Abstract
To analyze the miRNA expression profiles in Lawsonia intracellularis-infected porcine intestines, infected pigs were first identified using PCR (Polymerase Chain Reaction). Then, RNA from infected intestines and control tissues were isolated and subjected to microarray analysis and RT-PCR. Results showed that a total of 83 miRNAs were differentially expressed between the infected samples and controls, out of which 53 were upregulated and 30 were downregulated. Validation using RT-PCR showed a high degree of confidence for the obtained data. Using the current miRBase release 21.0, nine groups of miRNAs were located in the same cluster, and three groups of miRNAs were found to belong to the same family. Interestingly, except for ssc-miR-10a-5p, all clustered miRNAs and the family members exhibited the same expression patterns. Pathway analysis of the putative gene targets of the differentially expressed miRNAs showed that they were involved in the immune response, amino acid metabolism and cell communication/growth/motility. Thus, the results indicate that altered miRNA expression profiles can affect immunity, metabolism and cellular processes.
Keywords: intestine, Lawsonia intracellularis, miRNAs, pig
Lawsonia intracellularis (L. intracellularis) is the causative agent of porcine proliferative enteropathy (PPE), which is widely distributed in pig populations worldwide. PPE is an intestinal hyperplastic disease characterized by the thickening of the intestinal mucosa due to enterocyte proliferation [14]. This disease is associated with bloody diarrhea, sudden death, decreased food consumption and weight gain in affected pigs [9]. In addition, L. intracellularis is easily transmitted, so that a large numbers of pig herds are usually affected. The economic losses due to PPE can be at least 1-3US dollars per pig [10], which results in huge economic losses in livestock farming.
In animals, miRNAs regulate gene expression primarily via sequence-specific binding to the 3′ untranslated regions of target mRNAs and usually results in repression of gene expression [6]. More than 60% of mammalian genes are known to be targeted by miRNAs [4]. Recent studies demonstrate the role in porcine diseases, such as porcine reproductive and respiratory syndrome virus (PRRSV). miR-181 directly impairs PRRSV infection [5]; miR-125b interferes with PRRSV replication and viral gene expression by negatively regulating the NF-κB pathway [24]. Hoeke et al. [7] examined the interactions between miRNAs and their target mRNAs in Salmonella-infected piglet intestines using microarray analysis and showed that miR-29a regulates intestinal epithelial cell proliferation by targeting caveolin-2. The miRNA transcriptome of the porcine intestine has been published [20]. However, no studies identifying PPE-associated miRNAs have been carried out. We hypothesized that miRNA and mRNA expression profiles in the porcine intestine are altered following L. intracellularis infection. Thus, the aim of the present study was to investigate miRNA expression patterns in L. intracellularis-infected porcine intestines and identify potential disease regulators and their target genes.
MATERIALS AND METHODS
Ethics statement
All animal experiments were conducted in accordance with the guidelines of the Fujian Province on the Review of Welfare and Ethics of Laboratory Animals, which was approved by the Fujian Province Administration Office of Laboratory Animals.
Examination of intestinal samples
Feces and intestinal mucosa samples of pigs with diarrhea in herds were collected for DNA extraction. Next, infection with L. intracellularis was detected using PCR following the method of Suh et al. [22]. Briefly, PCR was routinely carried out using the species-specific primer pair (sense: 5′-GCAGCACTTGCAAACAATAAACT-3′ and anti-sense: 5′-TTCTCCTTTCTCATGTCCCATAA-3′). Positive intestinal samples yielded an L. intracellularis-specific PCR product of approximately 210 bp. Fecal samples from pigs without diarrhea were also collected and used as negative controls. All negative controls showed no PCR amplification.
All samples were also tested for infection with porcine epidemic diarrhea virus, transmissible gastroenteritis virus, porcine rotavirus and porcine circovirus type 2 using PCR (Table 1).
Table 1. Primers of each detected gene.
| Accession No. | Gene | Primer sequence 5′ to 3′ | Products size (bp) | |
|---|---|---|---|---|
| NC005148 | PCV2 | Forwards | TGACCTGTCTACTGCTGTGA | 570 |
| Reverse | CCGTGGATAGTTCTGTAGCA | |||
| FJ755618 | TGEV | Forwards | CAACCCTGAAACTAACGCAATTCT | 252 |
| Reverse | GCCCATCCAGTCGCACTACTT | |||
| AF353511 | RV | Forwards | AAATCCGCAACTATACTGTGACTA | 410 |
| Reverse | TTTGATCCGCCTACTTGAATGACT | |||
| FJ807867 | PEDV | Forwards | TCGAAGGAACGTGACCTCA | 140 |
| Reverse | CACGAACAGCCACATTACCA | |||
Sample preparation and RNA extraction
All samples tested negative for porcine epidemic diarrhea virus, transmissible gastroenteritis virus, porcine rotavirus and porcine circovirus type 2. Four 50-day-old L. intracellularis-infected DLY (Duroc × Landrace × Yorkshire) pigs and four control pigs were slaughtered in a legal slaughterhouse. Ileum tissues were collected within 30 min and immediately frozen in liquid nitrogen to preserve RNA quality. Total RNA was extracted from each sample using Trizol (Invitrogen, Carlsbad, CA, U.S.A.) reagent and mixed at equimolar amounts (four infected samples mixed together and four control samples mixed together). The quality of the mixed RNA was examined via 1.5% agarose gel electrophoresis and analyzed with BioPhotometer 6131 (Eppendorf, Hamburg, Germany).
Microarray assay with miRNA chips
miRNA expression was assessed using a GeneChip® miRNA 4.0 Array (Affymetrix, Santa Clara, CA, U.S.A.). Briefly, total RNA was purified using mirVana™ miRNA Isolation Kit (Affymetrix), after which poly (A) tails were added to the resulting small RNA samples. Biotin-labeled 3DNA® (Affymetrix) was ligated to the poly (A)-tailed RNA and hybridized to the microarray. Hybridization was performed at 48°C with rotation for 16 hr in the GeneChip Hybridization Oven 640 (Affymetrix). GeneChip arrays were washed and stained with streptavidin-phycoerythrin on an Affymetrix Fluidics Station 450 (Affymetrix) and subsequently scanned on a GeneChip Scanner 3000 (Affymetrix).
Data processing and analysis
Microarray data were analyzed to identify differentially expressed miRNAs. miRNAs were defined as upregulated and downregulated when the miRNA expression fold change relative to control samples was ≥2 at P-value <0.05. Cluster analysis of differentially expressed miRNAs was performed with the Gene Cluster 3.0 software by average linkage of hierarchical cluster analysis. Gene family and clustered miRNAs were analyzed based on the miRBase 21 release (http://www.mirbase.org/). miRNA target genes were predicted using the online software RNAhybrid [18]. Functional annotation of putative target genes was performed using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Molecule Annotation System Mas 3.0 (http://bioinfo.capitalbio.com/mas3/).
Real-time quantification of miRNAs using stem-loop RT-PCR
Stem-loop RT-PCR was performed as previously described [2]. Briefly, 1 µg of total RNA was reverse-transcribed into cDNA using the M-MLV reverse transcriptase (Promega, Madison, WI, U.S.A.) with specific stem-loop primers. Quantitative real-time PCR (qRT-PCR) was performed following standard protocols on a STRATAGENE Mx3005P sequence detection system. All reactions were run in duplicates, and a negative control without template was included for each gene. The Ct value was recorded for each reaction, and the amount of each miRNA relative to that of U6 RNA was described using the expression 2-(CtmiRNA-CtU6RNA). Primers were designed based on the sequenced miRNA using Premier 5.0.
Statistical analysis
Results are presented as mean ± SEM. Data were evaluated using Student’s t-test, and differences between groups were considered statistically significant at P<0.05. All statistical analyses were performed using SPSS 17.0 software.
RESULTS
Analysis of L. intracellularis-infected pig intestines
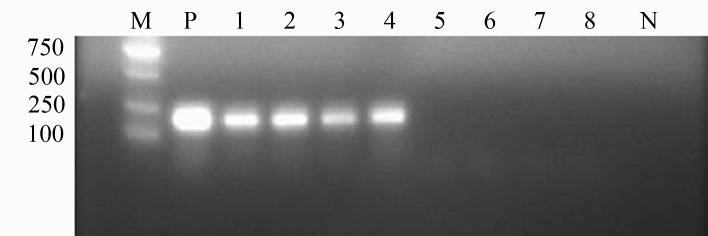
Out of all pigs that tested positive for L. intracellularis, four were slaughtered, and the ileum mucosa of each sample was collected and subjected to DNA extraction and PCR analysis. Intestines infected with L. intracellularis showed hyperplasia in the mucosal epithelia, one of which had an inconspicuous bleeding point. Accordingly, mucosal samples from the four L. intracellularis-positive pigs showed amplification of the 210-bp band, whereas samples from the four negative pigs showed no amplification (Fig. 1).
Fig. 1.
PCR detection of porcine intestines infected with L. intracellularis. M: DNA marker DL2000; P: positive control; 1-4: infected intestine samples; 5-8: uninfected intestine samples; N: negative control.
Differential expression of microRNAs between infected and normal intestinal tissues
To investigate miRNA expression profiles in L. intracellularis-infected intestines, total RNA of four infected samples and four control samples were extracted separately and mixed at equimolar amounts. miRNA microarray analyses were then performed after checking that the RNAs were completed. A total of 327 miRNAs were assessed, out of which 83 miRNAs showed significantly differential expression between the infected samples and the control. Out of 83 differentially expressed miRNAs, 53 were upregulated (Table 2), and 30 were downregulated (Table 3). Among the upregulated miRNAs, ssc-miR-486 (25.4-fold increase), ssc-miR-500 (14.3-fold increase) and ssc-miR-127 (12.3-fold increase) showed the most dramatic changes in expression levels. On the other hand, ssc-miR-215 (49.4-fold decrease), ssc-miR-194b-5p (32-fold decrease) and ssc-miR-122 (29.8-fold decrease) were the most downregulated miRNAs.
Table 2. Up-regulation of miRNA in infected intestine.
| Accession number | Name | Fold change (I/C) |
|---|---|---|
| MIMAT0013886 | ssc-miR-486 | 25.4 |
| MIMAT0013956 | ssc-miR-500 | 14.3 |
| MIMAT0013932 | ssc-miR-127 | 12.3 |
| MIMAT0018382 | ssc-miR-451 | 12 |
| MIMAT0013957 | ssc-miR-324 | 11.7 |
| MIMAT0010190 | ssc-miR-146b | 7.5 |
| MIMAT0013906 | ssc-miR-664-5p | 6.1 |
| MIMAT0013894 | ssc-miR-193a-5p | 6.1 |
| MIMAT0017966 | ssc-miR-4334-5p | 5.9 |
| MIMAT0015300 | ssc-miR-30a-3p | 5.4 |
| MIMAT0013869 | ssc-miR-133b | 5.4 |
| MIMAT0017377 | ssc-miR-92b-5p | 5 |
| MIMAT0002120 | ssc-miR-125b | 4.5 |
| MIMAT0010186 | ssc-miR-133a-3p | 4.1 |
| MIMAT0015210 | ssc-miR-24-1-5p | 4 |
| MIMAT0013895 | ssc-miR-193a-3p | 4 |
| MIMAT0006018 | ssc-miR-99b | 3.7 |
| MIMAT0007757 | ssc-miR-34a | 3.5 |
| MIMAT0013896 | ssc-miR-99a | 3.3 |
| MIMAT0007760 | ssc-miR-199b-3p | 3.2 |
| MIMAT0002147 | ssc-miR-214 | 3 |
| MIMAT0002144 | ssc-miR-181c | 2.9 |
| MIMAT0002156 | ssc-miR-124a | 2.9 |
| MIMAT0013875 | ssc-miR-199a-3p | 2.9 |
| MIMAT0013901 | ssc-miR-148b-3p | 2.9 |
| MIMAT0007762 | ssc-miR-221-3p | 2.9 |
| MIMAT0010189 | ssc-miR-503 | 2.9 |
| MIMAT0007758 | ssc-miR-130a | 2.7 |
| MIMAT0022922 | ssc-miR-30c-3p | 2.7 |
| MIMAT0017955 | ssc-miR-4331 | 2.7 |
| MIMAT0013885 | ssc-miR-10b | 2.6 |
| MIMAT0002151 | ssc-let-7c | 2.6 |
| MIMAT0013936 | ssc-miR-1307 | 2.6 |
| MIMAT0013945 | ssc-miR-708-5p | 2.6 |
| MIMAT0013879 | ssc-miR-143-3p | 2.5 |
| MIMAT0013897 | ssc-miR-125a | 2.5 |
| MIMAT0022962 | ssc-miR-421-3p | 2.4 |
| MIMAT0013903 | ssc-miR-885-3p | 2.4 |
| MIMAT0013866 | ssc-let-7e | 2.4 |
| MIMAT0013911 | ssc-miR-100 | 2.4 |
| MIMAT0018379 | ssc-miR-149 | 2.4 |
| MIMAT0025388 | ssc-miR-2366 | 2.4 |
| MIMAT0022957 | ssc-miR-455-5p | 2.3 |
| MIMAT0025361 | ssc-miR-132 | 2.3 |
| MIMAT0015709 | ssc-miR-22-5p | 2.3 |
| MIMAT0022960 | ssc-miR-490-3p | 2.2 |
| MIMAT0025376 | ssc-miR-490 | 2.2 |
| MIMAT0013955 | ssc-miR-335 | 2.2 |
| MIMAT0010191 | ssc-miR-181a | 2.1 |
| MIMAT0013929 | ssc-miR-331-5p | 2.1 |
| MIMAT0013887 | ssc-miR-152 | 2.1 |
| MIMAT0013940 | ssc-miR-532-5p | 2 |
| MIMAT0013921 | ssc-miR-424-3p | 2 |
Table 3. Down-regulation of miRNA in infected intestine.
| Accession number | Name | Fold change (C/I) |
|---|---|---|
| MIMAT0010192 | ssc-miR-215 | ‒49.4 |
| MIMAT0020365 | ssc-miR-194b-5p | ‒32 |
| MIMAT0002119 | ssc-miR-122 | ‒29.8 |
| MIMAT0017951 | ssc-miR-194b-3p | ‒23.1 |
| MIMAT0025389 | ssc-miR-2411 | ‒13.2 |
| MIMAT0002145 | ssc-miR-183 | ‒11.7 |
| MIMAT0015711 | ssc-miR-363 | ‒8.9 |
| MIMAT0002128 | ssc-miR-19a | ‒6.3 |
| MIMAT0025360 | ssc-miR-31 | ‒6 |
| MIMAT0025366 | ssc-miR-182 | ‒5.3 |
| MIMAT0025387 | ssc-miR-3613 | ‒5.2 |
| MIMAT0002141 | ssc-miR-7 | ‒5 |
| MIMAT0025368 | ssc-miR-194a | ‒4.8 |
| MIMAT0013910 | ssc-miR-192 | ‒4.8 |
| MIMAT0013944 | ssc-miR-342 | ‒4.3 |
| MIMAT0028147 | ssc-miR-7136-5p | ‒4.3 |
| MIMAT0025365 | ssc-miR-150 | ‒3.7 |
| MIMAT0002166 | ssc-miR-29c | ‒3.7 |
| MIMAT0013872 | ssc-miR-30e-5p | ‒3.4 |
| MIMAT0002165 | ssc-miR-21 | ‒3.2 |
| MIMAT0020585 | ssc-miR-18b | ‒2.9 |
| MIMAT0002161 | ssc-miR-18a | ‒2.8 |
| MIMAT0013884 | ssc-miR-10a-5p | ‒2.6 |
| MIMAT0002129 | ssc-miR-20a | ‒2.4 |
| MIMAT0020591 | ssc-miR-429 | ‒2.4 |
| MIMAT0022963 | ssc-miR-146a-5p | ‒2.2 |
| MIMAT0002127 | ssc-miR-184 | ‒2.2 |
| MIMAT0013950 | ssc-miR-19b | ‒2.1 |
| MIMAT0002137 | ssc-miR-29b | ‒2.1 |
| MIMAT0022959 | ssc-miR-155-5p | ‒2 |
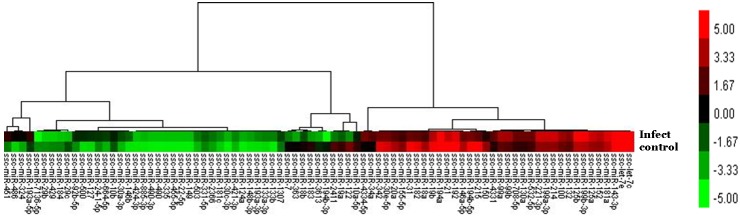
Based on the observed changes in expression patterns in these 83 miRNAs, a heat map was generated to distinguish the miRNA expression profiles between infected and normal intestinal tissues (Fig. 2).
Fig. 2.
Heat map of the expression profiles of differentially expressed microRNAs.
Chromosomal localization
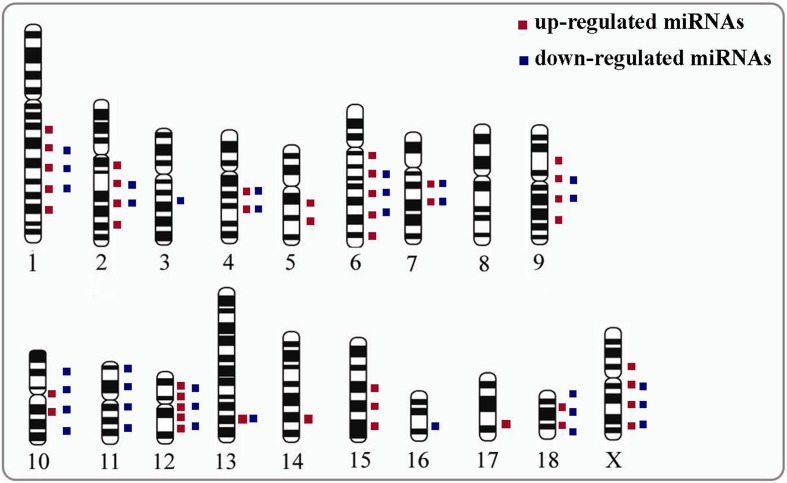
All differentially expressed miRNAs were mapped to pig chromosomes (sscorfa9) based on the sequence of the precursors of mature miRNAs. Consequently, 72 of the 83 miRNAs were mapped to 18 different pig chromosomes (Fig. 3 and Table S1), whereas 11 miRNAs did not map to any of the chromosomes in the available databases. Nine miRNAs had two copies in the genome, and 5 miRNAs were located in two different chromosomes.
Fig. 3.
Chromosomal localization of differentially expressed miRNAs. Red spots represent upregulated miRNAs, and blue spots indicate downregulated miRNAs.
miRNA expression analysis
Nine groups of miRNAs were clustered together based on microarray data. Among them, six groups consisted of two miRNAs, two groups consisted of three miRNAs, and one group comprised four miRNAs. Most miRNAs in the same cluster were located very close to each other, and several miRNAs were even located within 100 bp of each other. Interestingly, miRNAs from the same cluster also exhibited the same expression patterns, indicating synergistic effects in the regulation of the physiological functions of the intestine (Table 4).
Table 4. Expression pattern of differentially expressed miRNAs that located in the same cluster.
| Number | MiRNA | Chromosomal | Location | Expression |
|---|---|---|---|---|
| 1 | ssc-miR-500 | ChrX | 48632025-48632104 | up |
| ssc-miR-532-5p | 48637994-48638073 | |||
| 2 | ssc-miR-99b | Chr6 | 51858217-51858286 | up |
| ssc-miR-125a | 51858852-51858931 | |||
| ssc-let-7e | 51858376-51858455 | |||
| 3 | ssc-miR-99a | Chr13 | 191558610-191558689 | up |
| ssc-let-7c | 191559336-191559429 | |||
| 4 | ssc-miR-18b | ChrX | 126200019-126200101 | down |
| ssc-miR-19b-2 | 126199650-126199729 | |||
| ssc-miR-363-1 | 126199347-126199426 | |||
| 5 | ssc-miR-18a | Chr11 | 66610195-66610286 | down |
| ssc-miR-19a | 66610343-66610424 | |||
| ssc-miR-20a | 66610513-66610583 | |||
| ssc-miR-19b-1 | 66610642-66610721 | |||
| 6 | ssc-miR-29b-2 | Chr9 | 148552438-148552521 | down |
| ssc-miR-29c | 148552997-148553084 | |||
| 7 | ssc-miR-192 | Chr2 | 6416779-6416854 | down |
| ssc-miR-194a | 6416980-6417059 | |||
| 8 | ssc-miR-182 | Chr18 | 20035212-20035295 | down |
| ssc-miR-183 | 20030651-20030720 | |||
| 9 | ssc-miR-215 | Chr10 | 11963149-11963244 | down |
| ssc-miR-194b | 11786871-11786948 | |||
Additionally, several differentially expressed miRNAs belonged to the same gene family. Members of the miR-148 family were both upregulated, whereas miR-17 family members were all downregulated. Six of the miR-10 family members were upregulated in the infected tissues, except for ssc-miR-10a-5p (Table 5).
Table 5. Expression pattern of differentially expressed miRNAs that belong to the same family.
| Number | Family name | MiRNA | Expression |
|---|---|---|---|
| 1 | miR-10 family | ssc-miR-99a | up |
| ssc-miR-99b | |||
| ssc-miR-214 | |||
| ssc-miR-193a-3p | |||
| ssc-miR-10b | |||
| ssc-miR-10a-5p | down | ||
| 2 | miR-148 family | ssc-miR-152 | up |
| ssc-miR-148b-3p | |||
| 3 | miR-17 family | ssc-miR-18a | down |
| ssc-miR-18b | |||
| ssc-miR-20a | |||
Verification differential microRNA expression using stem-loop RT-PCR
Microarray results were validated via qRT-PCR analysis. Expression levels of eight differentially expressed miRNAs derived from microarray analysis were randomly selected and compared with results from qRT-PCR analysis. All the examined miRNAs, except for ssc-miR-192, showed the same expression patterns. In infected tissues, ssc-miR-125b, ssc-miR-143-3p, ssc-miR-181a and ssc-miR-214 were upregulated, whereas ssc-miR-194b, ssc-miR-215 and ssc-miR-342 were downregulated (Fig. 4).
Fig. 4.
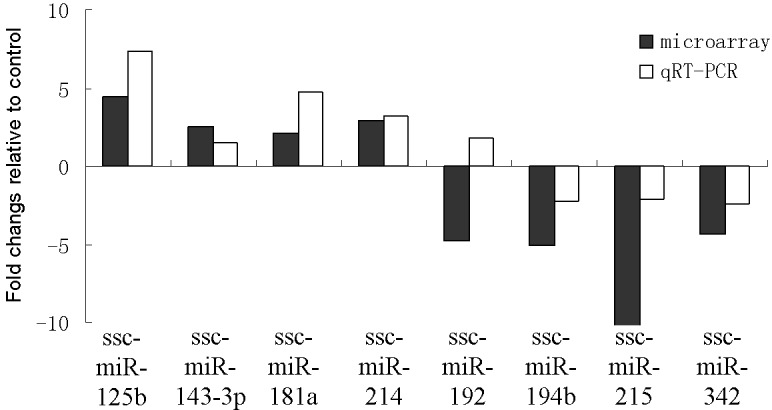
Relative expression levels of eight selected microRNAs in L. intracellularis-infected intestines and healthy tissues analyzed using miRNA microarray and quantitative reverse-transcription PCR (qRT–PCR) analyses. Eight differentially expressed miRNAs were randomly selected for qRT–PCR analysis. The vertical ordinate refers to the fold changes in the relative expression levels between infected and healthy tissues. Black bars indicate microarray results, and white bars indicate qRT–PCR results.
Prediction of target genes
Putative miRNA targets were analyzed using RNAhybrid, and 486 differentially expressed target genes were identified as negatively regulated by the 83 miRNAs isolated in the present study. KEGG pathway enrichment analysis showed that these predicted targets are involved in 44 pathways (Table 6) that are classified under Immunity (e.g., Leukocyte Transendothelial Migration), Cellular processes (e.g., Focal adhesion, Apoptosis and regulation of the Actin cytoskeleton), Signaling molecules and interaction (e.g., Cytokine-cytokine receptor interaction), Amino acid metabolism (e.g. Arginine and proline metabolism), Signal transduction (e.g., MAPK signaling pathway) and other related pathways.
Table 6. Possible pathways affected by differentially expressed miRNAs.
| Pathway | Count | P-value | Classification |
|---|---|---|---|
| Leukocyte transendothelial migration | 13 | 3.97E-15 | Immune system |
| Focal adhesion | 13 | 6.37E-12 | Cell communication |
| Complement and coagulation cascades | 12 | 2.06E-11 | Immune system |
| Hematopoietic cell lineage | 13 | 3.24E-10 | Immunes system |
| Cytokine-cytokine receptor interaction | 15 | 1.41E-08 | Signaling molecules and interaction |
| Apoptosis | 9 | 6.96E-08 | Cell growth and death |
| T cell receptor signaling pathway | 9 | 1.91E-07 | Immune system |
| Tryptophan metabolism | 6 | 5.96E-07 | Amino acid metabolism |
| Regulation of actin cytoskeleton | 8 | 7.34E-07 | Cell motility |
| MAPK signaling pathway | 8 | 8.35E-06 | Signal transduction |
| Cell adhesion molecules (CAMs) | 9 | 8.34E-06 | Signaling molecules and interaction |
| Arginine and proline metabolism | 8 | 9.09E-06 | Amino acid metabolism |
| ECM-receptor interaction | 8 | 1.98E-05 | Signaling molecules and interaction |
| Glycerolipid metabolism | 7 | 1.98E-05 | Amino acid metabolism |
| Glutathione metabolism | 8 | 3.80E-05 | Amino acid metabolism |
DISCUSSION
The pig (Sus scrofa) is a suitable model for biomedical studies [16], and research on porcine biological systems is relevant to the large-scale production industry. In this study, we analyzed the expression of miRNAs in L. intracellularis-infected porcine intestines and identified several miRNAs that are potentially involved in PPE. In this study, 53 out of the 327 miRNAs analyzed were upregulated in the infected intestines compared with normal tissues, and 30 miRNAs were downregulated. Among them, differential expression of eight randomly selected miRNAs was verified via qRT-PCR. Most of the qRT-PCR validation results were consistent with microarray data, indicating that PPE infection alters the expression of multiple miRNAs. Strong expression of miR-127 is associated with cell growth. Various studies have demonstrated that miR-127 inhibits cell proliferation by targeting various genes, such as p65 [8], Sept7 [30], Ski [12] and Setd8 [28]. Upregulation of miR-451 also suppressed cell proliferation [25,26,27]. Surprisingly, miR-127 and miR-451 were both in the top five miRNAs that were upregulated in the infected tissues, which exhibit thickening of the intestinal mucosa and proliferation of enterocyte. Upregulation of these two miRNAs can be caused by changes in the intestinal mucosa brought about by PPE infection. miR-127 and miR-451 were observed to act as tumor suppressors, suppressing cell proliferation in a carcinoma cell line. Thus, miR-127 and miR-451 can suppress cell proliferation in the ileum to prevent uncontrolled proliferation.
Based on previous reports, ssc-mir-146b showed the largest difference in expression between weaning piglets and suckling piglets. ssc-mir-146b showed higher expression in intestinal tissues of weaning piglets at days 4 and 7, whereas ssc-miR-215 showed higher upregulation in suckling piglets [23]. In the present study, ssc-mir-146b expression increased by 7.5-fold in infected intestine samples, and ssc-miR-215 showed higher levels in the control pigs, indicating the importance of these two miRNAs in intestinal function. Previous studies demonstrated that miR-146a/b regulates intestinal epithelial cell differentiation and the mucosal immune response by mediating the interleukin 1 receptor-associated kinase and transforming growth factor-β signaling pathways via negative feedback regulation [1, 15]. ssc-mir-146b and ssc-miR-215 have been suggested to negatively regulate epithelial cell differentiation.
miRNAs located within 10 kb of another miRNA belonged to the same cluster, suggesting expression from the same primary miRNA transcript (pri-miRNA). We identified nine groups of miRNAs that were located in the same cluster, most of which were found within several dozen bp of each other. Additionally, all clustered miRNAs tended to exhibit coordinated expression patterns, consistent with the results of Ruvkun et al. [19]. Our results suggest that the miRNAs in the same cluster have similar functions in the regulation of the intestine or may co-regulate target genes. Moreover, although genes from the same family always possess complementary action and may be involved in the same biological processes, some members of the same family exhibit different expression patterns. The expression patterns and regulatory mechanisms of the different members may have diverged during evolution [3]. In our study, ssc-miR-10a-5p was downregulated in infected tissues, while all other members of the miR-10 family were upregulated. miR-10a-5p was reported to suppress the expression of pro-inflammatory factors in the ileum [29]. Thus, downregulation of ssc-miR-10a-5p in infected intestines may result from an increase in the expression of pro-inflammatory factors.
Hoeke et al. [7] showed that Salmonella infections caused downregulation of caveolin-2 and upregulation of miR-29a in the intestine and verified caveolin-2 as the target of miR-29a. Additionally, caveolin-2 knockdown leads to suppressed proliferation of intestinal epithelial cells. In our study, miR-29b and miR-29c were found to be downregulated in the L. intracellularis-infected intestines. Bioinformatics prediction showed that caveolin-2 is a target of miR-29a/b/c, which can explain why miR-29b/c showed contrasting fold changes. In addition, miR-29b was reported to suppress cell proliferation in several cell lines [11, 13], suggesting that miR-29b/c is associated with cell proliferation in infected intestines.
Infection with L. intracellularis also alters the expression of genes involved in cell transport and the maintenance of mucosal integrity [21]. Oh et al. reported that genes involved in cell cycle, cell differentiation and cell structure were differentially expressed in infected mice [17]. Interestingly, we found that some of the differentially expressed genes were targets of the differentially expressed miRNAs. For example, Fbxo39, Syvn1 and sesn2 were upregulated in infected samples and are predicted to be targets of miR-20a, miR-155 and miR-122, which were downregulated in our study. By contrast, miR-424, miR-199a/b and miR-124 were upregulated in infected samples, and their corresponding target genes, namely, SLC6A4, S100G and Grb2, were all downregulated.
By analyzing the predicted targets of differentially expressed miRNA using KEGG pathway enrichment analysis, we found that most target genes were enriched in genes categorized under immune system, signaling molecules and interaction, and amino acid metabolism due to L. intracellularis infection. L. intracellularis stimulates proliferation of enterocytes in the intestine and results in hyperplasia, which in turn interferes with nutrient absorption. Target genes of the altered miRNAs were enriched in genes involved in leukocyte transendothelial migration, cytokine-cytokine receptor interaction, MAPK signaling pathway, and arginine and proline metabolism. Future studies will focus on the miRNAs that regulate cell proliferation, target genes of the MAPK signaling pathway and the actin cytoskeleton.
In summary, we identified 83 miRNAs that were differentially expressed between the L. intracellularis-infected intestines and control tissues. Among them, several miRNAs were located on the same chromosome or belonged to the same miRNA family. These groups of miRNAs all showed the same expression patterns (except for one). The altered miRNA expression patterns may play a role in the immune response. However, further studies are required to determine the functions of these miRNAs.
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of the Fujian Province, China (Grant No. 2014J05043 and 2015J01620), the Major projects of agricultural science and technology in Fujian Province (Grant No. 2014NZ01060015) and the PhD Start-up Fund of Longyan University (Grant No. LB2013012).
REFERENCES
- 1.Chassin C., Kocur M., Pott J., Duerr C. U., Gütle D., Lotz M., Hornef M. W.2010. miR-146a mediates protective innate immune tolerance in the neonate intestine. Cell Host Microbe 8: 358–368. doi: 10.1016/j.chom.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Chen C., Ridzon D. A., Broomer A. J., Zhou Z., Lee D. H., Nguyen J. T., Barbisin M., Xu N. L., Mahuvakar V. R., Andersen M. R., Lao K. Q., Livak K. J., Guegler K. J.2005. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 33: e179. doi: 10.1093/nar/gni178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esquela-Kerscher A., Slack F. J.2006. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer 6: 259–269. doi: 10.1038/nrc1840 [DOI] [PubMed] [Google Scholar]
- 4.Friedman R. C., Farh K. K., Burge C. B., Bartel D. P.2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19: 92–105. doi: 10.1101/gr.082701.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo X. K., Zhang Q., Gao L., Li N., Chen X. X., Feng W. H.2013. Increasing expression of microRNA 181 inhibits porcine reproductive and respiratory syndrome virus replication and has implications for controlling virus infection. J. Virol. 87: 1159–1171. doi: 10.1128/JVI.02386-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He L., Hannon G. J.2004. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5: 522–531. doi: 10.1038/nrg1379 [DOI] [PubMed] [Google Scholar]
- 7.Hoeke L., Sharbati J., Pawar K., Keller A., Einspanier R., Sharbati S.2013. Intestinal Salmonella typhimurium infection leads to miR-29a induced caveolin 2 regulation. PLoS ONE 8: e67300. doi: 10.1371/journal.pone.0067300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huan L., Bao C., Chen D., Li Y., Lian J., Ding J., Huang S., Liang L., He X.2015. Micro RNA-127-5p targets the biliverdin reductase B/nuclear factor-κB pathway to suppress cell growth in hepatocellular carcinoma cells. Cancer Sci. 107: 258–266. doi: 10.1111/cas.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson M., Fellström C., Jensen-Waern M.2010. Porcine proliferative enteropathy: an important disease with questions remaining to be solved. Vet. J. 184: 264–268. doi: 10.1016/j.tvjl.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 10.Jensen H. M.2006. Health management with reduced antibiotic use - experiences of a Danish pig vet. Anim. Biotechnol. 17: 189–194. doi: 10.1080/10495390600957142 [DOI] [PubMed] [Google Scholar]
- 11.Jia L. F., Huang Y. P., Zheng Y. F., Lyu M. Y., Wei S. B., Meng Z., Gan Y. H.2014. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN-AKT signaling pathway by targeting Sp1. Oral Oncol. 50: 1062–1071. doi: 10.1016/j.oraloncology.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 12.Jiang H., Jin C., Liu J., Hua D., Zhou F., Lou X., Zhao N., Lan Q., Huang Q., Yoon J. G., Zheng S., Lin B.2014. Next generation sequencing analysis of miRNAs: MiR-127-3p inhibits glioblastoma proliferation and activates TGF-β signaling by targeting SKI. OMICS 18: 196–206. doi: 10.1089/omi.2013.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano M., Tanaka K., Itonaga I., Iwasaki T., Tsumura H.2015. c-Myc Represses Tumor-Suppressive microRNAs, let-7a, miR-16 and miR-29b, and Induces Cyclin D2-Mediated Cell Proliferation in Ewing’s Sarcoma Cell Line. PLOS ONE 10: e0138560. doi: 10.1371/journal.pone.0138560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson G. H., Gebhart C. J.2000. Proliferative enteropathy. J. Comp. Pathol. 122: 77–100. doi: 10.1053/jcpa.1999.0347 [DOI] [PubMed] [Google Scholar]
- 15.Liao Y., Zhang M., Lönnerdal B.2013. Growth factor TGF-β induces intestinal epithelial cell (IEC-6) differentiation: miR-146b as a regulatory component in the negative feedback loop. Genes Nutr. 8: 69–78. doi: 10.1007/s12263-012-0297-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunney J. K.2007. Advances in swine biomedical model genomics. Int. J. Biol. Sci. 3: 179–184. doi: 10.7150/ijbs.3.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh Y. S., Lee J. B., McOrist S.2010. Microarray analysis of differential expression of cell cycle and cell differentiation genes in cells infected with Lawsonia intracellularis. Vet. J. 184: 340–345. doi: 10.1016/j.tvjl.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 18.Rehmsmeier M., Steffen P., Hochsmann M., Giegerich R.2004. Fast and effective prediction of microRNA/target duplexes. RNA 10: 1507–1517. doi: 10.1261/rna.5248604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruvkun G.2001. Molecular biology. Glimpses of a tiny RNA world. Science 294: 797–799. doi: 10.1126/science.1066315 [DOI] [PubMed] [Google Scholar]
- 20.Sharbati S., Friedländer M. R., Sharbati J., Hoeke L., Chen W., Keller A., Stähler P. F., Rajewsky N., Einspanier R.2010. Deciphering the porcine intestinal microRNA transcriptome. BMC Genomics 11: 275. doi: 10.1186/1471-2164-11-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith S. H., Wilson A. D., Van Ettinger I., MacIntyre N., Archibald A. L., Ait-Ali T.2014. Down-regulation of mechanisms involved in cell transport and maintenance of mucosal integrity in pigs infected with Lawsonia intracellularis. Vet. Res. (Faisalabad) 45: 55. doi: 10.1186/1297-9716-45-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh D. K., Lym S. K., Bae Y. C., Lee K. W., Choi W. P., Song J. C.2000. Detection of Lawsonia intracellularis in diagnostic specimens by one-step PCR. J. Vet. Sci. 1: 33–37. [PubMed] [Google Scholar]
- 23.Tao X., Xu Z.2013. MicroRNA transcriptome in swine small intestine during weaning stress. PLoS ONE 8: e79343. doi: 10.1371/journal.pone.0079343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D., Cao L., Xu Z., Fang L., Zhong Y., Chen Q., Luo R., Chen H., Li K., Xiao S.2013. MiR-125b reduces porcine reproductive and respiratory syndrome virus replication by negatively regulating the NF-κB pathway. PLoS ONE 8: e55838. doi: 10.1371/journal.pone.0055838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng T., Peng L., Chao C., Fu B., Wang G., Wang Y., Zhu X.2014. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int. J. Clin. Exp. Pathol. 7: 7653–7662. [PMC free article] [PubMed] [Google Scholar]
- 26.Zang W. Q., Yang X., Wang T., Wang Y. Y., Du Y. W., Chen X. N., Li M., Zhao G. Q.2015. MiR-451 inhibits proliferation of esophageal carcinoma cell line EC9706 by targeting CDKN2D and MAP3K1. World J. Gastroenterol. 21: 5867–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F., Huang W., Sheng M., Liu T.2015. MiR-451 inhibits cell growth and invasion by targeting CXCL16 and is associated with prognosis of osteosarcoma patients. Tumour Biol. 36: 2041–2048. doi: 10.1007/s13277-014-2811-2 [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Hou W., Chai M., Zhao H., Jia J., Sun X., Zhao B., Wang R.2016. MicroRNA-127-3p inhibits proliferation and invasion by targeting SETD8 in human osteosarcoma cells. Biochem. Biophys. Res. Commun. 469: 1006–1011. doi: 10.1016/j.bbrc.2015.12.067 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q., Xiao X., Li M., Li W., Yu M., Zhang H., Wang Z., Xiang H.2013. Acarbose reduces blood glucose by activating miR-10a-5p and miR-664 in diabetic rats. PLoS ONE 8: e79697. doi: 10.1371/journal.pone.0079697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J., Lu S., Yang S., Chen H., Shi H., Miao M., Jiao B.2014. MicroRNA-127 post-transcriptionally downregulates Sept7 and suppresses cell growth in hepatocellular carcinoma cells. Cell. Physiol. Biochem. 33: 1537–1546. doi: 10.1159/000358717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.