.3 ± 8.3 | <0.001 |
| Proportion with low HGS (%) | 28.2 | 49.0 | 0.002 | 24.8 | 44.8 | <0.001 | 20.6 | 49.3 | <0.001 | 21.2 | 55.9 | <0.001 |
| Gait speed (m/s) | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.002 | 1.0 ± 0.3 | 0.8 ± 0.3 | <0.001 | 1.0 ± 0.3 | 0.9 ± 0.3 | 0.001 | 1.0 ± 0.3 | 0.8 ± 0.3 | <0.001 |
| Proportion with slow walking speed (%) | 33.6 | 45.1 | 0.10 | 27.7 | 54.6 | <0.001 | 30.3 | 43.3 | 0.001 | 29.0 | 50.9 | <0.001 |
HGS, handgrip strength
Data are presented as mean ± SD.
Figure 1.
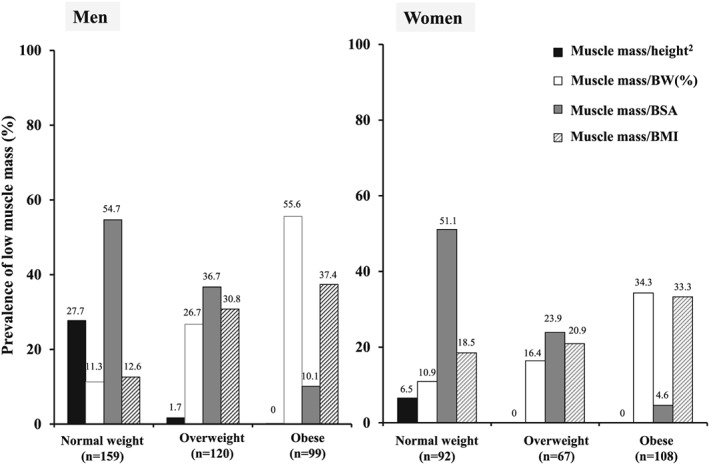
Prevalence of low muscle mass by international classification by body mass index (BMI). BMI < 25, 25 to <30 and ≥30 kg/m2 is normal weight, overweight, and obese, respectively.
Figure 1 shows the distribution of low muscle mass across categories of BMI. Low muscle mass indexed to height2 was present almost exclusively among normal or underweight patients. Indexing to BSA also showed an inverse association with BMI but did classify a substantial proportion of overweight and some obese patients as having low muscle mass. By contrast, indexing according to body weight and BMI classified more overweight and obese patients than normal or underweight patients as having low muscle mass relative to their size, with the gradient particularly remarkable for the percentage of body weight criterion, by which 55.6% of obese men and 34.3% of obese women had low muscle mass.
Associations between muscle mass, strength, and physical performance
Patients classified with low muscle mass had significantly lower handgrip strength than those with normal muscle mass by all methods except height2 (Table 2), but the difference was largest according to muscle mass/BMI (−6.84 kg, 95% CI −8.66 to −5.02, P < 0.001).
Handgrip strength was directly and modestly correlated with muscle mass normalized by percentage of body weight, BSA, and BMI (ρ = 0.43, 0.56 and 0.64, P < 0.001, respectively) and less so with muscle mass/height2 (ρ = 0.31 and P < 0.001) (Figure 2).
Figure 2.
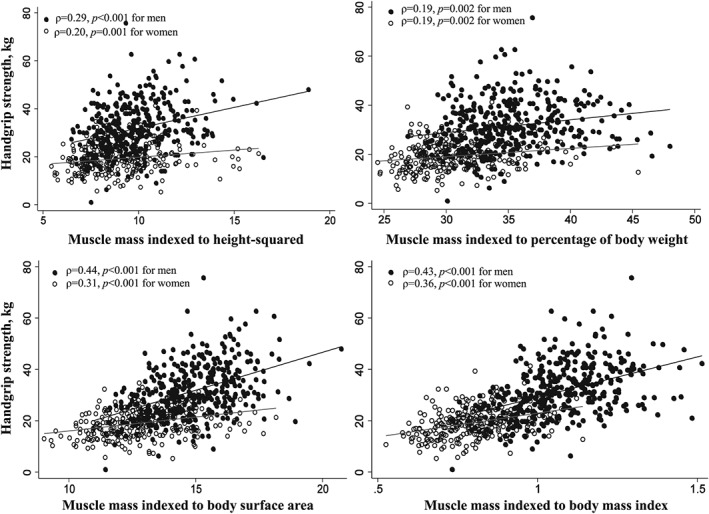
Scatter plot between total‐body muscle mass indexed to height2, percentage of body weight, body surface area, and body mass index and muscle strength (handgrip strength).
Patients with low muscle mass by all definitions were more likely to be weak than those with normal muscle mass (Table 3). However, low muscle mass indexed to height2 and percentage of body weight were not associated with higher odds of weakness after adjusting for age, sex, race, and diabetes. The association was strongest for muscle mass/BMI, such that patients with low muscle mass/BMI were almost five times more likely to be weak than patients with normal muscle mass/BMI (OR 4.72, 95% CI 3.23 to 6.91; Table 3) and this relationship persisted in multivariable logistic regression analysis (OR 1.82, 95% CI 1.13 to 2.94).
Table 3.
Linear and logistic regression analysis of the association of low muscle mass indexed by each metric with muscle strength and physical performance
| Measure of low muscle mass | Difference [95% CI] in handgrip strength (kg) or gait speed (m/s) | OR [95% CI] for having low handgrip strength or slow gait speed† | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted P | Adjusted# | P | Unadjusted | P | Adjusted# | P | ||
| Handgrip strength | ||||||||
| Muscle/height2 | −1.29 [−4.33, 1.75] | 0.40 | −2.72 [−5.08, −0.36] | 0.02 | 2.45 [1.37, 4.36] | 0.002 | 1.28 [0.63, 2.59] | 0.49 |
| Muscle/body weight (×100) | −2.78 [−4.66, −0.91] | 0.004 | 0.45 [−1.17, 2.07] | 0.59 | 2.46 [1.70, 3.57] | <0.001 | 0.93 [0.57, 1.51] | 0.76 |
| Muscle/BSA | −4.52 [−6.24, −2.80]* | <0.001 | −3.24 [−4.83, −1.65] | <0.001 | 3.73 [2.61, 5.34] | <0.001 | 1.86 [1.15, 2.99] | 0.01 |
| Muscle/BMI | −6.84 [−8.66, −5.02]* | <0.001 | −2.59 [−4.26, −0.93] | 0.002 | 4.72 [3.23, 6.91] | <0.001 | 1.82 [1.13, 2.94] | 0.01 |
| Gait speed | ||||||||
| Muscle/height2 | −0.13 [−0.21, −0.05] | 0.003 | −0.08 [−0.16, 0.00] | 0.05 | 1.63 [0.91, 2.89] | 0.09 | 1.35 [0.69, 2.64] | 0.37 |
| Muscle/body weight (×100) | −0.17 [−0.22, −0.12] | <0.001 | −0.09 [−0.15, −0.04] | 0.001 | 3.15 [2.18, 4.54] | <0.001 | 2.21 [1.38, 3.53] | 0.001 |
| Muscle/BSA | −0.08 [−0.13, −0.03] | 0.001 | 0.03 [−0.02, 0.08] | 0.28 | 1.76 [1.25, 2.47] | 0.001 | 0.97 [0.60, 1.54] | 0.89 |
| Muscle/BMI | −0.13 [−0.19, −0.08] | <0.001 | −0.07 [−0.13, −0.02] | 0.01 | 2.54 [1.76, 3.67] | <0.001 | 1.64 [1.003, 2.67] | 0.048 |
CIs, confident intervals; OR, odds ratio
Reference group is low muscle mass group by each metric.
Significant difference between men and women (P < 0.05). Difference in handgrip strength was −7.93 [−9.93, −5.93] in men and −3.16 [−4.75, −1.58] kg in women for muscle/BSA. For muscle/BMI, difference in handgrip strength was −8.88 [−11.10, −6.65] and −3.94 [−5.51, −2.37] kg in men and women, respectively.
Adjusted for age, race, sex, and diabetes.
Low grip strength defined as <16 kg for women and <26 kg for men; low gait speed defined as ≤0.8 m/s.
Although the correlation between muscle mass and gait speed was less robust, there were significant direct correlations with muscle mass indexed to percentage of body weight, BSA, and BMI but not with muscle mass indexed to height2 (Figure 3). Patients classified as having low muscle mass according to all indexing methods had slower walking speed, and low muscle mass was associated with higher odds of slow gait speed for all except the muscle mass/height2 metric in univariable regression analysis (Table 3).
Figure 3.
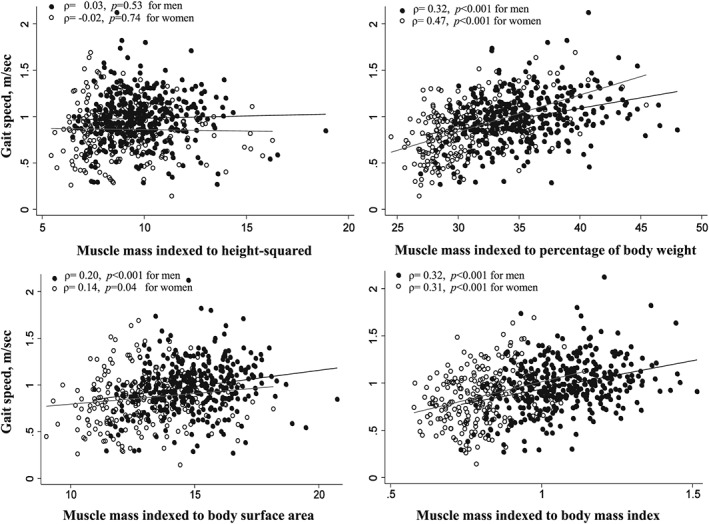
Scatter plot between total‐body muscle mass indexed to height2, percentage of body weight, body surface area, and body mass index and physical performance (gait speed).
Association of muscle mass and performance among subgroups of interest
For most metrics, associations of low muscle mass with strength and gait speed were similar among men and women. However, there were statistically significant interactions between sex and low muscle according to BSA and BMI. Men and women with low muscle mass were significantly weaker than those with normal muscle, but the absolute difference was larger for men. There were no significant interactions based on sex when considering the odds of weak grip strength, which may reflect the different standard cut points for men and women. For diabetes status, there were no significant interactions between association of low muscle mass with muscle strength or gait speed among patients with and without diabetes. Associations between muscle size and strength did not differ significantly among black and non‐black patients (Table 4). However, the association between gait speed and muscle mass did appear to differ according to race and was generally weaker among black patients. These differences were less pronounced after adjusting for other covariates and were no longer statistically significant except for muscle mass/height2 and muscle mass/body weight when gait speed was considered as a continuous variable.
Table 4.
Association between low muscle mass by each criterion with muscle strength and physical performance among non‐black and black patients
| Measure of low muscle mass | Difference [95% CI] in handgrip strength (kg) or gait speed (m/s) among patients with low vs. normal muscle | OR [95% CI] for having low handgrip strength or slow gait speed† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted# | Unadjusted | Adjusted# | |||||||||
| Non‐black | black | P | Non‐black | black | P | Non‐black | black | P | Non‐black | black | P | |
| Handgrip strength | ||||||||||||
| Muscle/height2 | −2.70 [−6.74, 1.33] | 0.20 [−3.96, 4.36] | 0.34 | −1.16 [−4.44, 2.13] | −4.12 [−7.43, −0.83] | 0.33 | 3.07 [1.16, 8.14] | 2.21 [0.96, 5.07] | 0.61 | 1.15 [0.39, 3.35] | 1.39 [0.54, 3.56] | 0.92 |
| Muscle/body weight | −3.57 [−5.99, −1.15] | −0.88 [−3.55, 1.78] | 0.16 | −0.74 [−3.08, 1.61] | 1.06 [−1.15, 3.28] | 0.25 | 3.13 [1.79, 5.48] | 1.48 [0.82, 2.68] | 0.07 | 1.14 [0.56, 2.32] | 0.76 [0.38, 1.51] | 0.28 |
| Muscle/BSA | −2.96 [−5.28, −0.63] | −4.65 [−7.04, −2.27] | 0.33 | −1.89 [−4.28, 0.51] | −4.26 [−6.38, −2.14] | 0.27 | 3.56 [2.08, 6.10] | 3.54 [2.07, 6.03] | 0.98 | 1.51 [0.73, 3.12] | 2.31 [1.21, 4.41] | 0.68 |
| Muscle/BMI | −4.91 [−7.17, −2.64] | −6.58 [−9.51, −3.65] | 0.38 | −2.19 [−4.39, 0.02] | −3.01 [−5.47, −0.54] | 0.58 | 3.53 [2.07, 6.03] | 3.60 [1.98, 6.58] | 0.96 | 1.53 [0.79, 2.97] | 2.16 [1.08, 4.31] | 0.74 |
| Gait speed | ||||||||||||
| Muscle/height2 | −0.31 [−0.43, −0.18] | −0.01 [−0.12, 0.10] | <0.001 | −0.18 [−0.30, −0.07] | −0.01 [−0.11, 0.09] | 0.01 | 4.18 [1.70, 10.30] | 0.84 [0.38, 1.87] | 0.01 | 1.91 [0.66, 5.52] | 1.01 [0.41, 2.47] | 0.11 |
| Muscle/body weight | −0.27 [−0.34, −0.20] | −0.12 [−0.19, −0.05] | 0.01 | −0.15 [−0.23, −0.07] | −0.07 [−0.13, 0.002] | 0.03 | 5.81 [3.19, 10.57] | 2.46 [1.51, 4.03] | 0.03 | 2.86 [1.31, 6.26] | 1.79 [0.98, 3.25] | 0.07 |
| Muscle/BSA | −0.16 [−0.23, −0.08] | −0.06 [−0.12, 0.01] | 0.04 | 0.001 [−0.09, 0.09] | 0.04 [−0.03, 0.10] | 0.12 | 2.44 [1.39, 4.28] | 1.61 [1.03, 2.51] | 0.26 | 0.88 [0.38, 2.01] | 1.00 [0.56, 1.78] | 0.27 |
| Muscle/BMI | −0.23 [−0.30, −0.17] | −0.14 [−0.21, −0.06] | 0.07 | −0.10 [−0.17, −0.02] | −0.04 [−0.12, 0.03] | 0.13 | 4.58 [2.55, 8.25] | 2.54 [1.46, 4.43] | 0.15 | 1.79 [0.85, 3.76] | 1.32 [0.68, 2.55] | 0.14 |
P for the difference between non‐black and black group for low muscle mass by each criterion.
Adjusted for age, sex, and diabetes.
Low grip strength defined as <16 kg for women and <26 kg for men; low gait speed defined as ≤0.8 m/s.
Discussion
We found that total‐body muscle mass normalized to height2 classified fewer patients as having low muscle mass, particularly among overweight and obese hemodialysis patients. The degree of correlation between muscle strength and muscle mass was highest for muscle mass standardized to BMI and lowest for height2. Low muscle mass normalized to height2 was not associated with higher likelihood of weakness.
In our cohort, the prevalence of low muscle mass varied from 8 to 32% with the lowest and highest frequency based on standardization method by height2 and BSA, respectively. The prevalence of low muscle mass in this study was slightly lower than in a Brazilian cohort16 that studied elderly patients on maintenance hemodialysis using various methods of estimating muscle mass, including anthropometric measurement, BIS, and dual energy X‐ray absorptiometry. In that study, all estimates were indexed to height2 and compared with norms using NHANES data. They reported the prevalence of low muscle mass measured by BIS was 14%, and the overall prevalence ranged from 4 to 74% depending on the method of body composition assessment and cutpoints applied. In a previous report by Kim and colleagues,15 the prevalence of low muscle mass using BIS‐derived lean tissue mass normalized to BSA in a Korean dialysis cohort was approximately 20% lower than low muscle mass by muscle mass/BSA criteria in ours. These observations are consistent with a systemic review32 conducted in community‐dwelling geriatric populations that highlighted the heterogeneity in prevalence of low muscle mass that arises because of lack of uniformity in the diagnostic criteria, cutpoints, and characteristics of the studied population as well as the choice of reference populations.
Using muscle mass divided by height2 resulted in a much lower prevalence of low muscle mass compared with other methods, especially among women. Obesity might account for these underestimated results.33, 34, 35 At equivalent height, individuals with higher body weight and BMI tend to have higher lean body mass. Obese patients who have low muscle mass relative to their body size, termed sarcopenic obesity, might not be identified as having low muscle mass by using normalization to height2. A study among healthy individuals also reported that implementation of body weight‐adjusted muscle mass criteria resulted in a higher prevalence of sarcopenia and sarcopenic obesity than when height‐based criteria were used.36 Newman et al.34 proposed the novel concept of using both height and fat mass adjustment to define low muscle mass by a linear regression model‐derived residuals method. Although this method may be complicated to use and is not useful for comparing across populations because it inherently relies on data from within the study population rather than normative data, the study did highlight the better performance of metrics that go beyond indexing to height alone.
Total‐body muscle mass was more closely related to muscle strength than physical performance (in this case, gait speed), regardless of the method of normalization. Our findings are similar to a previous report in the elderly population that muscle mass was more associated with grip strength than gait speed.19 In the present study, the likelihood and degree of weakness were associated with low muscle mass by most criteria, but the relation was weakest for muscle mass/height2 and strongest for the widely used weight adjusted for height indices BMI and BSA (Table 3). This is in concordance with a study by Estrada et al.,37 which showed that skeletal muscle mass normalized to percentage of body weight was a better predictor of mobility performance than muscle/height2 in healthy older women. A report from the FNIH sarcopenia project also found that muscle mass indexed to BMI, but not height or weight, was a potential discriminator of weakness.19
However, whereas muscle/height2 may underestimate low muscle mass, our data suggest that other methods, particularly muscle as a percentage of body weight, may overestimate low muscle mass among obese patients. On the other hand, low muscle mass by normalization to BSA and BMI, which include both body height and weight, was more closely associated with muscle strength and physical performance, with BMI performing slightly better than BSA and less influenced by race. Similarly, a recent study in the geriatric population concluded that criteria for low muscle mass that adjusted for both height and weight were more associated with weakness as well as poor physical performance than methods using either height or weight alone.38 Delmonico and colleagues20 also found that sarcopenia defined by a method adjusting for body fat and height was better at predicting decline in physical function than adjusting for height2.
The limitations of this study should be considered. Our cohort was slightly younger and had more black patients than the general US dialysis population. Thus, percentages of patients with low muscle mass in our cohort may not generalize to the whole US population. We did not include a healthy age‐matched control group. Thus, although our results are in general agreement with some studies in the general elderly population,19, 30, 31 we did not directly assess whether these results apply beyond patients on dialysis. We used total muscle mass rather than appendicular lean mass, which may be more closely associated with strength and gait speed. We assessed muscle mass using BIS prior to a dialysis session, which could lead to overestimation of muscle mass (and underestimation of the prevalence of low muscle) in the setting of overhydration39, and the lack of gold standard cutpoints of BIS‐derived total muscle mass to define low muscle mass should be aware. Nevertheless, whole body muscle mass assessment using BIS is more suitable for routine clinical practice than dual energy X‐ray absorptiometry or other techniques because of its simplicity and accessibility. Moreover, the equation for calculating skeletal muscle mass from BIS measurements used in this study was specifically derived from patients receiving hemodialysis (also mid‐week, before the dialysis session) and demonstrates an excellent correlation with a gold standard method by magnetic resonance imaging.28
In conclusion, skeletal muscle mass normalized to height‐squared may underestimate the prevalence of low muscle mass, particularly among overweight and obese patients on hemodialysis. Detection of sarcopenia among obese patients may require adjustment for body size. These results can be used to investigate associations of sarcopenia with outcomes in the dialysis population and to test interventions to address the important problem of sarcopenia.
Conflict of interest
The authors have no conflicts of interest to declare.
Disclaimer
The interpretation and reporting of the data presented herein are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Supporting information
Table S1. Patient characteristics of the study cohort comparing patients with and without BIS data.
Supporting info item
Acknowledgement
The study complies with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.40
This work was supported through contracts N01‐DK‐7‐0005 and KD‐7‐5004 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Johansen's effort was also supported by K24DK085153 from the NIDDK. Dr. Delgado's work is supported by the Department of Veterans Affairs, Clinical Science Research and Development Program under Career Development Award 1IK2CX000527‐01A2. Her contribution is the result of work supported with the resources and the use of facilities at the San Francisco VA Medical Center. This work has been made possible in part by an International Society of Nephrology funded Fellowship to Dr. Kittiskulnam. Dr. Carrero‐Roig acknowledges grant support from Stockholm County Council and the Swedish Research Council.
Kittiskulnam, P. , Carrero, J. J. , Chertow, G. M. , Kaysen, G. A. , Delgado, C. , and Johansen, K. L. (2017) Sarcopenia among patients receiving hemodialysis: weighing the evidence. Journal of Cachexia, Sarcopenia and Muscle, 8: 57–68. doi: 10.1002/jcsm.12130.
References
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med 2011;27:337–339. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 4. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 5. Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 2008;12:433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014;9:1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SS, Molnar MZ, Tayek JA, Ix JH, Noori N, Benner D, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross‐sectional study and review of literature. J Cachexia Sarcopenia Muscle 2013;4:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Streja E, Rhee CM, Soohoo M, Feng M, Brunelli SM, et al. Lean body mass and survival in hemodialysis patients and the roles of race and ethnicity. J Ren Nutr 2016;26:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 12. Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, et al. An evidence‐based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 2014;69:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 2016;doi:10.1016/j.kint.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 14. Ren H, Gong D, Jia F, Xu B, Liu Z. Sarcopenia in patients undergoing maintenance hemodialysis: incidence rate, risk factors and its effect on survival risk. Ren Fail 2016;38:364–371. [DOI] [PubMed] [Google Scholar]
- 15. Kim JK, Choi SR, Choi MJ, Kim SG, Lee YK, Noh JW, et al. Prevalence of and factors associated with sarcopenia in elderly patients with end‐stage renal disease. Clin Nutr 2014;33:64–68. [DOI] [PubMed] [Google Scholar]
- 16. Lamarca F, Carrero JJ, Rodrigues JC, Bigogno FG, Fetter RL, Avesani CM. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging 2014;18:710–717. [DOI] [PubMed] [Google Scholar]
- 17. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 18. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159:413–421. [DOI] [PubMed] [Google Scholar]
- 19. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014;69:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 21. Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent‐Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int 2003;63:291–297. [DOI] [PubMed] [Google Scholar]
- 22. Marcus RL, LaStayo PC, Ikizler TA, Wei G, Giri A, Chen X, et al. Low Physical Function in Maintenance Hemodialysis Patients Is Independent of Muscle Mass and Comorbidity. J Ren Nutr 2015;25:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. US Renal Data System . USRDS 2011 Annual Data Report, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011.
- 24. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–311. [PubMed] [Google Scholar]
- 25. Moissl U, Arias‐Guillen M, Wabel P, Fontsere N, Carrera M, Campistol JM, et al. Bioimpedance‐guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol 2013;8:1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol (1985) 1997;82:1542–1558. [DOI] [PubMed] [Google Scholar]
- 27. Matthie JR. Second generation mixture theory equation for estimating intracellular water using bioimpedance spectroscopy. J Appl Physiol (1985) 2005;99:780–781. [DOI] [PubMed] [Google Scholar]
- 28. Kaysen GA, Zhu F, Sarkar S, Heymsfield SB, Wong J, Kaitwatcharachai C, et al. Estimation of total‐body and limb muscle mass in hemodialysis patients by using multifrequency bioimpedance spectroscopy. Am J Clin Nutr 2005;82:988–995. [DOI] [PubMed] [Google Scholar]
- 29. National Health and Nutrition Examination Survey. Available from: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes03_04.aspx. Accessed 3 June 2015.
- 30. Alley DE, Shardell MD, Peters KW, McLean RR, Dam TT, Kenny AM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci 2014;69:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pagotto V, Silveira EA. Methods, diagnostic criteria, cutoff points, and prevalence of sarcopenia among older people. ScientificWorldJournal 2014;doi:10.1155/2014/231312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009;33:885–892. [DOI] [PubMed] [Google Scholar]
- 34. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 35. Domiciano DS, Figueiredo CP, Lopes JB, Caparbo VF, Takayama L, Menezes PR, et al. Discriminating sarcopenia in community‐dwelling older women with high frequency of overweight/obesity: the Sao Paulo Ageing & Health Study (SPAH). Osteoporos Int 2013;24:595–603. [DOI] [PubMed] [Google Scholar]
- 36. Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci 2012;67:1107–1113. [DOI] [PubMed] [Google Scholar]
- 37. Estrada M, Kleppinger A, Judge JO, Walsh SJ, Kuchel GA. Functional impact of relative versus absolute sarcopenia in healthy older women. J Am Geriatr Soc 2007;55:1712–1719. [DOI] [PubMed] [Google Scholar]
- 38. Meng NH, Li CI, Liu CS, Lin WY, Lin CH, Chang CK, et al. Sarcopenia defined by combining height‐ and weight‐adjusted skeletal muscle indices is closely associated with poor physical performance. J Aging Phys Act 2015;23:597–606. [DOI] [PubMed] [Google Scholar]
- 39. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000;89:465–471. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics of the study cohort comparing patients with and without BIS data.
Supporting info item


