Abstract
Background
Although muscle mass declines with testosterone deficiency in men, previous studies of muscle function have not demonstrated consistent deficits, likely due to relatively insensitive methodology. Our objective was to determine the effects of testosterone deprivation on the biomechanical function of individual lower‐limb muscles.
Methods
We conducted a 12‐month prospective, observational case–control study of 34 men newly commencing androgen deprivation treatment (ADT) for prostate cancer and 29 age‐matched prostate cancer controls. Participants were assessed at 0, 6, and 12 months while walking in a biomechanics laboratory. We combined video‐based motion capture and ground reaction force data with computerized musculoskeletal modelling to assess the following primary outcomes: (i) peak joint torques at the hip, knee and ankle, and corresponding individual muscle forces; (ii) individual muscle contributions to acceleration of the body's centre of mass; and (iii) walking speed, stride length, and step width. A linear mixed model was used to compare mean differences between groups.
Results
Compared with controls over 12 months, men receiving ADT had a mean reduction in total testosterone level from 14.1 to 0.4 nmol/L, and demonstrated more marked decreases in peak hip flexor torque by 14% [mean difference −0.11 N/kg (−0.19, −0.03), P = 0.01] and peak knee extensor torque by 16% [−0.11 N/kg (−0.20, −0.02), P = 0.02] of the initial mean value. Correspondingly, iliopsoas force decreased by 14% (P = 0.006), and quadriceps force decreased by 11%, although this narrowly missed statistical significance (P = 0.07). Soleus decreased contribution to forward acceleration of the body's centre of mass by 17% [mean difference −0.17 m/s2 (−0.29, −0.05), P < 0.01]. No significant changes between groups were observed in other joint torques or individual muscle contributions to acceleration of the body. Step width increased by 18% [mean adjusted difference 1.4 cm (0.6, 27.4), P = 0.042] in the ADT group compared with controls, with no change in stride length or walking speed.
Conclusions
Testosterone deprivation selectively decreases lower‐limb muscle function, predominantly affecting muscles that support body weight, accelerate the body forwards during walking, and mediate balance. Future exercise and pro‐myogenic interventional studies to mitigate ADT‐associated sarcopenia should target these deficits.
Keywords: Androgen deprivation, Balance, Falls, Kinematics, Muscle function, Prostate cancer, Sarcopenia
Introduction
Sarcopenia in the aging population has been associated with frailty, mobility limitation, and increased mortality.1, 2, 3 Sarcopenia occurs almost universally in men receiving androgen deprivation therapy (ADT) for prostate cancer, consequent to profound testosterone deficiency. Developing interventions to minimize long‐term adverse effects of ADT in men with localized prostate cancer is paramount, particularly as 5‐year cancer‐specific survival rates exceed 95%.4
Muscle mass declines by 2–4% per year with ADT and occurs maximally in the first 6 to 12 months.5, 6, 7 This has been associated with subjective reports of increased fatigue and decreased quality of life.8 However, whether this translates into deficits in muscle strength or function is not known, with inconsistent results demonstrated. This may be because previous tests of muscle function (such as leg‐press or chair‐rise time) are relatively insensitive and may not be assessing functional deficits affected by ADT. It is also unclear whether ADT uniformly decreases muscle mass and function, or if individual muscles differ in testosterone sensitivity as seen in rodents.9
Video‐based motion capture technology can digitally record human movements in three dimensions with high precision.10, 11, 12 When these data are combined with recordings of ground reaction force and computerized musculoskeletal modelling, it is possible to generate detailed biomechanical analyses of muscle function, including the quantification of individual lower‐limb joint torques as well as estimates of individual muscle forces and their contributions to the functional tasks of support, propulsion, and mediolateral balance during walking.12 Extensively validated and used for over two decades in the biomechanics field, this approach has applications in animation, sports science, and robotics. The combination of computerized musculoskeletal modelling and three‐dimensional gait analysis has not previously been applied to longitudinal studies in humans. We used this methodology in this prospective controlled study to assess skeletal muscle function in men undergoing ADT.
We hypothesized that ADT leads to abnormalities in lower‐limb biomechanical function during gait and has differential effects on individual muscles.
Materials and methods
Methods
We conducted a prospective 12‐month case–control study of 63 age‐matched and radiotherapy‐matched, ADT‐naïve, ambulatory men with localized non‐metastatic prostate cancer recruited from an outpatient clinic for men with prostate cancer at a tertiary referral hospital (Austin Health, Melbourne, Australia). The study was approved by Human Research Ethics Committees at Austin Health and the University of Melbourne (Victoria, Australia). All participants provided written and informed consent.
Cases were 34 men newly commencing ADT planned for >12 months. All cases received gonadotropin‐releasing hormone agonists as ADT. Controls were 29 men not receiving ADT over 12 months. Prostate cancer controls were specifically recruited to control for a cancer diagnosis and ascertain the specific effect of testosterone deficiency alone. We did not include a control group of healthy older men, because this study was designed to specifically examine the effects of ADT. Groups were matched for age, body mass index, medical co‐morbidities, radiotherapy treatment, and baseline testosterone level. All men were independently living in the community, ambulant, fully active, unrestricted in their physical activities with normal performance status (Eastern Co‐operative Oncology Group performance status 0), and had no evidence of metastases on staging investigations at entry to the study. Men were excluded if they had evidence of androgen deficiency (baseline total testosterone <10 nmol/L), neuromuscular disease, limitation in their exercise tolerance, active cardiac, respiratory or joint disease, or if they required a walking aid. Subjects underwent assessments at 0, 6, and 12 months. Twenty‐nine men receiving ADT and 24 controls had complete gait analysis data available at all time points.
Each assessment included biomechanical gait analysis, serum biochemistry, hand‐grip strength (kilogram) (Jamar Hand Dynamometer, S.I. instruments, Adelaide, Australia), and Minnesota Leisure‐time Physical Activity Questionnaire.13 Lean mass was measured by dual‐energy X‐ray absorptiometry (Prodigy version 7.51; GE Lunar, Madison, WI, USA) at 0 and 12 months (coefficient of variation <2% for repeated scans).14 Dual‐energy X‐ray absorptiometry was not performed at 6 months to minimize radiation exposure. All men received general lifestyle education for prostate cancer and were encouraged to exercise regularly and maintain healthy dietary habits.
Biochemical assays
Fasting serum total testosterone was determined using an immunometric testosterone assay (Access, Beckman Coulter, Inc.) with a minimum detection limit of 0.4 nmol/L and an inter‐assay variation of 5.7% at 4.7 nmol/L. The reference range was 10.0–27.6 nmol/L, derived from an independent reference panel.15 Prostate‐specific antigen (PSA) was determined using electrochemiluminescent immunoassay (Cobas e602, Roche Diagnostics) with a minimum detection limit of 0.03 µg/L and an inter‐assay variation of 1.83% at 0.63 µg/L.
Biomechanical gait experiments
Participants performed level walking in the Biomotion Laboratory at the University of Melbourne (Australia). Two assessors performed all gait experiments at each visit (A. G. S. and A. S. C.). Experimental data were measured and processed as previously described and accepted in the biomechanics field.16, 17 Briefly, the three‐dimensional positions of 45 reflective markers attached to the bony prominences of each subject were recorded with nine cameras positioned circumferentially around the laboratory using a video motion capture system (VICON, Oxford Metrics Ltd., Oxford) sampling at a rate of 120 Hz (Figure 1). Ground reaction forces were measured using three force plates (AMTI, Watertown, MA) embedded in the floor. Each subject performed five walking trials (self‐selected speed), and the mean of two trials where the feet landed within the boundaries of the force plates were analysed. Data were recorded across one full gait cycle (ipsilateral heel‐strike to ipsilateral heel‐strike) and further sub‐divided into stance and swing phases (Figure 2). Lower‐limb joint angles represented in the sagittal plane and ground reaction forces are highly reproducible (coefficients of multiple correlations 0.968–0.995 and 0.942–0.997, respectively).10, 11 The coefficient of variation for repeated measurements of step width, stride length, and walking speed is 1.7–6.1%.11
Figure 1.
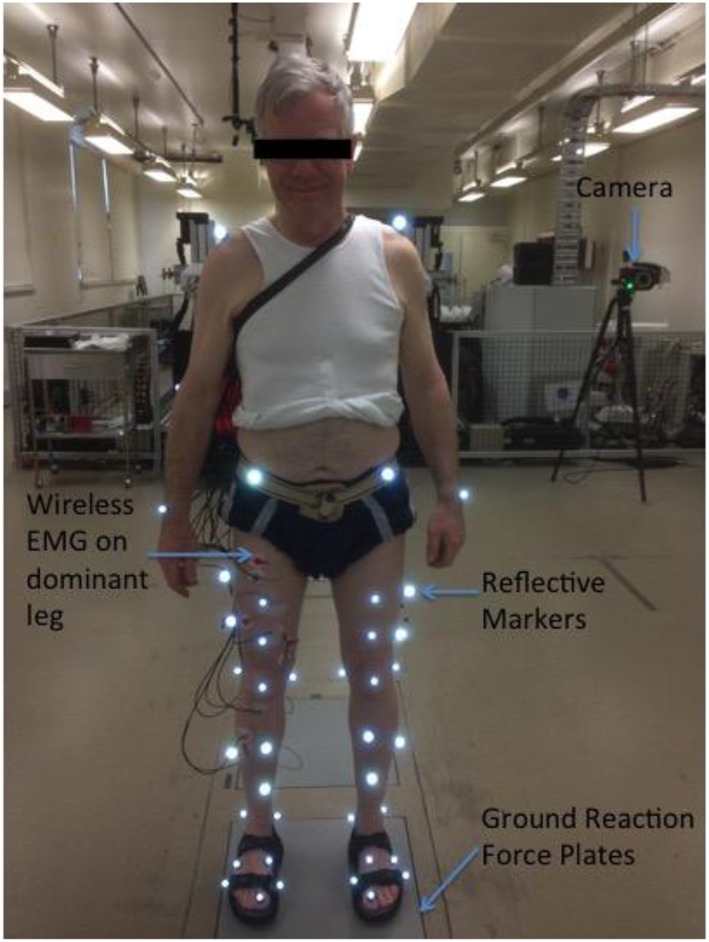
Representative participant in the Biomotion Laboratory. Anterior view of subject fitted with 45 reflective markers standing on one of the three ground reaction force plates embedded in the laboratory floor. Nine cameras were positioned circumferentially around the laboratory. Subject photographed has provided full consent for publication of image.
Figure 2.
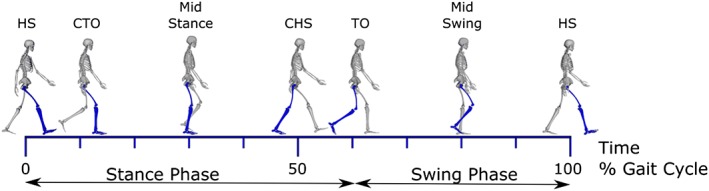
The gait cycle. Depicted is one gait cycle, with the right leg dominant, beginning at right heel strike and ending at the next right heel strike. HS, heel strike; CTO, contralateral toe off the ground; CHS, contralateral heel strike; TO, toe off. The stance phase is defined as the time the dominant (right) foot is on the ground, which is 62% of one gait cycle. The swing phase is the time the dominant foot is off the ground and comprises 38% of one gait cycle. Muscle activation varies for each individual muscle as a function of time during the gait cycle. The gluteus maximus, gluteus medius, hamstrings, and quadriceps are active during early stance (from right heel strike to just after contralateral toe‐off) to generate support whereas the iliopsoas, gastrocnemius, soleus, and plantarflexor invertors are active during mid‐late stance to accelerate the body forward in preparation for the subsequent swing phase.
Musculoskeletal model of the body
Biomechanical gait experiments provided precise position, dimensions, ground reaction forces, velocity, and acceleration of each subject and each subject's joints and body segments. This raw data then underwent computerized musculoskeletal modelling to determine outcomes of lower‐limb joint torques, individual muscle forces, and individual muscle contributions to acceleration of the body's centre of mass.18 Using physics‐based simulation of the human body, computerized musculoskeletal modelling can delineate how individual muscles and joints interact to produce movement. Three‐dimensional, muscle‐actuated simulations can accurately reproduce gait and other movement dynamics of individual patients and can be used to determine maximum isometric force and joint torque that a muscle can develop at any position and analyse how muscles contribute to motions (induced accelerations of the body's centre of mass) during walking or running. We used a generic three‐dimensional musculoskeletal model implemented in an open‐source biomechanics simulation software package to calculate net joint torques and lower‐limb muscle forces.19 The model is primarily of the lower extremities with the skeleton modelled as 14 segments with 23 degrees of freedom and 92 muscle‐tendon actuators to represent 76 muscles in the lower limbs and torso (Figure 3). Generic models were scaled to individual participant's body dimensions to develop subject‐specific models.
Figure 3.
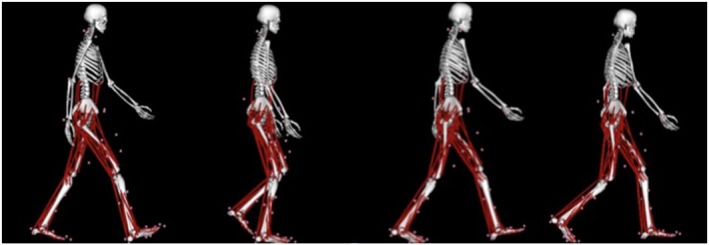
Muscle‐tendon actuators in a musculoskeletal model. Muscle‐tendon actuators shown in red used in the computerized musculoskeletal model representing lower‐limb and torso muscles of a subject during stance phase beginning with right heel strike through to right toe‐off.
Net joint torques and lower‐limb muscle forces
Net torques measured in Newton‐metre represent the sum of all the forces of the muscles acting to rotate a joint. The net torques developed about the hip, knee, and ankle joints were calculated using a standard inverse dynamics approach in the computerized musculoskeletal model. The joint torques were then decomposed into individual muscle forces (measured in Newtons) using a mathematical theory known as static optimization, which minimized the sum of all muscle activations squared, subject to each muscle's force–length–velocity properties.19
Individual muscle contributions to centre of mass accelerations
The function of an individual muscle can be described by how it contributes to moving the body during a task (such as walking), that is, by its contribution to acceleration of the body's centre of mass. The body accelerates in three directions during walking; vertically (upwards to maintain an erect position), anteroposteriorly (braking followed by propulsion), and mediolaterally (sideways). Using the computerized musculoskeletal model, a ‘pseudo‐inverse force decomposition’ method was used to compute the contribution of each muscle force to the acceleration of the centre of mass of the body in these three directions.20 All results were time‐normalized to the stance phase and averaged separately across all participants. Muscle forces were normalized to each participant's body weight (BW) whereas joint torques were normalized to BW multiplied by height (BW × H).
Step width, walking speed, and stride length
Step width was calculated based on the mediolateral distance between the right heel marker at right heel strike and the left heel marker at left heel strike. Walking speed was taken as the average speed of the reflective marker attached to the sacrum. Stride length was calculated by dividing the average speed calculated between consecutive heel strikes by time.
Sample size determination
Power calculations were based on walking speed, knee flexion torque, and hand‐grip strength as main outcomes. For 80% power (5% significance) to demonstrate a difference in joint torque of 0.3 N/m2 (SD 0.2), walking speed of 0.13 m/s (SD 0.07), and hand grip strength of 4.0 kg (SD 5.0), the sample size required for each cohort was 7, 5, and 25 men, respectively.21, 22, 23 It was estimated that 30 men per cohort would be required to allow for 20% drop‐out.
Statistical analysis
Non‐normally distributed clinical variables including testosterone, PSA, and physical activity are presented as median and interquartile range (IQR) (Tables 1 and 2). For these parameters, comparisons between groups at baseline were made using Wilcoxon rank‐sum test for continuous variables or χ2 test for frequencies. Gait variables were normally distributed and are presented as mean ± standard deviation (Tables 3 and 4). A linear mixed model was used to measure the difference in the change from baseline to 12 months between the ADT group and the control group. The fixed effects in the model were group, time, and their interaction, and the random effect was subject identity. The model accounts for between‐subject and within‐subject effects, where the between‐subject effect is group, within‐subject effect is time, and the interaction between group and time represents the change across groups over time. As a summary measure, the mean difference (95% confidence interval) which is the difference in the change across groups over time, and the corresponding P value are reported. A P < 0.05 was considered significant. Statistical analysis was performed using spss Statistics software (version 22.0.0.0 for Mac) (IBM Corporation, New York, USA).
Table 1.
Baseline characteristics of the study participants
| Baseline characteristic | ADT group | Controls | P value |
|---|---|---|---|
| n = 34 | n = 29 | ||
| Age (years) | 67.6 (64.6, 72.0) | 70.6 (65.3, 72.9) | 0.48 |
| Body mass index (kg/m2) | 27.8 (25.4, 31.5) | 27.2 (26.0, 31.8) | 0.75 |
| Prostate cancer Gleason score | 9 (8, 9) | 7 (7, 7) | <0.001 |
| Concurrent radiotherapy treatment | 94.1% | 89.7% | 0.51 |
| Total testosterone (nmol/L) | 14.1 (10.2, 17.6) | 15.0 (11.1, 16.9) | 0.91 |
| PSA (µg/L) | 3.62 (0.21, 18.7) | 0.05 (0.03, 0.28) | <0.001 |
| Haemoglobin (g/L) | 149 (140, 157) | 150 (142, 155) | 0.66 |
| Medical co‐morbidities | |||
| Ischaemic heart disease | 17.6% | 17.2% | 1.00 |
| Diabetes mellitus | 14.7% | 17.2% | 1.00 |
| Liver disease | 0% | 0% | 1.00 |
| Chronic kidney disease | 0% | 0% | 1.00 |
| Hypertension | 58.8% | 58.6% | 1.00 |
ADT, androgen deprivation treatment; PSA, prostate‐specific antigen.
Data presented are median (interquartile range) or proportions (%). P < 0.05 were considered statistically significant between groups (Wilcoxon‐sum rank test or χ2 test for frequencies). Prostate cancer Gleason score <7, low‐moderate risk; 7, intermediate risk; 8–10, high risk prostate cancer.
Table 2.
Differences in clinical parameters across groups over time
| Clinical values | ADT group (n = 34) | Controls (n = 29) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Total testosterone (nmol/L) | ||||
| 0 months | 14.1 (10.2, 17.6) | 15.0 (11.1, 16.9) | ||
| 6 months | 0.40 (0.30, 0.57)*** | 14.3 (9.90, 17.2) | ||
| 12 months | 0.40 (0.30, 0.50)*** | 14.8 (11.2, 15.6) | −13.0 (−15.4, −10.7) | <0.001 |
| PSA (µg/L) | ||||
| 0 months | 3.62 (0.21, 18.7) | 0.05 (0.03, 0.28) | ||
| 6 months | 0.03 (0.03, 0.11)*** | 0.03 (0.03, 0.21) | ||
| 12 months | 0.03 (0.03, 0.04)*** | 0.03 (0.03, 0.28) | −21.3 (−35.1, −8.2) | 0.002 |
| Handgrip strength (kg) | ||||
| 0 months | 41.5 (36.0, 44.0) | 40.0 (34.0, 45.0) | ||
| 6 months | 36.0 (32.0, 42.0)*** | 41.0 (34.0, 46.0) | ||
| 12 months | 38.0 (31.0, 43.0)*** | 43.0 (37.0, 48.0) | −4.66 (−7.09, −2.25) | <0.001 |
| Physical activity (kcal/week) | ||||
| 0 months | 1600 (1160, 3305) | 1599 (866, 2452) | ||
| 6 months | 1668 (688, 3260) | 1336 (516, 2449) | ||
| 12 months | 1525 (925, 2744) | 1195 (780, 1049) | 105 (−301, 511) | 0.73 |
| Lean mass (g) | ||||
| 0 months | 55029 (50 571, 60 589) | 55 302 (51 380, 60 516) | ||
| 12 months | 53 187 (49 423, 55 785)* | 54 485 (51 551, 58 669) | −1453 (−190, −2716) | 0.03 |
ADT, androgen deprivation treatment; CI, confidence interval; PSA, prostate‐specific antigen.
Data are presented as median (interquartile range). Mean Difference refers to the between group difference, which is the difference in the change in the mean of the ADT group over change in the mean of the control group over 12 months and is presented with [95% confidence interval (CI)] and P value (determined from the interaction effect of the mixed model). P < 0.05 were considered statistically significant and refers to overall significance of the change between groups during follow‐up.
P < 0.05.
P < 0.01.
P < 0.001 refers to a significant within group difference in the mean 12 month value and the mean 0 month value within the group (determined from the time effect of the mixed model and paired t‐test). Handgrip strength was measured in the dominant hand, best of three attempts. Self‐reported physical activity was measured by the Minnesota Leisure Time Physical Activity Questionnaire.13 Lean mass was only measured at 0 and 12 months.
Table 3.
Peak joint torques
| Peak torque (N m/kg) | ADT group (n = 29) | Controls (n = 24) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Hip flexion during late stance | ||||
| 0 months | 0.806 ± 0.124 | 0.801 ± 0.164 | ||
| 6 months | 0.683 ± 0.152 | 0.691 ± 0.172 | ||
| 12 months | 0.623 ± 0.140* | 0.728 ± 0.208* | −0.108 (−0.189, −0.028) | 0.009 |
| Hip extension during early stance | ||||
| 0 months | 0.872 ± 0.247 | 0.931 ± 0.193 | ||
| 6 months | 0.892 ± 0.185 | 0.997 ± 0.248 | ||
| 12 months | 0.988 ± 0.169*** | 0.996 ± 0.201*** | 0.050 (−0.033, 0.134) | 0.229 |
| Hip abduction during stance | ||||
| 0 months | 0.777 ± 0.096 | 0.804 ± 0.096 | ||
| 6 months | 0.825 ± 0.098 | 0.812 ± 0.100 | ||
| 12 months | 0.809 ± 0.073* | 0.831 ± 0.085* | 0.006 (−0.048, 0.059) | 0.836 |
| Knee extension during early stance | ||||
| 0 months | 0.686 ± 0.198 | 0.619 ± 0.192 | ||
| 6 months | 0.593 ± 0.231 | 0.507 ± 0.178 | ||
| 12 months | 0.504 ± 0.191*** | 0.546 ± 0.150*** | −0.109 (−0.199, −0.018) | 0.019 |
| Ankle subtalar inversion during stance | ||||
| 0 months | 0.245 ± 0.072 | 0.274 ± 0.088 | ||
| 6 months | 0.275 ± 0.086 | 0.259 ± 0.082 | ||
| 12 months | 0.270 ± 0.082 | 0.273 ± 0.092 | 0.026 (−0.006, 0.059) | 0.113 |
| Ankle plantar flexion during late stance | ||||
| 0 months | 1.334 ± 0.112 | 1.410 ± 0.100 | ||
| 6 months | 1.338 ± 0.120 | 1.453 ± 0.100 | ||
| 12 months | 1.399 ± 0.100** | 1.437 ± 0.116** | 0.039 (−0.022, 0.099) | 0.203 |
ADT, androgen deprivation treatment; CI, confidence interval.
Peak torque (Newton‐metres) was adjusted for body weight (kilogram) and leg length. Data are presented as mean ± standard deviation. P < 0.05 was considered statistically significant. Mean difference refers to the between group difference, which is the difference in the change in the mean of the ADT group over change in the mean of the control group over 12 months and is presented with [95% confidence interval (CI)] and P value (determined from the interaction effect of the mixed model). There were no statistically significant interactions between 0 and 6 months.
P < 0.05.
P < 0.01.
P < 0.001 refers to a significant within group difference in the mean 12 month value and the mean 0 month value within the group (determined from the time effect of the mixed model and paired t‐test).
Table 4.
Individual muscle contributions to acceleration of the body's centre of mass in the vertical, anteroposterior, and mediolateral directions
| Accelerations m/s | ADT group (n = 29) | Controls (n = 24) | Mean difference (95% CI) | P value |
|---|---|---|---|---|
| Vertical (upwards) acceleration | ||||
| Peak gluteus medius during early stance | ||||
| 0 months | 2.49 ± 0.41 | 2.61 ± 0.41 | ||
| 6 months | 2.61 ± 0.42 | 2.66 ± 0.47 | ||
| 12 months | 2.61 ± 0.41** | 2.73 ± 0.44** | −0.001 (−0.14, 0.14) | 0.987 |
| Peak gluteus maximus during early stance | ||||
| 0 months | 2.74 ± 0.92 | 2.92 ± 0.69 | ||
| 6 months | 3.07 ± 0.85 | 3.27 ± 0.85 | ||
| 12 months | 3.23 ± 0.76*** | 3.32 ± 0.91*** | 0.10 (−0.29, 0.49) | 0.624 |
| Peak hamstrings during early stance | ||||
| 0 months | 0.84 ± 0.45 | 0.89 ± 0.44 | ||
| 6 months | 0.81 ± 0.36 | 0.88 ± 0.46 | ||
| 12 months | 0.96 ± 0.48 | 0.89 ± 0.48 | 0.12 (−0.05, 0.29) | 0.160 |
| Peak quadriceps during early stance | ||||
| 0 months | 4.45 ± 1.41 | 4.07 ± 1.10 | ||
| 6 months | 3.96 ± 1.59 | 3.58 ± 1.10 | ||
| 12 months | 3.58 ± 1.26*** | 3.73 ± 1.04*** | −0.53 (−1.09, 0.03) | 0.063 |
| Peak gastrocnemius during late stance | ||||
| 0 months | 1.91 ± 0.66 | 1.84 ± 0.55 | ||
| 6 months | 1.92 ± 0.78 | 2.10 ± 0.54 | ||
| 12 months | 2.13 ± 0.83*** | 2.10 ± 0.59*** | −0.04 (−0.29, 0.21) | 0.737 |
| Peak soleus during late stance | ||||
| 0 months | 6.75 ± 0.52 | 6.83 ± 0.62 | ||
| 6 months | 6.54 ± 0.62 | 6.82 ± 0.59 | ||
| 12 months | 6.73 ± 0.50 | 6.89 ± 0.56 | −0.08 (−0.35, 0.19) | 0.558 |
| Anteroposterior acceleration (anterior/forwards = propulsion, posterior/backwards = braking) | ||||
| Peak gluteus medius contribution to braking | ||||
| 0 months | 0.32 ± 0.11 | 0.38 ± 0.14 | ||
| 6 months | 0.38 ± 0.12 | 0.39 ± 0.16 | ||
| 12 months | 0.39 ± 0.16** | 0.44 ± 0.19** | 0.02 (−0.06, 0.10) | 0.663 |
| Peak gluteus maximus contribution to braking | ||||
| 0 months | 0.58 ± 0.20 | 0.67 ± 0.20 | ||
| 6 months | 0.66 ± 0.17 | 0.76 ± 0.21 | ||
| 12 months | 0.72 ± 0.19*** | 0.76 ± 0.18*** | 0.06 (−0.06, 0.17) | 0.328 |
| Peak quadriceps contribution to braking | ||||
| 0 months | 1.86 ± 0.50 | 1.78 ± 0.38 | ||
| 6 months | 1.70 ± 0.57 | 1.57 ± 0.39 | ||
| 12 months | 1.58 ± 0.44*** | 1.63 ± 0.37*** | −0.14 (−0.34, 0.05) | 0.140 |
| Peak iliopsoas contribution to propulsion | ||||
| 0 months | 0.44 ± 0.21 | 0.50 ± 0.17 | ||
| 6 months | 0.49 ± 0.16 | 0.55 ± 0.21 | ||
| 12 months | 0.55 ± 0.25** | 0.54 ± 0.16** | 0.07 (−0.04, 0.18) | 0.221 |
| Peak gastrocnemius contribution to propulsion | ||||
| 0 months | 0.54 ± 0.15 | 0.58 ± 0.15 | ||
| 6 months | 0.52 ± 0.15 | 0.55 ± 0.14 | ||
| 12 months | 0.55 ± 0.13 | 0.57 ± 0.16 | 0.02 (−0.05, 0.08) | 0.628 |
| Peak Soleus contribution to propulsion | ||||
| 0 months | 1.00 ± 0.24 | 0.82 ± 0.17 | ||
| 6 months | 0.86 ± 0.30 | 0.73 ± 0.14 | ||
| 12 months | 0.78 ± 0.26*** | 0.78 ± 0.19*** | −0.17 (−0.29, −0.05) | 0.005 |
| Mediolateral (sideways) accelerationa | ||||
| Peak gluteus medius | ||||
| 0 months | −1.09 ± 0.14 | −1.11 ± 0.14 | ||
| 6 months | −1.11 ± 0.14 | −1.12 ± 0.14 | ||
| 12 months | −1.11 ± 0.14 | −1.10 ± 0.15 | −0.03 (−0.09, 0.03) | 0.28 |
ADT, androgen deprivation treatment; CI, confidence interval.
Data are presented as mean ± standard deviation. P < 0.05 was considered statistically significant. Mean difference refers to the between group difference, which is the difference in the change in the mean of the ADT group over change in the mean of the control group over 12 months and is presented with [95% confidence interval (CI)] and P value (determined from the interaction effect of the mixed model). There were no statistically significant interactions between 0 and 6 months.
P < 0.05.
P < 0.01.
P < 0.001 refers to a significant within group difference in the mean 12 month value and the mean 0 month value within the group (determined from the time effect of the mixed model and paired t‐test).
Negative values refer to medial accelerations and positive values refer to lateral accelerations.
Results
Baseline characteristics of both groups prior to ADT commencement are shown in Table 1. At baseline, all men had normal testosterone levels for age and were matched for age, radiotherapy status, and medical co‐morbidities. Inevitably, the two groups differed in their prostate cancer characteristics, consistent with indications for ADT treatment; the ADT group had high‐risk prostate cancer, whereas controls predominantly had intermediate‐risk disease (Table 1). These different risks are solely based on the Gleason score and PSA levels and were not expected to impact on physical performance.
In the ADT treated group, total testosterone levels and consequently PSA, lean body mass and hand‐grip strength significantly declined compared with controls over 12 months (Table 2). Self‐reported physical activity levels were unchanged over time in both groups.
Net joint torques and lower‐limb muscle forces
Mean differences between the change in the ADT group and the change in the control group are presented in Table 3 with corresponding P values. Asterisks in Table 3 highlight significance within group differences from 0 to 12 months for each calculated joint torque. Compared with controls over 12 months, men receiving ADT had a significant reduction in two joint torques; hip flexion and knee extension.
Peak hip flexor torque during late stance reduced by 14% [−0.108 (−0.189, −0.028) N m/kg, P = 0.009] over 12 months relative to the initial mean value (Table 3) mediated by a reduction in iliopsoas force in the ADT group (1.72 ± 0.37 times BW to 1.38 ± 0.36 times BW) compared with controls (1.66 ± 0.40 times BW to 1.56 ± 0.46 times BW), with a mean difference of −0.24 (−0.41, −0.07) times BW, P = 0.006. For the mean subject participant BW of 85 kg, this equated to a reduction of 20 kg in iliopsoas force (25%).
Peak knee extension torque during midstance also had a mean reduction of 16% [−0.109 (−0.199, −0.018) N m/kg, P = 0.019] over 12 months mediated predominantly by quadriceps. While the decrease in quadriceps force in the ADT group (1.54 ± 0.48 times BW to 1.25 ± 0.44 times BW) compared with controls (1.39 ± 0.34 times BW to 1.28 ± 0.35 times BW) was more marked [mean difference −0.17 (−0.36, 0.02) times BW, P = 0.073], this finding narrowly missed statistical significance. There were no other significant differences in individual muscle forces (data not shown).
No statistically significant between group changes in joint torques were observed at 6 months.
Individual muscle contributions to centre of mass accelerations
Individual muscle contributions to acceleration of the body's centre of mass in three directions are shown in Table 4. Compared with controls, the ADT group had decreased contribution from the soleus to anterior acceleration (forward propulsion) of the body's centre of mass by 17% of the ADT group's initial mean value over 12 months (Table 4). No significant changes were observed at 6 months.
Step width, walking speed, and stride length
Compared with controls over 12 months, the ADT group had a mean increase in step width of 18% (Figure 4C). There were no significant changes in walking speed, stride length (Figure 4A and 4B) or ground reaction forces over time (data not shown).
Figure 4.
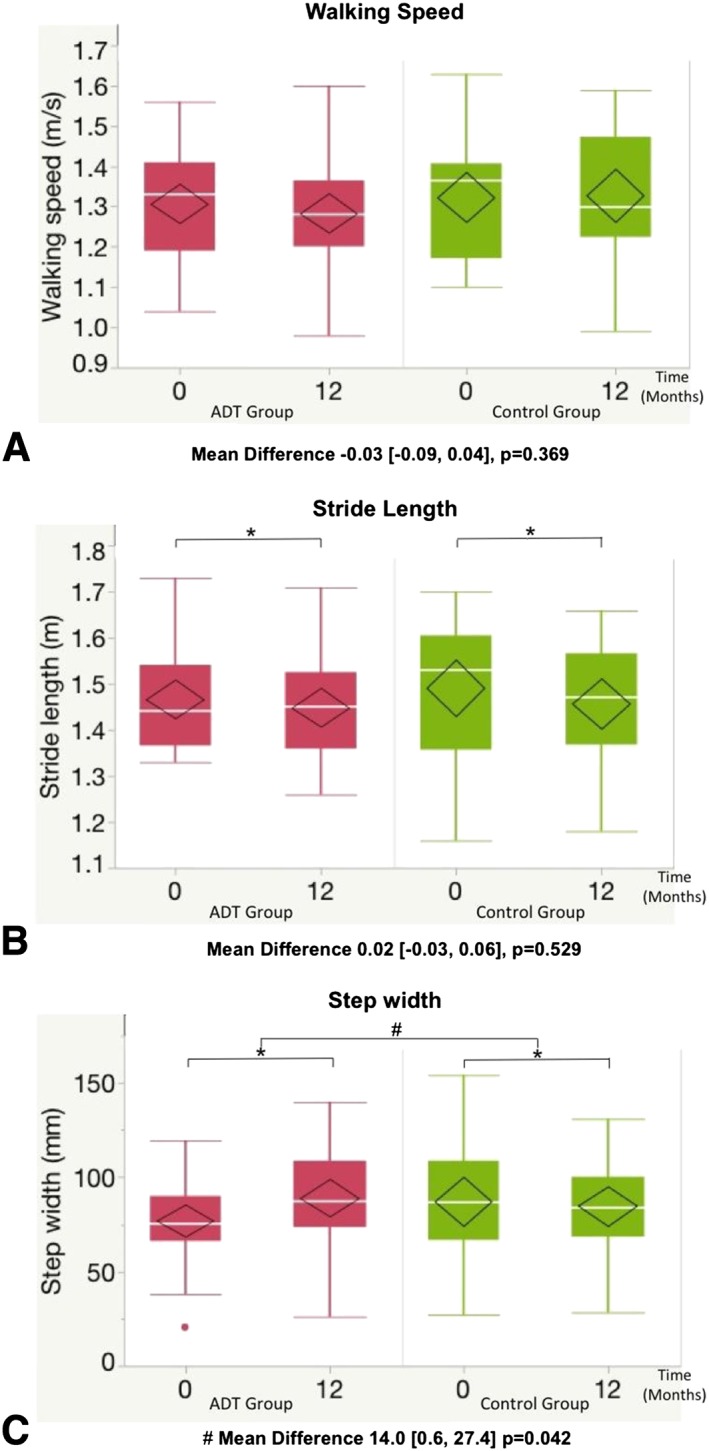
Temporal‐spatial parameters. Mean difference refers to the difference in the change in the mean of the ADT group over change in the mean of the control group over 12 months and is presented with (95% confidence interval) and P value. # P < 0.05 represents a significant difference in the change in the mean of the ADT group compared with the change in the control group over 12 months (main outcome). * P < 0.05 represents a significant difference between the mean 12‐month value and mean baseline value within the same group. No significant interactions were observed from 0 to 6 months. Box plots demonstrate median, interquartile range, and range. The diamond represents the mean and the 95% confidence interval of the mean.
Discussion
Using quantitative gait analysis in conjunction with computerized musculoskeletal modelling, we report that ADT was associated with selective decrements in lower‐limb muscle function; specifically, peak hip flexor torque reduced by 14% in the ADT group, mediated by a reduction in iliopsoas force; knee extensor torque reduced by 16%, mediated by a reduction in quadriceps force; soleus' contribution to forward acceleration (propulsion) of the body reduced by 17%; and step width increased by 18%. These biomechanical changes were evident for walking at self‐selected speeds, a key functional activity of daily living but became detectable only after 12 months of androgen deprivation. The objective deficits in muscle function associated with ADT over time demonstrated here are in keeping with subjective reports of decreased physical aspects of quality of life demonstrated in prior studies.8
Despite causing systemic testosterone deprivation, and a decrease in total body lean mass, ADT did not affect all lower‐limb muscles equally. Instead, testosterone deprivation led to selective impairments in lower limb muscle function, predominantly affecting muscles involved in generating vertical support, accelerating the body forward (propulsion), and preparing the leg for the swing phase; namely, quadriceps, soleus, and iliopsoas, respectively. Selective effects are similarly seen in mouse models of testosterone deficiency, where these animals show more marked reductions in levator ani and hind‐limb muscles.24, 25
We found that men receiving ADT walked with a wider base of support by increasing their step width by 14 mm. This magnitude is considered a clinically meaningful change.26 This finding may provide an explanation for observational studies that have associated low testosterone with balance impairment and self‐reported falls.27 Interestingly, noting that testosterone levels gradually decline with aging, increased step width has been shown to be a good discriminator between older and younger adults in cross‐sectional studies.28, 29
Despite previous studies demonstrating that loss of muscle mass occurs early, within the first 3 to 6 months of ADT,5, 30 we observed no change in muscle function at 6 months and significant decrements were only evident after 12 months of androgen deprivation. Changes in muscle mass do not necessarily equate to changes in muscle function supporting the importance of distinguishing between these two parameters.31
Our findings may have implications for mitigating adverse effects for men receiving ADT for non‐metastatic prostate cancer, as well as for aging‐associated functional limitations. The functional deficits reported here identify specific targets for clinical trials to improve ADT and potentially aging‐associated sarcopenia. Strengthening of the knee extensor muscles may be particularly important, consistent with previous studies demonstrating that functional deficits in quadriceps are associated with mobility limitation and the age‐associated testosterone decline.32
There are limitations to this study. Our gait experiments did not assess strenuous activity or maximal voluntary muscle capacity. It is possible that ADT may lead to even more significant functional deficits with strenuous activity or endurance, which cannot be assessed with the methodology used here. However, we performed an in‐depth analysis of muscle function during walking, the most common and relevant activity of daily living for older adults. Secondly, a group of healthy controls was not included as the aim of the study was to specifically examine the effects of ADT. Hence, we used prostate cancer controls matched for co‐morbidities including cancer diagnosis, age, and radiotherapy status. Indications for ADT inevitably limited the ability to match for grade of prostate cancer. Therefore, men receiving ADT had, at baseline, higher histological grade (Gleason score 9 vs. 7 in controls) and higher PSA levels (3.62 vs. 0.05 µg/L). We cannot exclude the possibility that the between group differences in prostate cancer grade could have different effects on sarcopenia and muscle function over time. However, consequent to radiotherapy, PSA levels decreased in ADT‐treated men at 6 months and remained low at 12 months, with no difference to controls (Table 2). Most importantly, all study participants had localized disease without evidence of metastases on baseline staging investigations, were fully active, unrestricted in their physical activities, and had a normal performance status. Thirdly, physical activity was not standardized. While self‐reported questionnaires demonstrated no change in leisure‐time physical activity levels, we cannot exclude the possibility that the mechanisms by which ADT led to the gait impairments included reduced physical activity.
However, the strengths of this study are significant and include the first application of this methodology to longitudinal studies in humans. This allowed the identification of specific deficits in gait biomechanics, which provide new insights into the biology of androgen action in human skeletal muscle. Identification of a precise method to study the effects of ADT and possibly androgens in age‐related frailty is valuable, given the contradictory results of previous functional studies and that there are currently no effective treatment strategies, which have demonstrated a consistent benefit on function. Application of this methodology to interventional trials has the potential to develop strategies targeted to the specific deficits to prevent loss of muscle mass and functional deficits associated with ADT, to ultimately improve physical functioning, an important aspect of quality of life for many patients with prostate cancer.
In summary, this longitudinal study has demonstrated that ADT leads to selective decrements in lower‐limb muscle function, particularly affecting muscles that generate support and forward propulsion of the body, with muscles responsible for balance also adversely affected. The gait phenotype identified here may provide a rational basis to guide exercise and promyogenic pharmacotherapy in clinical trials to mitigate ADT‐associated sarcopenia and improve outcomes for men with prostate cancer.
Author contributions
A. S. C., M. P., and M. G. designed the research study. A. S. C. and D. L. J. recruited all participants. A. S. C. and A. G. S. conducted all experiments and acquired the data. H. G. processed the data and performed computerized musculoskeletal modelling with assistance from A. G. S. and M. P., A. S. C., R. H., and H. G. performed statistical analysis of the results. A. S. C. and M. G. wrote the manuscript. All co‐authors revised and approved the current manuscript.
Conflict of interest
The authors have declared that no conflict of interest exists.
Acknowledgements
This study was supported by a National Health and Medical Research Council of Australia (NHMRC) project grant (1006407). A. S. C. is supported by a NHMRC postgraduate scholarship (1017233) to undertake this project as part of a PhD. M. G. is supported by a NHMRC Career Development Fellowship (1024139). Support provided by an Australian Research Council Discovery Projects grant (DP1095366) and an Innovation Fellowship provided by the Victorian Endowment for Science, Knowledge, and Innovation to M. G. P. are also gratefully acknowledged.
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia, and Muscle: update 2015.33
Cheung, A. S. , Gray, H. , Schache, A. G. , Hoermann, R. , Lim Joon, D. , Zajac, J. D. , Pandy, M. G. , and Grossmann, M. (2017) Androgen deprivation causes selective deficits in the biomechanical leg muscle function of men during walking: a prospective case–control study. Journal of Cachexia, Sarcopenia and Muscle, 8: 102–112. doi: 10.1002/jcsm.12133.
References
- 1. Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, Yeap BB. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab 2010;95:3165–3172. [DOI] [PubMed] [Google Scholar]
- 2. Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, Zhang A, Coviello A, Kelly‐Hayes M, D'Agostino RB, et al. Free testosterone levels are associated with mobility limitation and physical performance in community‐dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010;95:2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett‐Connor E, Fink HA, Hoffman AR, Lau E, Lane NE, et al. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab 2009;94:3806–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorff TB, Flaig TW, Tangen CM, Hussain MH, Swanson GP, Wood DP Jr, Sakr WA, Dawson NA, Haas NB, Crawford ED, et al. Adjuvant androgen deprivation for high‐risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol 2011;29:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab 2005;90:6410–6417. [DOI] [PubMed] [Google Scholar]
- 6. Boxer RS, Kenny AM, Dowsett R, Taxel P. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male 2005;8:207–212. [DOI] [PubMed] [Google Scholar]
- 7. Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, Leder BZ, Goessl C. Sarcopenia during androgen‐deprivation therapy for prostate cancer. J Clin Oncol 2012;30:3271–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheung AS, Zajac JD, Grossmann M. Muscle and bone effects of androgen deprivation therapy: current and emerging therapies. Endocr Relat Cancer 2014;21:R371–394. [DOI] [PubMed] [Google Scholar]
- 9. Rana K, Fam BC, Clarke MV, Pang TP, Zajac JD, MacLean HE. Increased adiposity in DNA binding‐dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab 2011;301:E767–778. [DOI] [PubMed] [Google Scholar]
- 10. McGinley JL, Baker R, Wolfe R, Morris ME. The reliability of three‐dimensional kinematic gait measurements: a systematic review. Gait Posture 2009;29:360–369. [DOI] [PubMed] [Google Scholar]
- 11. Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GV. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J Orthopaedic Res 1989;7:849–860. [DOI] [PubMed] [Google Scholar]
- 12. Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng 2010;12:401–433. [DOI] [PubMed] [Google Scholar]
- 13. Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–755. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim‐Joon D, Bolton D, Zajac JD, Grossmann M. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf) 2011;74:377–383. [DOI] [PubMed] [Google Scholar]
- 15. Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, Handelsman DJ. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab 2005;90:5928–5936. [DOI] [PubMed] [Google Scholar]
- 16. Lin YC, Fok LA, Schache AG, Pandy MG. Muscle coordination of support, progression and balance during stair ambulation. J Biomech 2015;48:340–347. [DOI] [PubMed] [Google Scholar]
- 17. Pandy MG, Lin YC, Kim HJ. Muscle coordination of mediolateral balance in normal walking. J Biomech 2010;43:2055–2064. [DOI] [PubMed] [Google Scholar]
- 18. Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open‐source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 2007;54:1940–1950. [DOI] [PubMed] [Google Scholar]
- 19. Anderson FC, Pandy MG. Dynamic optimization of human walking. J Biomech Eng 2001;123:381–390. [DOI] [PubMed] [Google Scholar]
- 20. Lin Y‐CY, Kim HJH, Pandy MMG. A computationally efficient method for assessing muscle function during human locomotion. Int J Num Methods Biomed Eng 2011;27:436–449. [Google Scholar]
- 21. Persch LN, Ugrinowitsch C, Pereira G, Rodacki AL. Strength training improves fall‐related gait kinematics in the elderly: a randomized controlled trial. Clin Biomech 2009;24:819–825. [DOI] [PubMed] [Google Scholar]
- 22. Bento PC, Pereira G, Ugrinowitsch C, Rodacki AL. Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech 2010;25:450–454. [DOI] [PubMed] [Google Scholar]
- 23. Soyupek F, Soyupek S, Perk H, Ozorak A. Androgen deprivation therapy for prostate cancer: effects on hand function. Urol Oncol 2008;26:141–146. [DOI] [PubMed] [Google Scholar]
- 24. Axell AM, MacLean HE, Plant DR, Harcourt LJ, Davis JA, Jimenez M, Handelsman DJ, Lynch GS, Zajac JD. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab 2006;291:E506–516. [DOI] [PubMed] [Google Scholar]
- 25. MacLean HE, Chiu WS, Notini AJ, Axell AM, Davey RA, McManus JF, Ma C, Plant DR, Lynch GS, Zajac JD. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J 2008;22:2676–2689. [DOI] [PubMed] [Google Scholar]
- 26. Brach JS, Perera S, Studenski S, Katz M, Hall C, Verghese J. Meaningful change in measures of gait variability in older adults. Gait Posture 2010;31:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szulc P, Claustrat B, Marchand F, Delmas PD. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab 2003;88:5240–5247. [DOI] [PubMed] [Google Scholar]
- 28. Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech 2004;37:935–938. [DOI] [PubMed] [Google Scholar]
- 29. Schrager MA, Kelly VE, Price R, Ferrucci L, Shumway‐Cook A. The effects of age on medio‐lateral stability during normal and narrow base walking. Gait Posture 2008;28:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, Kantoff PW. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002;87:599–603. [DOI] [PubMed] [Google Scholar]
- 31. de Rooy C, Grossmann M, Zajac JD, Cheung AS. Targeting muscle signaling pathways to minimize adverse effects of androgen deprivation. Endocr Relat Cancer 2016;23:R15–R26. [DOI] [PubMed] [Google Scholar]
- 32. Hsu B, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ. Longitudinal relationships of circulating reproductive hormone with functional disability, muscle mass, and strength in community‐dwelling older men: the Concord Health and Ageing in Men project. J Clin Endocrinol Metab 2014;99:3310–3318. [DOI] [PubMed] [Google Scholar]
- 33. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


