Abstract
An alkaline agent, namely food additive grade calcium hydroxide (FdCa (OH)2) in the powder form, was evaluated for its bactericidal efficacies in chicken feces at pH 13. The point for this evaluation was neutralization of the alkaline agent’s pH at the time of bacterial recovery, since otherwise the results are substantially misleading. Without neutralization of the FdCa (OH)2 pH, the spiked bacteria were killed within min at the time of recovery in aqueous phase, but not in the solid form in feces, hence, it has been demonstrated that when bacteria were in solid, it took longer time than in liquid for the alkaline agent to inactivate them down to the acceptable level (≥3 log10 CFU/ml).
Keywords: food additive grade calcium hydroxide, infection control/disinfection, livestock biosecurity, poultry production
Disinfectants and their application comprise an essential part of infection control strategies at livestock farms. Currently, various sources of antimicrobial products are available [8, 23]. Among them, alkaline agents (especially lime) are well known for their strong bactericidal activity and are most frequently applied for the control and prevention of biological hazards at farms, because they can inactivate bacteria under contamination of organic materials [1, 2, 17, 20, 26]. Bactericidal efficacy of lime has been evaluated by researchers, but during recovery of spiked microorganism, these researchers have used distilled water, phosphate buffered saline or medium that would raise the pH value of the mixture in the aqueous phase and result in inactivation of the treated microorganisms during their recovery, which is a misleading result [1, 2, 20, 21, 24, 26, 29]. Such incorrectness of the results may cause outbreaks of infectious diseases in the poultry industry despite application of lime as disinfectant and consequently bring about huge economic loses, along with animal and public health’s concerns.
Among the bacterial infections, salmonellosis and colibacillosis are very common, and they are most frequently present at farms, causing high morbidity and mortality, and reducing productivity of the chicks and hatchability of the eggs [5, 9, 11, 13, 16, 19]. In addition to that, billion tons of poultry bedding materials are harvested from the poultry farms, annually, which are highly contaminated with various kinds of pathogens [15, 28], including Salmonella spp. and E. coli [7, 22], and farmers are using these bedding materials for fertilization of their farm lands as a chosen method of litter disposal [4, 12]. Several studies have demonstrated the role of poultry litter or their wastes, in the contamination of surface water and environments around [3, 6, 17, 18, 27]. Thus, Salmonella spp. and E. coli constitute a meaningful public health concern as well.
To enhance the biosecurity at farms and to prevent contamination of farms and the environments around, it is worthwhile to establish a perfect evaluation method for the alkaline agents, in order to find the required exact concentration and time for the alkaline agents to inactivate pathogens in feces and litter, and finally to suggest their proper application at farms, for enhancement of the biosecurity.
Food additive grade calcium hydroxide (FdCa (OH)2) powder, that is a novel product at pH 13, made of natural calcium carbonates derived from limestone through calcination process, with the average diameter of the powder size at 10 µm, was kindly provided by Fine Co., Ltd. (Tokyo, Japan). Chicken feces were collected from chickens less than three weeks old kept in our laboratory and autoclaved at 121°C for 15 min; then, they were heated at 80°C for 60 min, in order to reduce their humidity and stored at 4°C until use in the experiments.
Bacterial suspensions of E. coli strain NBRC106373 and S. Infantis were prepared and enumerated as described previously [10].
Bacterial inactivation was considered as reduction factor (RF) [14] and was calculated using the equation below after conversion of bacterial titer to the log10 CFU/ml:
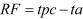 |
In the above equation, tpc is the titer of bacteria from untreated sample in log10 CFU/ml, while ta is the titer of recovered bacteria from treated samples. The inactivation rate was acceptable when the RF was greater than or equal to 3 [10, 14, 25, 26].
For the recovery of spiked bacteria from feces using phosphate buffered saline (PBS: 0.14 M NaCl, 2 mM KCl, 3 mM Na2HPO4 and 1.5 m KH2PO4, pH 7.4), the autoclaved feces were measured in weight and added into 50 ml conical centrifuge tubes according to the experiments design, in order to make final concentrations of 0, 5, 10 and 20% (w/w), in the total weight of 0.5 g with FdCa (OH)2 powder. Hundred microliters of E. coli or S. Infantis were inoculated in the measured feces and vortexed to mix well; then, FdCa (OH)2 powder was added on them, followed by mixing by vortex mixer. Soon after, 10 ml PBS was added on the mixture to harvest the spiked bacteria from feces through mixing by vortex for about 1 min. Serial tenfold dilution was prepared immediately per sample and plated on DHL agar as described [10].
For the recovery of spiked bacteria from feces after neutralization of pH, the experiments were designed the same as bacterial recovery from feces using PBS, but after adding FdCa (OH)2 powder to the contaminated feces, followed by proper mixing, samples were incubated for different exposure times (3, 6 and 20 hr) at room temperature (25 ± 2°C) in a dark place. Then, 10 ml 1M Tris-HCl (pH 7.2) was added on the mixture to stop FdCa (OH)2 powder activities and to harvest the remained bacteria from feces through mixing by vortex for about 1 min. Then, serial tenfold dilution was prepared per sample and plated on DHL agar as described above.
In order to confirm whether Tris-HCl is able to stop bactericidal activities of FdCa (OH)2 powder, 10 ml of Tris-HCl was added on the inoculated feces, after its incubation for the certain time periods with the control and then, FdCa (OH)2 powder was added, and the same procedure was conducted with the other samples. As there was no contact between bacteria with FdCa (OH)2 powder, it was recorded as 0 min contact times.
Table 1 shows bactericidal efficacies of different concentrations of FdCa (OH)2 powder on the bacteria during their recovery by PBS. When FdCa (OH)2 treated bacteria were recovered by PBS, these bacteria were inactivated with RF=4.53 (E. coli), and RF=4.59 (S. Infantis) within min, down to the detectable level (≤3.6 log10 CFU/ml), even at its low concentration (5%).
Table 1. Inactivation of bacteria by FdCa (OH)2 in feces during their recovery by PBS, within min.
| Concentration of FdCa (OH)2 | RFa) | |
|---|---|---|
| E. coli | S. Infantis | |
| 5% | ≥4.59 ± 0.00b) | ≥4.53 ± 0.00 |
| 10% | ≥4.59 ± 0.00b) | ≥4.53 ± 0.00 |
| 20% | ≥4.59 ± 0.00b | ≥4.53 ± 0.00 |
Chicken feces were inoculated with bacteria in conical centrifuge tubes, and then, different amounts of FdCa (OH)2 powder was added on them and mixed properly, and soon thereafter, the remaining bacteria were harvested by adding 10 ml PBS. a) Reduction factor=log10 (titer of control/ml) −log10 (titer of treated samples/ml). b) Data represent mean ± standard deviation of three individual reactions.
In a preliminary experiment, at 1 hr exposure time, even application of 30% concentrated FdCa (OH)2 powder reduced titer of the tested bacteria only with RF=2.1, less than the acceptable level (RF≥3.0), if bacteria were recovered after neutralization (data not shown). Table 2 illustrates efficacy of different percentage of FdCa (OH)2 powder on E. coli and S. Infantis in feces. In the 0 min contact time, FdCa (OH)2 powder could not reduce titer of the tested bacteria at all (RF=0.0), but within 3 hr of exposure time, its 5% concentration reduced the titer of E. coli (RF=2.81), and S. Infantis (RF=2.88), which is under the acceptable level (RF≥3); however, when incubation time was increased to 6 hr, it was able to inactivate both tested bacteria down to the detectable level (RF≥4.5). Furthermore, FdCa (OH)2 powder in the higher concentration (10%) required 3 hr exposure time to decrease titer of tested bacteria down to the detectable limit (RF≥4.3).
Table 2. Bactericidal effects of FdCa (OH)2 powder towards the bacteria present in feces.
| Concentration of FdCa (OH)2 | Bacteria | RFa) | ||
|---|---|---|---|---|
| 0b) min | 3 hr | 6 hr | ||
| 5% | E. coli | 0.00 ± 0.00 | 2.81 ± 1.31c) | ≥4.53 ± 0.00 |
| S. Infantis | 0.00 ± 0.00 | 2.88 ± 1.60 | ≥4.73 ± 0.00 | |
| 10% | E. coli | 0.00 ± 0.00 | ≥4.33 ± 0.00 | ≥4.53 ± 0.00 |
| S. Infantis | 0.00 ± 0.00 | ≥4.73 ± 0.00 | ≥4.73 ± 0.00 | |
Chicken feces were inoculated with bacteria in conical centrifuge tubes, and then, different amounts of FdCa (OH)2 powder was added on them and mixed properly, kept for different exposure times, and then, the remaining bacteria were harvested by 10 ml of 1 M Tris-HCl. a) Reduction factor=log10 (titer of control/ml) −log10 (titer of treated samples/ml). b) Incubation times. c) Data represent mean ± standard deviation of three different reactions.
Pathogens in contaminated feces play a critical role in the transmission of infectious diseases from one to other animals, as well as in the contamination of surrounding environments. Inactivation of pathogens present in the feces plays a fundamental role in the prevention of fecal-oral transmission of infections and in the enhancement of biosecurity at the livestock farms.
FdCa (OH)2 powder is a novel product, which is demonstrated as excellent bactericidal efficacies in chicken feces in the present study. FdCa (OH)2 powder efficacy was highly related to its pH value. When it was resuspended in PBS, the aqueous phase pH was found ≥13, but resuspended in 10 ml Tris-HCl, and as in the 0 min contact time, its pH decreased to around 8, and reduction of its pH resulted in ceasing of its efficacy (Table 2); as there was no reduction observed in the titer of exposed bacteria, it confirms the accuracy of our evaluation system.
Demonstration of an accurate evaluation system for appraising disinfectants is very important to prevent misleading results. Data on Tables 1 and 2 show a very considerable gap between the capacities of an alkaline agent to inactivate bacteria, as a result of inaccurate evaluation method. Inaccurate reports regarding bactericidal competence will cause outbreaks of diseases despite of application of alkaline agents as disinfectant at farms. Accurate evaluation method would minimize such severe error during application of disinfectants at farms. In addition to that, it will help farmers to design a better strategy for disease prevention and control, and finally would minimize the animal and public health concerns about infectious diseases.
In conclusion, the present study highlighted the misleading results of the alkaline agent’s evaluations as bactericides, their requirement for long exposure time in solid and the accuracy of the established evaluation methods for alkaline agents. The findings of this study can also help farmers to properly apply alkaline agents in appropriate concentrations and exposure times in their farms, in order to prevent and control infectious diseases outbreaks and to enhance biosecurity. Finally, the findings may help farmers to implement better strategies for controlling infections in their livestock farms.
Acknowledgments
The authors thank Fine Co., Ltd., for providing FdCa (OH)2, used in this experiment. This study was supported in part by Regulatory Science, Ministry of Agriculture, Forestry and Fisheries, Japan (MAFF) 2016.
REFERENCES
- 1.Bean C. L., Hansen J. J., Margolin A. B., Balkin H., Batzer G., Widmer G.2007. Class B alkaline stabilization to achieve pathogen inactivation. Int. J. Environ. Res. Public Health 4: 53–60. doi: 10.3390/ijerph2007010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett D. D., Higgins S. E., Moore R. W., Beltran R., Caldwell D. J., Byrd J. A., Hargis B. M.2003. Effects of lime on Salmonella Enteritidis survival in vitro. J. Appl. Poult. Res. 12: 65–68. doi: 10.1093/japr/12.1.65 [DOI] [Google Scholar]
- 3.Bolan N. S., Szogi A. A., Chuasavathi T., Seshadri B., Rothrock M. J., Panneerselvam P.2010. Uses and management of poultry litter. Worlds Poult. Sci. J. 66: 673–698. doi: 10.1017/S0043933910000656 [DOI] [Google Scholar]
- 4.Bujoczek G., Oleszkiewicz J., Sparling R., Cenkowski S.2000. High solid anaerobic digestion of chicken manure. J. Agric. Eng. Res. 76: 51–60. doi: 10.1006/jaer.2000.0529 [DOI] [Google Scholar]
- 5.Center for Diseases Prevention and Control2013. Antibiotic resistance threat in the United States. Available from; http://www.cdc.gov/drugresistance/threat-report-2013(Accesed 2014).
- 6.Chen Z., Jiang Z.2014. Microbiological safety of chicken litter or chicken litter-based organic fertilizers: A review. Agriculture 4: 1–29. doi: 10.3390/agriculture4010001 [DOI] [Google Scholar]
- 7.Diarrassouba F., Diarra M. S., Bach S., Delaquis P., Pritchard J., Topp E., Skura B. J.2007. Antibiotic resistance and virulence genes in commensal Escherichia coli and Salmonella isolates from commercial broiler chicken farms. J. Food Prot. 70: 1316–1327. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak G.2008. Disinfection 101. Center for food security and public health: 1–20. Available from; http://www.cfsph.iastate.edu/Disinfection/Assets/ Disinfection 101 .pdf.
- 9.Grant A., Hashem F., Parveen S.2016. Salmonella and Campylobacter: Antimicrobial resistance and bacteriophage control in poultry. Food Microbiol. 53Pt B: 104–109. doi: 10.1016/j.fm.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Hakim H., Alam M. S., Sangsriratanakul N., Nakajima K., Kitazawa M., Ota M., Toyofuku C., Yamada M., Thammakarn C., Shoham D., Takehara K.2016. Inactivation of bacteria on surfaces by sprayed slightly acidic hypochlorous acid water: in vitro experiments. J. Vet. Med. Sci. 78: 1123–1128. doi: 10.1292/jvms.16-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwabuchi E., Maruyama N., Hara A., Nishimura M., Muramatsu M., Ochiai T., Hirai K.2010. Nationwide survey of salmonella prevalence in environmental dust from layer farms in Japan. J. Food Prot. 73: 1993–2000. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher B. P., Leahy J. J., Henihan A. M., O’Dwyer T. F., Sutton D., Leahy M. J.2002. Advances in poultry litter disposal technology--a review. Bioresour. Technol. 83: 27–36. doi: 10.1016/S0960-8524(01)00133-X [DOI] [PubMed] [Google Scholar]
- 13.Kim J. H., Kim K. S.2010. Hatchery hygiene evaluation by microbiological examination of hatchery samples. Poult. Sci. 89: 1389–1398. doi: 10.3382/ps.2010-00661 [DOI] [PubMed] [Google Scholar]
- 14.Lombardi M. E., Ladman B. S., Alphin R. L., Benson E. R.2008. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. 52: 118–123. doi: 10.1637/8055-070907-Reg [DOI] [PubMed] [Google Scholar]
- 15.Lu J., Sanchez S., Hofacre C., Maurer J. J., Harmon B. G., Lee M. D.2003. Evaluation of broiler litter with reference to the microbial composition as assessed by using 16S rRNA and functional gene markers. Appl. Environ. Microbiol. 69: 901–908. doi: 10.1128/AEM.69.2.901-908.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutful Kabir S. M.2010. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 7: 89–114. doi: 10.3390/ijerph7010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire R. O., Hesterberg D., Gernat A., Anderson K., Wineland M., Grimes J.2006. Liming poultry manures to decrease soluble phosphorus and suppress the bacteria population. J. Environ. Qual. 35: 849–857. doi: 10.2134/jeq2005.0339 [DOI] [PubMed] [Google Scholar]
- 18.Nayak B., Weidhaas J., Harwood V. J.2015. LA35 poultry fecal marker persistence is correlated with that of indicators and pathogens in environmental waters. Appl. Environ. Microbiol. 81: 4616–4625. doi: 10.1128/AEM.00444-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nógrády N., Kardos G., Bistyák A., Turcsányi I., Mészáros J., Galántai Z., Juhász A., Samu P., Kaszanyitzky J. E., Pászti J., Kiss I.2008. Prevalence and characterization of Salmonella infantis isolates originating from different points of the broiler chicken-human food chain in Hungary. Int. J. Food Microbiol. 127: 162–167. doi: 10.1016/j.ijfoodmicro.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Nyberg K. A., Vinnerås B., Lewerin S. S., Kjellberg E., Albihn A.2011. Treatment with Ca(OH)2 for inactivation of Salmonella Typhimurium and Enterococcus faecalis in soil contaminated with infected horse manure. J. Appl. Microbiol. 110: 1515–1523. doi: 10.1111/j.1365-2672.2011.05006.x [DOI] [PubMed] [Google Scholar]
- 21.Ota M., Toyofuku C., Thammakarn C., Sangsriratanakul N., Yamada M., Nakajima K., Kitazawa M., Hakim H., Alam M. S., Shoham D., Takehara K.2016. Calcinated egg shell as a candidate of biosecurity enhancement material. J. Vet. Med. Sci. 78: 831–836. doi: 10.1292/jvms.16-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roll V. F. B., Dai Prá M. A., Roll A. P.2011. Research on Salmonella in broiler litter reused for up to 14 consecutive flocks. Poult. Sci. 90: 2257–2262. doi: 10.3382/ps.2011-01583 [DOI] [PubMed] [Google Scholar]
- 23.Russell H., and Ayliffe, 2013. Type of microbicidal and microbistatic agents, pp. 5–70. In: Russell, Hugo and Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization, 5th ed. (Adam, F. P., Jean-Yves, M. and Sattar, S. A. eds.), Wiley-Blackwell, A John Wiley and Sons, Ltd., Publication, Chichester.
- 24.Stringfellow K., Caldwell D., Lee J., Byrd A., Carey J., Kessler K., McReynolds J., Bell A., Stipanovic R., Farnell M.2010. Pasteurization of chicken litter with steam and quicklime to reduce Salmonella Typhimurium. J. Appl. Poult. Res. 19: 380–386. doi: 10.3382/japr.2009-00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takehara K., Chinen O., Jahangir A., Miyoshi Y., Ueno Y., Ueda S., Takada Y., Ruenphet S., Mutoh K., Okamura M., Nakamura M.2009. Ceramic powder made from chicken feces: anti-viral effects against avian influenza viruses. Avian Dis. 53: 34–38. doi: 10.1637/8382-062008-Reg.1 [DOI] [PubMed] [Google Scholar]
- 26.Thammakarn C., Tsujimura M., Satoh K., Hasegawa T., Tamura M., Kawamura A., Ishida Y., Suguro A., Hakim H., Ruenphet S., Takehara K.2015. Efficacy of scallop shell powders and slaked lime for inactivating avian influenza virus under harsh conditions. Arch. Virol. 160: 2577–2581. doi: 10.1007/s00705-015-2517-9 [DOI] [PubMed] [Google Scholar]
- 27.Venglovsky J., Martinez J., Placha I.2006. Hygienic and ecological risks connected with utilization of animal manures and biosolids in agriculture. Livest. Sci. 102: 197–203. doi: 10.1016/j.livsci.2006.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Videnska P., Rahman M. M., Faldynova M., Babak V., Matulova M. E., Prukner-Radovcic E., Krizek I., Smole-Mozina S., Kovac J., Szmolka A., Nagy B., Sedlar K., Cejkova D., Rychlik I.2014. Characterization of egg laying hen and broiler fecal microbiota in poultry farms in Croatia, Czech Republic, Hungary and Slovenia. PLOS ONE 9: e110076. doi: 10.1371/journal.pone.0110076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong J. W., Selvam A.2009. Reduction of indicator and pathogenic microorganisms in pig manure through fly ash and lime addition during alkaline stabilization. J. Hazard. Mater. 169: 882–889. doi: 10.1016/j.jhazmat.2009.04.033 [DOI] [PubMed] [Google Scholar]


