Abstract
It remains unclear whether epithelial cell-derived cytokines, including interleukin (IL)-25, IL-33 and thymic stromal lymphopoietin (TSLP), contribute to development of canine chronic enteropathy (CE), which includes antibiotic-responsive enteropathy (ARE), food-responsive enteropathy (FRE) and inflammatory bowel disease (IBD). In the present study, we examined mRNA expression of il-25, il-33 and tslp in the duodenal and colonic mucosae of dogs with ARE, FRE and IBD. Real-time PCR analysis revealed that mRNA expression of il-33 was significantly lower in the duodenum in dogs with FRE than in healthy dogs. The results suggest that epithelial cell-derived cytokines may not be an inducer of Th2-type immunity in the gut of dogs with CE, and decreased expression of IL-33 may be involved in induction of FRE. Further studies are required to clarify roles of epithelial cell-derived cytokines, especially IL-33, in the pathogenesis of canine CE.
Keywords: chronic enteropathy, dog cytokines, epithelial cell-derived cytokines, food-responsive enteropathy, inflammatory bowel disease
Chronic enteropathy (CE) in dogs is characterized by persistent or recurrent gastrointestinal (GI) signs and mucosal inflammation in the intestine [1, 4]. CE is a group of disorders comprised of antibiotic-responsive enteropathy (ARE) that responds to antimicrobial therapy [5], food-responsive enteropathy (FRE) that responds to dietary trials [3] and inflammatory bowel disease (IBD) that incompletely responds to antibiotic and dietary trials and requires anti-inflammatory or immunosuppressive agents for management [22]. It has been suggested that mucosal barrier dysfunction, bacterial flora abnormalities and/or inappropriate reactions to dietary components lead to chronic dysregulation of mucosal immune responses in the intestines of dogs with CE [3,4,5, 22]. However, detailed mechanisms for the development of canine ARE, FRE and IBD are not yet fully elucidated.
Intestinal epithelial cells (IECs) form a biochemical and physical barrier that segregates luminal microbiota and dietary elements from the mucosal immune system [14]. IECs were also shown to regulate innate and adaptive immunity at mucosal sites by expressing pattern-recognition receptors for microorganisms and producing a variety of humoral factors, including cytokines [14]. Thus, IECs play a pivotal role in intestinal homeostasis.
Epithelial cell-derived cytokines, including interleukin (IL)-25, IL-33 and thymic stromal lymphopoietin (TSLP), are released from IECs in response to tissue damage, allergen stimulation and helminth infection [6, 16]. These cytokines have been shown to enhance Th2-type immunity by stimulating dendritic cells, innate lymphoid cells, basophils and mast cells in mice and humans [6, 16]. It is also reported that IL-25, IL-33 and TSLP exhibit anti-inflammatory or immunoregulatory functions to maintain homeostatic conditions in the intestine [16]. Previous studies demonstrated aberrant expression of epithelial cell-derived cytokines, such as decreased expression of IL-25 [19] and TSLP [15], and increased expression of IL-33 [9], in human patients with IBD. These findings indicate that epithelial cell-derived cytokines contribute to the development of mucosal inflammation in the intestine by promoting Th2-type immune responses or dysregulating intestinal immune homeostasis in human patients with IBD. The pathological and immunological features of CE in dogs are in part similar to those of IBD; however, a distinct Th2 polarization has not been demonstrated in dogs with CE [7]. Thus, it is possible that aberrant expression of epithelial cell-derived cytokines might be implicated in the pathogenesis of CE in dogs by disturbing the intestinal homeostasis. In the present study, as a first step to clarify the significance of epithelial cell-derived cytokines in canine CE, we analyzed mRNA expression of il-25, il-33 and tslp in the duodenal and colonic mucosae of dogs with CE.
Twenty-one dogs diagnosed with CE at the Tokyo University of Agriculture and Technology Animal Medical Center were included in this study. For diagnosis of CE, inclusion criteria were chronic GI signs, such as vomiting and/or diarrhea, over a duration of >3 weeks and histopathological evidence of inflammation in the duodenal and/or colonic mucosae obtained by endoscopic biopsy. Exclusion criteria were to rule out other causes of chronic GI signs, including metabolic disease, infection, parasitic disease, pancreatic insufficiency, hepatic disease and renal disease, by complete blood count, serum biochemistry profile, fecal examination, urinalysis, abdominal radiography and ultrasonography. Alimentary lymphoma was excluded by histopathological evaluation and negative results of polymerase chain reaction (PCR) for antigen receptor gene rearrangements for the T-cell receptor gamma-chain gene and the immunoglobulin heavy-chain gene (Canine-Lab, Tokyo, Japan) in duodenal and/or colonic mucosal samples. After confirmation of CE, ARE, FRE and IBD were differentially diagnosed by antibiotic and dietary trials in each dog according to a previous report [18]. As an antibiotic trial, metronidazole (Flagyl; Shionogi & Co., Ltd., Osaka, Japan) (10–15 mg/kg, q12 hr) was orally administered to each dog for 2 weeks. When dogs did not respond to metronidazole, tylosin (Tylan; Eli Lilly Japan K.K., Kobe, Japan) (20 mg/kg, q12 hr) was orally administered for 2 weeks. ARE was diagnosed by complete resolution of GI signs with the antibiotic trial. After exclusion of ARE, a dietary trial was performed for diagnosis of FRE. Based on the dietary history in each dog, at least two different kinds of diets were selected from Anallergenic, Hypoallergenic, Selected Protein, Gastrointestinal Low Fat (Royal Canin Japon Inc., Tokyo, Japan), w/d [Hill’s-Colgate (Japan) Ltd., Tokyo, Japan] and home-made diets. A first diet was administered for 2 weeks. When dogs did not respond to the first diet, a second diet was administered for 2 weeks. FRE was diagnosed by complete resolution of GI signs with the dietary trial. After exclusion of ARE and FRE, dogs were finally diagnosed with IBD. GI signs of dogs with IBD were partially or completely improved by oral administration of prednisolone (Pfizer, Tokyo, Japan) (0.5–2.0 mg/kg, q24 hr). Clinical severity of 21 dogs with CE was scored according to the canine chronic enteropathy clinical activity index (CCECAI) [1].
Six healthy intact male beagles were used as a control group. The mean age ± standard deviation (SD) of the control dogs was 3.8 ± 2.4 years (range, 1.2 to 6.4 years), and mean body weight ± SD was 11.1 ± 0.5 kg (range, 10.1 to 11.6 kg). Dogs were housed in individual cages and fed a commercial diet [Science Diet Adult; Hill’s-Colgate (Japan) Ltd.] once daily. Water was provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Tokyo University of Agriculture and Technology.
Dogs were prepared for endoscopy by withholding food for at least 24 hr. Mucosal biopsy specimens were obtained from all dogs with endoscopic biopsy forceps under general anesthesia. Duodenal samples were collected from all dogs; colonic samples were collected from all healthy control dogs, and 16 out of 21 dogs with CE. More than six tissue specimens were obtained from each region. The duodenal and/or colonic biopsy specimens were subjected to histopathological analysis and graded according to the guideline of the world small animal veterinary association (WSAVA) international gastrointestinal standardization group [22]. A portion of the biopsy sample was immediately stored in RNAlater® Solution (Thermo Fisher Scientific, Waltham, MA, U.S.A.) according to the manufacturer’s instruction for preservation until RNA extraction.
Total RNA was extracted from biopsy specimens using NucleoSpin® RNA (Takara Bio, Otsu, Japan) and reverse-transcribed into cDNA using PrimeScriptTM RT Master Mix (Takara Bio). cDNA samples were subjected to real-time PCR analysis as described previously [11]. Primers for real-time PCR (Supplementary Table 1) were designed by a perfect real time support system (Takara Bio) and according to previous reports [8, 20]. In the duodenal mucosa, glyceraldehyde 3-phosphate dehydrogenase (gapdh), TATA-binding protein (tbp) and succinate dehydrogenase complex, subunit A (sdha) were used as reference genes according to a previous report [13]. In the colonic mucosa, gapdh, tbp and hydroxymethylbilane synthase (hmbs) were selected as reference genes as described previously [20]. Relative mRNA expression of the target genes was determined by the 2-ΔCt method, wherein each value is presented as an n-fold difference relative to the geometric mean of the three reference genes [21].
The normality of all data was analyzed by the Shapiro-Wilk test. Data between two groups were compared by the unpaired t test or the Mann-Whitney U test, depending on the normality. Data among groups were compared by one-way analysis of variance or the Kruskal-Wallis test, depending on the normality. When a significant difference was detected among groups, data between each pair were analyzed by the Tukey-Kramer test or the Steel-Dwass test as a post hoc analysis. The correlation between expression levels of il-33 mRNA and CCECAI or WSAVA score was evaluated by the Spearman’s rank correlation coefficient. Statistical analyses were performed using BellCurve for Excel software (Social Survey Research Information Co., Ltd., Tokyo, Japan). P<0.05 was considered statistically significant.
Among 21 dogs with CE, 3, 7 and 11 were diagnosed with ARE, FRE and IBD, respectively. The clinical and histopathological characteristics are summarized in Supplementary Table 2.
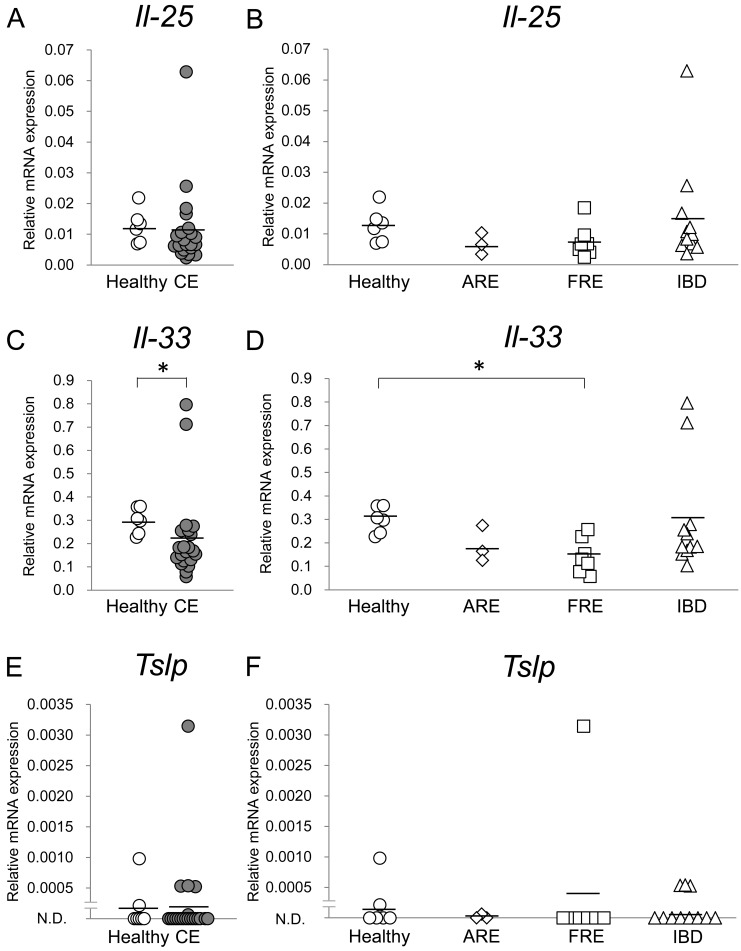
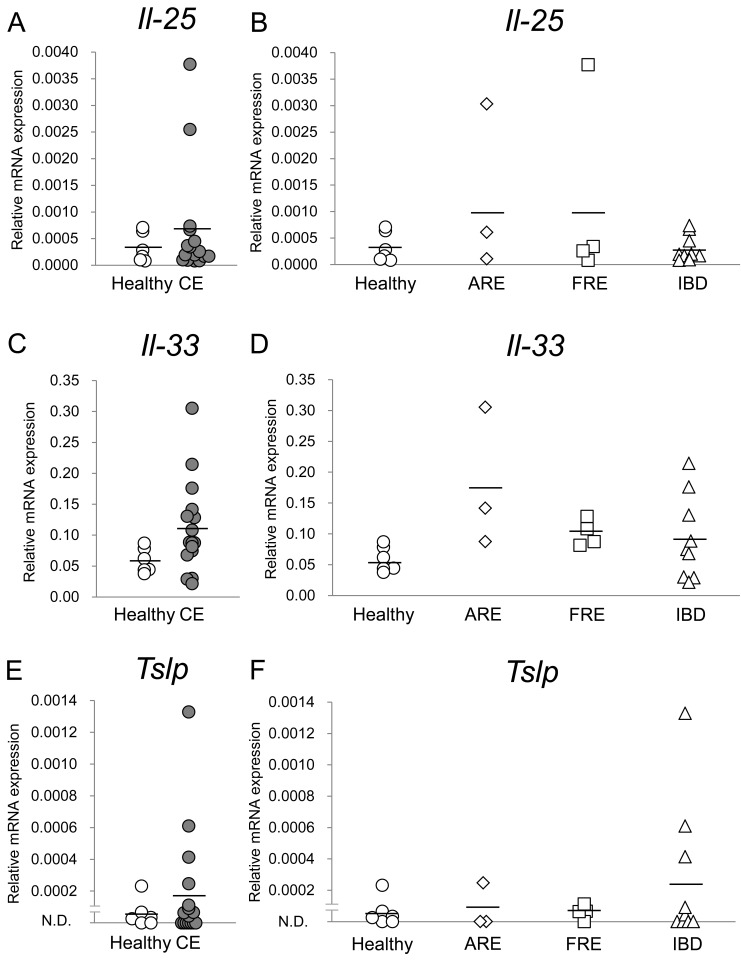
Expression levels of il-25, il-33 and tslp mRNA in the duodenal and colonic mucosae were compared between healthy dogs and dogs with CE; those were then compared among healthy dogs and dogs with ARE, FRE or IBD. In the duodenal mucosa, whereas expression levels of il-25 and tslp mRNA were not significantly different between healthy dogs and dogs with CE (Fig. 1A and 1E; P>0.05, respectively), those of il-33 mRNA in dogs with CE were significantly lower than those in healthy dogs (Fig. 1C; P<0.05). Among healthy dogs and dogs with ARE, FRE or IBD, whereas expression levels of il-25 and tslp mRNA were not significantly different (Fig. 1B and 1F; P>0.05, respectively), those of il-33 mRNA in dogs with FRE were significantly lower than those in healthy dogs in the duodenal mucosa (Fig. 1D; P<0.05). In the colonic mucosa, expression levels of il-25, il-33 and tslp mRNA were not significantly different between healthy dogs and dogs with CE (Fig. 2A, 2C and 2E; P>0.05, respectively) and among healthy dogs and dogs with ARE, FRE or IBD (Fig. 2B, 2D, and 2F; P>0.05, respectively).
Fig. 1.
Relative mRNA expression of epithelial cell-derived cytokines in the duodenal mucosa of dogs with chronic enteropathy (CE). Expression levels of il-25 (A and B), il-33 (C and D) and tslp (E and F) mRNAs were analyzed by real-time PCR in healthy dogs (n=6) and dogs with CE (n=21), which included dogs with antibiotic-responsive enteropathy (ARE) (n=3), food-responsive enteropathy (FRE) (n=7) and inflammatory bowel disease (IBD) (n=11). The horizontal lines in each group represent the mean value. Relative mRNA expression was compared between healthy dogs and dogs with CE by the Mann-Whitney U test and was compared among healthy dogs and dogs with ARE, FRE or IBD by the Kruskal-Wallis test, followed by the Steel-Dwass test. *P<0.05
Fig. 2.
Relative mRNA expression of epithelial cell-derived cytokines in the colonic mucosa of dogs with chronic enteropathy (CE). Expression levels of il-25 (A and B), il-33 (C and D) and tslp (E and F) mRNAs were analyzed by real-time PCR in healthy dogs (n=6) and dogs with CE (n=16), which included dogs with antibiotic-responsive enteropathy (ARE) (n=3), food-responsive enteropathy (FRE) (n=4) and inflammatory bowel disease (IBD) (n=9). The horizontal lines in each group represent the mean value. Relative mRNA expression was compared between healthy dogs and dogs with CE by the Mann-Whitney U test for il-25 and tslp and by the unpaired t test for il-33. Relative mRNA expression was compared among healthy dogs and dogs with ARE, FRE or IBD by the Kruskal-Wallis test for il-25 and tslp and by one-way analysis of variance for il-33.
We next examined whether expression levels of il-33 mRNA in the duodenal mucosa correlated with the clinical and histopathological severity in dogs with FRE. No significant correlations were detected between expression levels of il-33 mRNA and CCECAI score (Supplementary Fig. 1A; rs=0.51, P>0.05) or between those of il-33 mRNA and WSAVA score (Supplementary Fig. 1B; rs=0.09, P>0.05) in the duodenal mucosa of dogs with FRE.
Accumulating evidence in mice and humans has revealed that IL-25, IL-33 and TSLP secreted by epithelial cells initiate Th2-type immune responses in the skin, trachea and gut [6, 16]. In dogs with CE, no predominant Th1-, Th2- or Th17-type immunity has been demonstrated previously [7]. Consistent with this finding, the present study demonstrated that none of il-25, il-33 and tslp genes was upregulated in the duodenum and colon in dogs with ARE, FRE or IBD compared with that in the healthy controls. These results suggest that epithelial cell-derived cytokines, such as IL-25, IL-33 and TSLP, may not be an inducer of Th2-type immune responses in the gut of dogs with CE.
IL-33 functions as a danger signal or alarmin in IECs in response to tissue damage [12]. Several studies revealed that expression of IL-33 was increased at the mRNA and protein levels in IECs of human patients with IBD, especially ulcerative colitis (UC), which is characterized by predominant Th2-type immunity, suggesting a possible role of IL-33 in the induction of UC [9]. In murine models for intestinal inflammation, IL-33 has been shown to have both detrimental and anti-inflammatory roles, depending on the phase of inflammation [9, 12]. During the acute phase of dextran sodium sulfate (DSS)-induced colitis in mice, IL-33 released by necrotic or damaged IECs recruited neutrophils, resulting in the onset of intestinal inflammation [12]. In contrast, during the recovery phase of DSS-induced colitis, IL-33 promoted wound healing and improved epithelial regeneration [10, 12]. An anti-inflammatory role for IL-33 was also demonstrated in trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice, in which administration of IL-33 substantially ameliorated tissue injury and clinical signs of TNBS-mediated colitis [2]. It is suggested that anti-inflammatory effects of IL-33 were partially mediated by induction of regulatory T cells [2, 12]. A recent study also reported that IL-33 promoted the expansion and function of regulatory T cells in the intestine in mice [17]. The present study demonstrated that mRNA expression of il-33 was significantly lower in the duodenum in dogs with FRE than in healthy dogs. Considering anti-inflammatory roles for IL-33 during the recovery phase of intestinal inflammation, it can be assumed that normal expression of IL-33 may be implicated in the maintenance of homeostasis in intestinal mucosal immunity in dogs, whereas decreased expression of IL-33 could induce chronic inflammation in the gut of dogs with FRE through dysregulated recovery of the intestinal barrier function and impaired expansion and function of regulatory T cells. However, it remains unclear why mRNA expression of il-33 was decreased only in the duodenal mucosa of dogs with FRE and not in those with other forms of CE.
A major limitation of the present study was that only mRNA expression of il-25, il-33 and tslp was examined in the intestinal mucosa of a small number of dogs with CE. Further studies are required to clarify distribution and cellular sources of IL-25, IL-33 and TSLP at protein levels in the canine intestine using a larger sample size. In addition, since the current study found that mRNA expression of il-33 was significantly lower in the duodenum in dogs with FRE than in healthy dogs, it would also be intriguing to elucidate how IL-33 differentially regulates the pathogenesis of ARE, FRE and IBD in dogs.
Supplementary Material
REFERENCES
- 1.Allenspach K., Wieland B., Gröne A., Gaschen F.2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21: 700–708. doi: 10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- 2.Duan L., Chen J., Zhang H., Yang H., Zhu P., Xiong A., Xia Q., Zheng F., Tan Z., Gong F., Fang M.2012. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3+ regulatory T-cell responses in mice. Mol. Med. 18: 753–761. doi: 10.2119/molmed.2011.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaschen F. P., Merchant S. R.2011. Adverse food reactions in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 41: 361–379. doi: 10.1016/j.cvsm.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 4.German A. J., Hall E. J., Day M. J.2003. Chronic intestinal inflammation and intestinal disease in dogs. J. Vet. Intern. Med. 17: 8–20. doi: 10.1111/j.1939-1676.2003.tb01318.x [DOI] [PubMed] [Google Scholar]
- 5.Hall E. J.2011. Antibiotic-responsive diarrhea in small animals. Vet. Clin. North Am. Small Anim. Pract. 41: 273–286. doi: 10.1016/j.cvsm.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Hammad H., Lambrecht B. N.2015. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 43: 29–40. doi: 10.1016/j.immuni.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 7.Heilmann R. M., Suchodolski J. S.2015. Is inflammatory bowel disease in dogs and cats associated with a Th1 or Th2 polarization? Vet. Immunol. Immunopathol. 168: 131–134. doi: 10.1016/j.vetimm.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 8.Maeda S., Ohno K., Uchida K., Igarashi H., Goto-Koshino Y., Fujino Y., Tsujimoto H.2014. Intestinal protease-activated receptor-2 and fecal serine protease activity are increased in canine inflammatory bowel disease and may contribute to intestinal cytokine expression. J. Vet. Med. Sci. 76: 1119–1127. doi: 10.1292/jvms.14-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes T., Bernardazzi C., de Souza H. S.2014. Interleukin-33 and inflammatory bowel diseases: lessons from human studies. Mediators Inflamm. 2014: 423957. doi: 10.1155/2014/423957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oboki K., Ohno T., Kajiwara N., Arae K., Morita H., Ishii A., Nambu A., Abe T., Kiyonari H., Matsumoto K., Sudo K., Okumura K., Saito H., Nakae S.2010. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. U.S.A. 107: 18581–18586. doi: 10.1073/pnas.1003059107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohmori K., Nishikawa S., Oku K., Oida K., Amagai Y., Kajiwara N., Jung K., Matsuda A., Tanaka A., Matsuda H.2013. Circadian rhythms and the effect of glucocorticoids on expression of the clock gene period1 in canine peripheral blood mononuclear cells. Vet. J. 196: 402–407. doi: 10.1016/j.tvjl.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 12.Pastorelli L., De Salvo C., Vecchi M., Pizarro T. T.2013. The role of IL-33 in gut mucosal inflammation. Mediators Inflamm. 2013: 608187. doi: 10.1155/2013/608187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters I. R., Peeters D., Helps C. R., Day M. J.2007. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol. 117: 55–66. doi: 10.1016/j.vetimm.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 14.Peterson L. W., Artis D.2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14: 141–153. doi: 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- 15.Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G. M., Nespoli A., Viale G., Allavena P., Rescigno M.2005. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6: 507–514. doi: 10.1038/ni1192 [DOI] [PubMed] [Google Scholar]
- 16.Saenz S. A., Taylor B. C., Artis D.2008. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226: 172–190. doi: 10.1111/j.1600-065X.2008.00713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiering C., Krausgruber T., Chomka A., Fröhlich A., Adelmann K., Wohlfert E. A., Pott J., Griseri T., Bollrath J., Hegazy A. N., Harrison O. J., Owens B. M., Löhning M., Belkaid Y., Fallon P. G., Powrie F.2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513: 564–568. doi: 10.1038/nature13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson K. W., Jergens A. E.2011. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet. Clin. North Am. Small Anim. Pract. 41: 381–398. doi: 10.1016/j.cvsm.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Su J., Chen T., Ji X. Y., Liu C., Yadav P. K., Wu R., Yang P., Liu Z.2013. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm. Bowel Dis. 19: 720–728. doi: 10.1097/MIB.0b013e3182802a76 [DOI] [PubMed] [Google Scholar]
- 20.Tamura Y., Ohta H., Yokoyama N., Lim S. Y., Osuga T., Morishita K., Nakamura K., Yamasaki M., Takiguchi M.2014. Evaluation of selected cytokine gene expression in colonic mucosa from dogs with idiopathic lymphocytic-plasmacytic colitis. J. Vet. Med. Sci. 76: 1407–1410. doi: 10.1292/jvms.13-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F.2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: RESEARCH0034. [DOI] [PMC free article] [PubMed]
- 22.Washabau R. J., Day M. J., Willard M. D., Hall E. J., Jergens A. E., Mansell J., Minami T., Bilzer T. W., WSAVA International Gastrointestinal Standardization Group2010. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 24: 10–26. doi: 10.1111/j.1939-1676.2009.0443.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.