Abstract
Bisphenol A (BPA), a well-known endocrine disruptor, is metabolized and eliminated rapidly from the body in adult animals. However, many authors have reported that perinatal BPA exposure alters development of the brain, reproductive system and behavior in the next generation. Recently, BPA substitutes, especially bisphenol F (BPF), have been used because of concerns about the influence of BPA on children, although the actual effects on the next generation are unknown. In this study, we observed behavioral adverse effects of the offspring of mice exposed to BPA or BPF in fetal period. Female C57BL/6 mice were given oral BPA or BPF (0 or 10 mg/kg body weight) daily from gestational day 11.5 to 18.5. The open field test, the elevated plus maze test and the forced swim test were performed at postnatal week 10. BPF exposure altered offspring behavior significantly, resulting in increases in anxiety and depressive state. The influence of BPF was stronger than that of BPA. We demonstrated novel evidence that BPF influences the behavior of offspring.
Keywords: anxiety behavior, bisphenol A, bisphenol F, depression, endocrine disruptors
Numerous studies have reported the effects of unintentional gestational exposure to environmental endocrine disruptors, including bisphenol A (BPA, 2,2-bis[4-hydroxyphenyl]propane), on the fetus. BPA is an estrogen-mimicking industrial chemical. Exposure to even low doses of BPA may impact human health [47, 49]. BPA is used to manufacture polycarbonate plastics and epoxy resins, which are used in various materials in contact with food. Human exposure to BPA is widespread, and more than 90% of Americans [5] and Canadians [3] have detectable levels of urinary BPA. In addition, BPA has been detected not only in adult blood but also in fetal samples, including the placenta [13, 42]. A number of reports have suggested that BPA exposure may affect the fetus, infants and young children in terms of development of the reproductive system [33, 41, 45], brain [21, 32, 39] and behavior [49] via its action on steroid receptors. A number of studies indicate that perinatal BPA exposure has some influence on anxiety in rodents [49]. These reports have led several countries to ban BPA in plastic baby bottles.
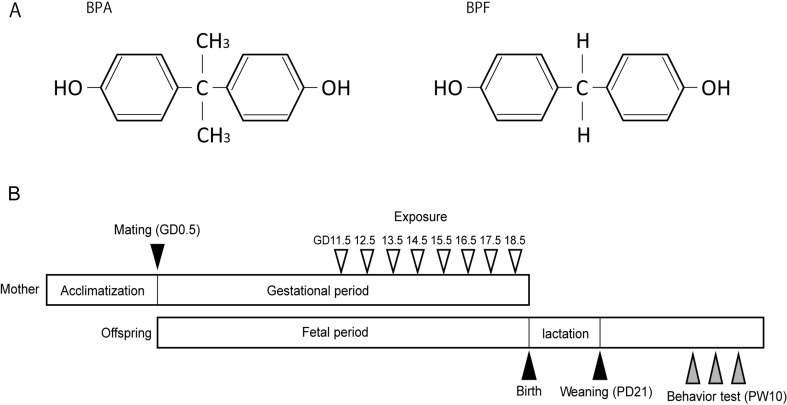
As evidence of the danger of BPA increases, several structural substitutes, such as bisphenol B (2,2-bis[4-hydroxyphenyl]butane), bisphenol F (BPF, bis[4-hydroxyphenyl]methane), bisphenol S (bis[4-hydroxyphenyl]sulfone) and bisphenol AF (4,4-[hexafluoroisopropylidene]diphenol), are being used worldwide without restriction to replace BPA [24, 25]. Little is known about the risk of these structural substitutes, especially their influence on the fetus. These structural substitutes of BPA have been detected in beverages, canned foodstuffs and human serum [7, 24]. One of the most commonly used structural substitutes for BPA is BPF, having a structure in which 2 methyl groups from the middle of BPA are removed (Fig. 1A). According to some reports, BPF shows estrogenic activity, including affinity for estrogen receptors and anti-androgenic activity in vitro, which is slightly different from BPA [20, 34, 37, 38]. This suggests that BPA-like adverse effects can also be hypothesized for BPF. However, in contrast to BPA, no study has reported the influence of BPF exposure on the behavior of the fetus.
Fig. 1.
The structure of bisphenol A (BPA) and bisphenol F (BPF) and the experimental procedure schema for behavior tests. (A) The structural difference of BPA and BPF. (B) Dams were given olive oil, BPA or BPF orally during late pregnancy. Behavior tests were performed at postnatal week (PW) 10.
Many studies of gestational BPA exposure have suggested its influence on anxiety in rodents, and the dosing period given BPA to pregnant rodents has been different in each study. For example, studies have treated rodents with BPA throughout pregnancy and lactation [23, 40], from the middle of pregnancy until a few days after birth [11, 14] or from the period prior to mating until weaning [17]. Neonates show the effects of BPA even when dams were exposed only during a short period of lactation [12, 31]. The effects of BPA exposure limited to pregnancy have also been reported [8, 50]. Some reports suggest that BPA is transmitted via the placenta [2, 30, 44]. Interestingly, BPF residues were detected in fetal tissues, including the brain, after oral administration of BPF to pregnant rats in a late stage of gestation (gestational day [GD] 17) [4]. In the present study, we limited the dosing period of BPA and BPF to late pregnancy and used the open field test (OFT), the elevated plus maze test (EPM) and the forced swimming test (FST) to evaluate adult behavior of the offspring.
This report is the first to examine whether BPF, a structural substitute for BPA, has adverse effects on behavior of offspring in later life.
MATERIALS AND METHODS
Animals and treatments
Male and female C57BL/6NCrSlc mice 7 weeks of age were purchased from Sankyo Lab Co. (Tokyo, Japan). All mice were maintained in an environment with controlled temperature (22 ± 2°C) and humidity (50 ± 5%) and on a 12-hr light (7:00 AM−7:00 PM) and dark (7:00 PM−7:00 AM) cycle. Food and water were freely available. After acclimatizing for 2 weeks, female mice were placed with males. The morning on which a vaginal plug was detected was considered GD 0.5. Dams were isolated from males. In this study, dams were assigned to 1 of 3 treatment groups: oil vehicle as a control, BPA (10 mg/kg body weight (BW)/d, Kanto Chemical Co., Tokyo, Japan) dissolved in olive oil or BPF (10 mg/kg BW/d, Kanto Chemical Co.,) dissolved in olive oil. The BPA and BPF were dissolved in olive oil to become 1 mg/ml, and they were administered with a required amount to be 10 mg/kg BW of treatment mice. The 10 mg/kg BW dose was between the adult systemic “no observed adverse effect level” (NOAEL) of BPA (5 mg/kg/d) in rats and the reproductive and offspring toxicity NOAEL of BPA (50 mg/kg/d) in rats [46]. We adopted this dose of BPF for initial risk assessment, because the NOAEL of BPF has not been determined. Each treatment group consisted of 6 dams, and litters from one treatment group did not come from the same breeder male. Oil vehicle, BPA and BPF were orally (gavage) administered daily from GD 11.5 to 18.5 (late pregnancy; Fig. 1B). All offspring remained with their dams until postnatal day (PD) 21. After weaning, they were group housed with same-sex littermates. Offspring consisted of controls (n=23 males and n=20 females), BPA group (n=24 males and n=21 females) and BPF group (n=26 males and n=25 females). Behavioral tests were conducted at postnatal week (PW) 10. All experimental procedures were in accordance with the guidelines of the Committee for Animal Welfare at Rakuno Gakuen University. These guidelines are based on the Directive 2010/63/EU (revising Directive 86/609/EEC) on the protection of animals used for scientific purposes. (Ethics Committee protocol approval number VH25A4, approved 26 June 2013; and VH15A2, approved 23 June 2015.)
Behavioral tests
We examined the adult behavior of offspring of dams exposed in late pregnancy to BPA and BPF by using the OFT, the EPM and the FST conducted on 3 consecutive days at PW 10. The behavior was recorded and automatically analyzed by a computer with software (TimeOFCR1 for OFT, TimeEP1 for EPM and TimeFZ1 for FST) purchased from O’Hara & Co., Ltd. (Tokyo, Japan). All animals were handled for 1 min per day for the 3 days preceding the OFT to decrease their anxiety levels, while they were carried from their cages to the experimental apparatus. On the examination day, animals with their cages were placed in the behavioral test room at least 1 hr before the test for habituation. All tests were conducted between 1:00–6:00 PM. Each apparatus was cleaned with 70% ethanol, wiped dry and then cleaned with water after each animal. Figure 1B shows the schema of the experimental procedure for behavior tests. Offspring that slept or remained immobile for most of the test duration were excluded from the analysis of the OFT and the EPM.
Open field test
Open field test is often used as an initial screening for anxiety-related behavior in rodents [1]. The OFT apparatus was a square field (50 × 50 × 30 cm). Each mouse was placed into the right corner of the arena and was allowed to freely explore the arena for the duration of the test session. Their behavior was recorded for 10 min under 100-lux light intensity. Behavioral variables measured included the total distance moved and the time spent in the center region, indicators of locomotor activity and anxiety-related behavior. In addition, we recorded and counted the frequency of rearing episodes (standing on hind legs with or without contact with the wall), an indicator of exploratory behavior; the frequency of self-grooming episodes (licking, scratching or cleaning any part of the head or body), an indicator of anxiety-related behavior; and the frequency of stretching episodes (moving only the upper body freely to scan and sniff surroundings without moving the hind legs), an indicator of anxiety-related behavior. Heightened anxiety was inferred from increased the time spent in the center region, the frequency of self-grooming episodes and/or that of stretching episodes. As a result of excluding inappropriate data, the results of OFT consisted of controls (n=23 males and n=20 females), BPA group (n=23 males and n=21 females) and BPF group (n=26 males and n=24 females).
Elevated plus maze test
Sex differences in anxiety behavior are reliably assessed in rodents using the EPM, a well-established paradigm [1]. The EPM has 2 open arms (25 × 5 × 15 cm), 2 closed arms (25 × 5 × 15 cm) and a central square area (5 × 5 cm) binding these 4 arms into a plus shape. The platform was elevated 45 cm above the floor. Each mouse was placed onto the central square area at the beginning of the test, and behavior was recorded for 5 min under 110-lux light intensity. Behavioral variables measured were the total distance moved (a measure of locomotor activity), the time spent in open or closed arms, and the number of open or closed arm entries (a measure of anxiety). Heightened anxiety was inferred from increased time spent in closed arms and/or decreased time spent in open arms. The decreasing of the percentage of open arm entries in total entries also be validated as a measure of heightened anxiety. As a result of excluding inappropriate data, the results of EPM consisted of controls (n=16 males and n=17 females), BPA group (n=18 males and n=18 females) and BPF group (n=21 males and n=22 females).
Forced swimming test
The FST, initially developed by Porsolt et al. [36], is one of the most commonly used tests for assessing the depressive state in rodents and is often used in BPA studies [12, 18]. For the FST, we used a water tank consisting of a transparent cylindrical container (22 × 12 cm) filled to a depth of 15 cm with water at 22 ± 2°C. We placed the water tank in the middle of a box that was controlled at a 200-lux light intensity. Each mouse was placed individually in the water for 6 min, and the percentages of total immobile time were measured. At the same time, the immobile time per 0.5-min was analyzed by a computer. The time of immobility represented a depressive state. Immobility was defined as mice floating motionless with only small movements to keep their heads above the surface of the water. The results of FST consisted of controls (n=23 males and n=20 females), BPA group (n=24 males and n=21 females) and BPF group (n=26 males and n=25 females).
Statistical analysis
Comparisons between open-arm and closed-arm results of the EPM within the same group were analyzed by the Student t test. Comparisons of behavioral effects between controls and BPA group, and between controls and BPF group were analyzed by Dunnett’s one-sided test. A P value of 0.05 was considered significant. All results are presented as means ± standard errors (SE).
RESULTS
Open field test results
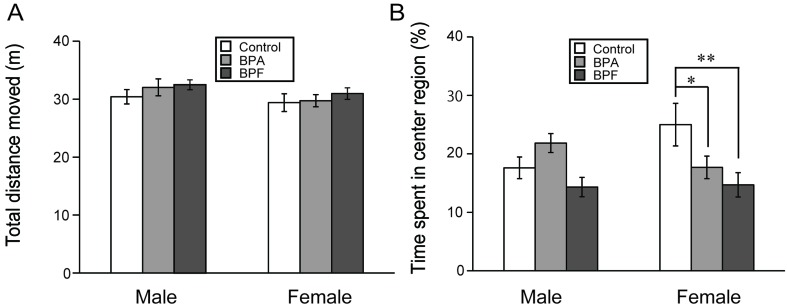
Neither BPA nor BPF exposure significantly altered the total distance moved (Fig. 2A), the frequency of rearing and self-grooming (Table 1) as compared with controls. For male mice in the BPA and BPF groups, time spent in the center region was not significantly different from those in the control group (Fig. 2B). However, for female mice, significant increases of stretching episodes were found in both of BPA (P<0.05) and BPF groups (P<0.05) as compared with controls (Table 1). In addition, BPA and BPF groups significantly decreased the time spent in the center region as compared with controls (P<0.05 and P<0.01, respectively; Fig. 2B).
Fig. 2.
Effects of BPA or BPF exposure in late pregnancy on locomotor activity and anxiety-like behavior of offspring in the open field test (OFT). The parameters are the total distance moved (A) and the time spent in the center region (B) (mean ± standard error [SE]). *P<0.05 and **P<0.01 compared controls with BPF group.
Table 1. Influence of BPA or BPF on each behavioral frequency during the open field test.
| Control | BPA | BPF | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Rearing | 64.39 ± 4.89 | 52.2 ± 5.87 | 71.65 ± 4.55 | 51.24 ± 4.07 | 65.07 ± 3.94 | 58.12 ± 2.85 |
| Grooming | 4.57 ± 0.71 | 3.75 ± 0.40 | 4.04 ± 0.51 | 4.29 ± 0.44 | 4.37 ± 0.51 | 3.42 ± 0.35 |
| Stretching | 3.00 ± 0.55 | 1.70 ± 0.46 | 2.26 ± 0.38 | 3.29 ± 0.48a) | 2.15 ± 0.41 | 3.17 ± 0.53b) |
Data are mean ± SE, a)P<0.05 compared controls with BPA group, b)P<0.05 compared controls with BPF group.
Elevated plus maze test results
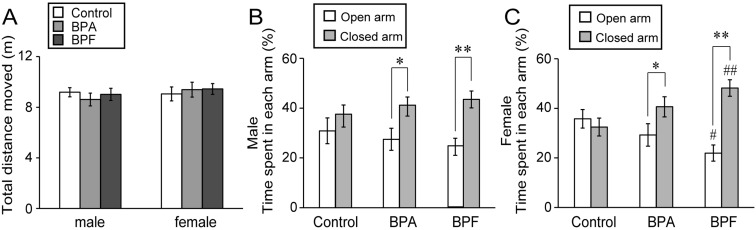
Neither BPA nor BPF exposure significantly altered the total distance moved in the EPM as compared with controls (Fig. 3A). In males, significant difference in the comparison between groups was not found in the result of the time spent in each arm. However, as a result of the comparison of the time spent in open arms with the time spent in closed arms in the same group, significant difference was found in the BPA and BPF groups (P<0.05 and P<0.01, respectively; Fig. 3B), indicating increased anxiety. In females, the result of the comparison of the time spent in open arms with the time spent in closed arms in the same group showed significant difference in the BPA and BPF groups (P<0.05 and P<0.01, respectively; Fig. 3C). The percentage of open arm entries in total entries also showed a significant decrease in the BPF group (P<0.01; Fig. S1). In addition, BPF administration induced a significant increase in the time spent in closed arms (P<0.01) and a significant decrease in the time spent in open arms (P<0.05) as compared with controls (Fig. 3C). These finding indicate that mice in the BPF group experienced more anxiety than those in the control and BPA groups.
Fig. 3.
Effects of BPA or BPF exposure in late pregnancy on locomotor activity and anxiety-like behavior of offspring in the elevated plus maze test (EPM). Parameters are total distance moved (A) and the time spent in each arm of males (B) and females (C) (mean ± SE). *P<0.05 and **P<0.01 compared the time spent in open arms with the time spent in closed arms in the same group. #P<0.05 and ##P<0.01 compared controls with BPF group.
Forced swimming test results
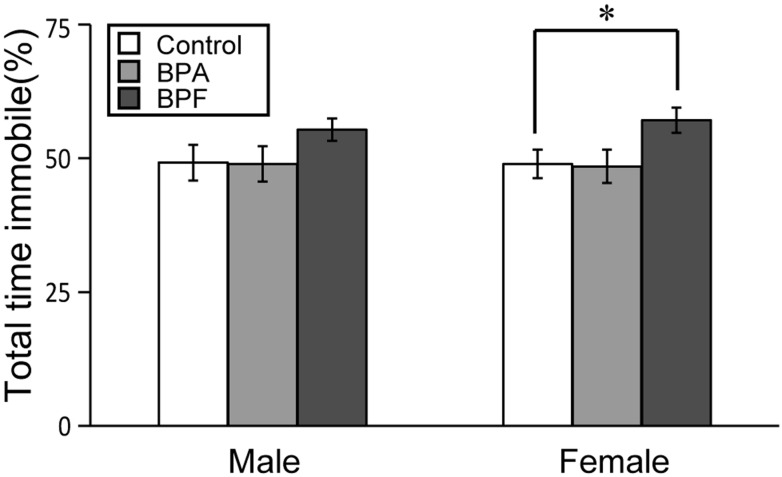
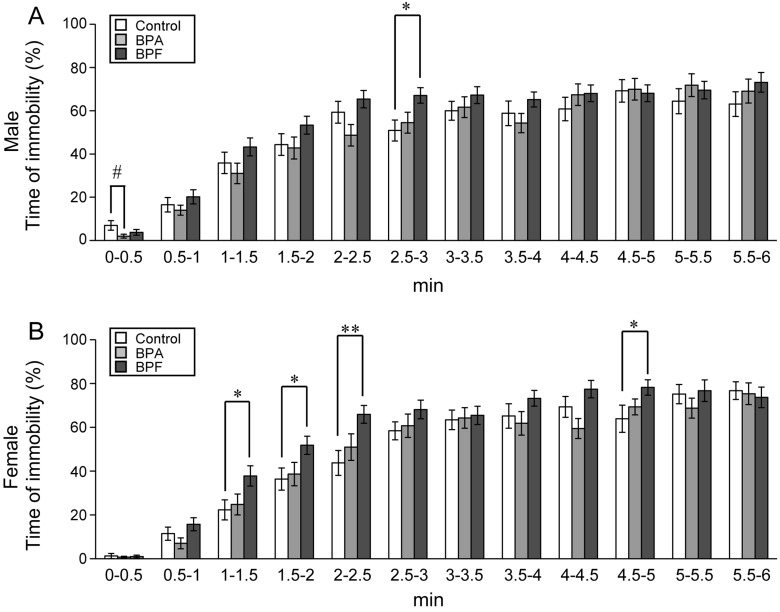
The FST was conducted for 6 min on the third day of testing. We compared the percentage of immobile time, considered to be a depressive state, between experimental and control groups. In females, BPF group had significant increased immobility compared with controls (P<0.05; Fig. 4). BPA did not affect the percentage of immobility time for either sex. Observation of immobility during each 0.5-min revealed that immobility significantly increased in both sexes in the BPF group early in the examination (Fig. 5). In males, a significant increase of immobility was found between controls and BPF groups at 2.5–3 min (P<0.05; Fig. 5A). In females, significant increases of immobility were found in the BPF group at 1–1.5 (P<0.05), 1.5–2 (P<0.05), 2–2.5 (P<0.01) and 4.5–5 (P<0.05) min as compared with controls (Fig. 5B).
Fig. 4.
Effects of BPA or BPF exposure in late pregnancy on depression-like behavior of offspring in the forced swimming test (FST). The total time of immobility during the 6-min FST is shown for males and females (mean ± SE). *P<0.05 compared controls with BPF group.
Fig. 5.
Effects of BPA or BPF exposure in late pregnancy on depression-like behavior during each 0.5-min increment in the FST. The total time of immobility per 0.5-min is shown for males (A) and females (B) (mean ± SE). *P<0.05 and **P<0.01 compared controls with BPF group. #P<0.05 compared controls with BPA group.
DISCUSSION
We administered BPA and BPF to dams orally, because the usual route in humans is oral. The administration period was from GD 11.5 to 18.5 (Fig. 1B). Supplemental table 1 shows the number of offspring and ratio of males to females, indicating no significant differences. Supplemental Fig. 2 shows the body weights of offspring of the control and BPF groups; there were no significant differences. There were also no significant differences in the BPA group, as reported in a previous study [51].
In all behavioral tests in the present study, BPF exposure changed the behavior of offspring more significantly than BPA exposure. Significant influences of BPF were found in all behavioral tests, especially in females. In the BPF group, anxiety increased compared with controls (Figs. 2, 3 and S1). Male mice typically display more anxiety than females [16], as was the case in our control group. After BPF exposure, however, the results were similar for both sexes, indicating the abolishment of typical sex differences. In addition, BPF increased depressive states compared with controls, which was not the case with BPA (Fig. 4). Although BPF is increasingly used worldwide as a substitute for BPA, these findings suggest that BPF induces more negative effects in the fetus than BPA.
Notably, BPA and BPF exposure affected offspring, although the dosing period was restricted to late pregnancy. This period is important for brain function, because the brain undergoes structural and functional changes that continue into the neonatal period. The sex steroids play an essential role in sexual differentiation of the brain, and estrogenic activity of BPA appears to be involved in some sexually dimorphic areas of the brain [49], for instance, the anteroventral periventricular nucleus of the hypothalamus (AVPV) and the locus ceruleus (LC). It was reported that sex differences of these brain areas were affected by BPA and resulted in abolishment of behavioral sex differences in open field test [22, 23, 39]. Interestingly, our data showed that BPF had a stronger influence than BPA on behavior and the apparent loss of sexual dimorphism, although the estrogenic activity and estrogen receptor activity of BPF are slightly less potent than those of BPA [20, 34, 37, 38]. When considering only estrogenicity, the severe adverse effect of BPF on the fetus could be explained by a larger amount of BPF being transmitted to the fetus via the placenta. However, when considering factors other than estrogenicity, a conceivable cause for the disruption of sexual dimorphism is the greater steroidogenesis of BPF compared to BPA; this causes an increase of progesterone that is not seen with BPA [38]. Progesterone regulates neuroendocrine functions mediated primarily by progesterone receptors in the central nervous system and also plays a role in sexual differentiation [27, 35, 48]. Because few studies have examined the hormonal actions, especially in vivo, of BPF and other BPA substitutes, more investigations are necessary.
In one of the few reports of the influence on the brain by BPF, it was reported that BPF altered multiple gene expression related with dopamine-serotonin system in the prefrontal cortex of juvenile female rats [6]. Dopamine and serotonin have been known to be related to psychiatric disorders [29]. There is a possibility that changes of these gene expression brought the behavioral disruption we showed in this study. Recently, a number of studies have linked the effects of BPA to an epigenetic mechanism disruption, particularly DNA methylation which plays an important role in the regulation of gene expression [9, 15, 43, 49]. BPF has not yet been examined for this effect. To determine the mechanism of BPF influence, we should note that there are possible explanations other than estrogenicity and other hormonal actions for the disruption of offspring behavior.
In adult humans, the metabolism of BPA is primarily mediated by UGT2B7, an orthologue of the rodent UGT2B1 gene [26]. BPF is also metabolized by UGT in human hepatocytes [10]. In a rodent study, fetal hepatic UGT activity on BPF is very weak, and the activity level increases gradually with fetal age, similar to activity on BPA [52]. UGT is weakly expressed in fetuses of rodents and humans [19, 28, 30], indicating that fetuses are more susceptible to BPA and BPF than adults. We think that human fetuses have possibility to be affected by BPF because long pregnant period may increase the risk to be exposed by BPF even if the exposure level is low. In order to think association with human health, it is necessary to investigate more details, such as other influence and relationship between dose and influence.
In summary, we have revealed for the first time that behavioral disruption of offspring is an adverse effect of BPF administration during pregnancy. In addition, the adverse effects of BPF were larger in magnitude than those of BPA. This study is an important first step toward understanding the effects of BPF. The use of BPF has been increasing, but more investigations are necessary to determine the safety of BPF as a substitute for BPA.
Supplementary Material
Acknowledgments
This study was supported in part by the Health Science Research Grant H23-Kagaku-004 from the Ministry of Health, Labour and Welfare, Japan, and a Feasibility Study among Fundamental Studies under the framework of EXTEND 2010 (Extended Tasks on Endocrine Disruption 2010) funded by the Ministry of the Environment, JAPAN.
REFERENCES
- 1.Bailey K. R., Crawley J. N.2009. Methods of Behavior Analysis in Neuroscience. pp. 77–101 In: Anxiety-Related Behaviors in Mice, 2nd ed. (Buccafusco, J.J. ed.), CRC Press/Taylor & Francis LCC, Boca Raton. [Google Scholar]
- 2.Balakrishnan B., Henare K., Thorstensen E. B., Ponnampalam A. P., Mitchell M. D.2010. Transfer of bisphenol A across the human placenta. Am. J. Obstet Gynecol. 202:393. e1-7. [DOI] [PubMed] [Google Scholar]
- 3.Bushnik T., Haines D., Levallois P., Levesque J., Van Oostdam J., Viau C.2010. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 21: 7–18. [PubMed] [Google Scholar]
- 4.Cabaton N., Chagnon M. C., Lhuguenot J. C., Cravedi J. P., Zalko D.2006. Disposition and metabolic profiling of bisphenol F in pregnant and nonpregnant rats. J. Agric. Food Chem. 54: 10307–10314. doi: 10.1021/jf062250q [DOI] [PubMed] [Google Scholar]
- 5.Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L.2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 116: 39–44. doi: 10.1289/ehp.10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro B., Sánchez P., Torres J. M., Ortega E.2015. Bisphenol A, bisphenol F and bisphenol S affect differently 5α-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environ. Res. 142: 281–287. doi: 10.1016/j.envres.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 7.Cobellis L., Colacurci N., Trabucco E., Carpentiero C., Grumetto L.2009. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 23: 1186–1190. doi: 10.1002/bmc.1241 [DOI] [PubMed] [Google Scholar]
- 8.Cox K. H., Gatewood J. D., Howeth C., Rissman E. F.2010. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm. Behav. 58: 754–761. doi: 10.1016/j.yhbeh.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolinoy D. C., Huang D., Jirtle R. L.2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. U.S.A. 104: 13056–13061. doi: 10.1073/pnas.0703739104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumont C., Perdu E., de Sousa G., Debrauwer L., Rahmani R., Cravedi J. P., Chagnon M. C.2011. Bis(hydroxyphenyl)methane-bisphenol F-metabolism by the HepG2 human hepatoma cell line and cryopreserved human hepatocytes. Drug Chem. Toxicol. 34: 445–453. doi: 10.3109/01480545.2011.585651 [DOI] [PubMed] [Google Scholar]
- 11.Farabollini F., Porrini S., Dessì-Fulgherit F.1999. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol. Biochem. Behav. 64: 687–694. doi: 10.1016/S0091-3057(99)00136-7 [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto T., Kubo K., Nishikawa Y., Aou S.2013. Postnatal exposure to low-dose bisphenol A influences various emotional conditions. J. Toxicol. Sci. 38: 539–546. doi: 10.2131/jts.38.539 [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg G., Rice D. C.2009. Does rapid metabolism ensure negligible risk from bisphenol A? Environ. Health Perspect. 117: 1639–1643. doi: 10.1289/ehp.0901010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gioiosa L., Fissore E., Ghirardelli G., Parmigiani S., Palanza P.2007. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm. Behav. 52: 307–316. doi: 10.1016/j.yhbeh.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Ho S. M., Tang W. Y., Belmonte de Frausto J., Prins G. S.2006. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 66: 5624–5632. doi: 10.1158/0008-5472.CAN-06-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imhof J. T., Coelho Z. M. I., Schmitt M. L., Morato G. S., Carobrez A. P.1993. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav. Brain Res. 56: 177–180. doi: 10.1016/0166-4328(93)90036-P [DOI] [PubMed] [Google Scholar]
- 17.Jašarević E., Sieli P. T., Twellman E. E., Welsh T. H., Jr, Schachtman T. R., Roberts R. M., Geary D. C., Rosenfeld C. S.2011. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. U.S.A. 108: 11715–11720. doi: 10.1073/pnas.1107958108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones B. A., Watson N. V.2012. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Horm. Behav. 61: 605–610. doi: 10.1016/j.yhbeh.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 19.King C. D., Rios G. R., Assouline J. A., Tephly T. R.1999. Expression of UDP-glucuronosyltransferases (UGTs) 2B7 and 1A6 in the human brain and identification of 5-hydroxytryptamine as a substrate. Arch. Biochem. Biophys. 365: 156–162. doi: 10.1006/abbi.1999.1155 [DOI] [PubMed] [Google Scholar]
- 20.Kitamura S., Suzuki T., Sanoh S., Kohta R., Jinno N., Sugihara K., Yoshihara S., Fujimoto N., Watanabe H., Ohta S.2005. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 84: 249–259. doi: 10.1093/toxsci/kfi074 [DOI] [PubMed] [Google Scholar]
- 21.Komada M., Asai Y., Morii M., Matsuki M., Sato M., Nagao T.2012. Maternal bisphenol A oral dosing relates to the acceleration of neurogenesis in the developing neocortex of mouse fetuses. Toxicology 295: 31–38. doi: 10.1016/j.tox.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 22.Kubo K., Arai O., Ogata R., Omura M., Hori T., Aou S.2001. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neurosci. Lett. 304: 73–76. doi: 10.1016/S0304-3940(01)01760-8 [DOI] [PubMed] [Google Scholar]
- 23.Kubo K., Arai O., Omura M., Watanabe R., Ogata R., Aou S.2003. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 45: 345–356. doi: 10.1016/S0168-0102(02)00251-1 [DOI] [PubMed] [Google Scholar]
- 24.Liao C., Kannan K.2013. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 61: 4655–4662. doi: 10.1021/jf400445n [DOI] [PubMed] [Google Scholar]
- 25.Liao C., Liu F., Guo Y., Moon H. B., Nakata H., Wu Q., Kannan K.2012. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ. Sci. Technol. 46: 9138–9145. doi: 10.1021/es302004w [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie P. I., Owens I. S., Burchell B., Bock K. W., Bairoch A., Bélanger A., Fournel-Gigleux S., Green M., Hum D. W., Iyanagi T., Lancet D., Louisot P., Magdalou J., Chowdhury J. R., Ritter J. K., Schachter H., Tephly T. R., Tipton K. F., Nebert D. W.1997. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7: 255–269. doi: 10.1097/00008571-199708000-00001 [DOI] [PubMed] [Google Scholar]
- 27.Mani S. K., Oyola M. G.2012. Progesterone signaling mechanisms in brain and behavior. Front. Endocrinol. (Lausanne) 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto J., Yokota H., Yuasa A.2002. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ. Health Perspect. 110: 193–196. doi: 10.1289/ehp.02110193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niederkofler V., Asher T. E., Dymecki S. M.2015. Functional Interplay between Dopaminergic and Serotonergic Neuronal Systems during Development and Adulthood. ACS Chem. Neurosci. 6: 1055–1070. doi: 10.1021/acschemneuro.5b00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H.2010. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 118: 1196–1203. doi: 10.1289/ehp.0901575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patisaul H. B., Bateman H. L.2008. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm. Behav. 53: 580–588. doi: 10.1016/j.yhbeh.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 32.Patisaul H. B., Polston E. K.2008. Influence of endocrine active compounds on the developing rodent brain. Brain Res. Brain Res. Rev. 57: 352–362. doi: 10.1016/j.brainresrev.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 33.Peretz J., Vrooman L., Ricke W. A., Hunt P. A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H. S., Swan S. H., VandeVoort C. A., Flaws J. A.2014. Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environ. Health Perspect. 122: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez P., Pulgar R., Olea-Serrano F., Villalobos M., Rivas A., Metzler M., Pedraza V., Olea N.1998. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ. Health Perspect. 106: 167–174. doi: 10.1289/ehp.98106167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen S. L., Intlekofer K. A., Moura-Conlon P. J., Brewer D. N., Del Pino Sans J., Lopez J. A.2013. Novel progesterone receptors: neural localization and possible functions. Front. Neurosci. 7: 164. doi: 10.3389/fnins.2013.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porsolt R. D., Bertin A., Jalfre M.1977. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229: 327–336. [PubMed] [Google Scholar]
- 37.Rochester J. R., Bolden A. L.2015. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 123: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenmai A. K., Dybdahl M., Pedersen M., Alice van Vugt-Lussenburg B. M., Wedebye E. B., Taxvig C., Vinggaard A. M.2014. Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci. 139: 35–47. doi: 10.1093/toxsci/kfu030 [DOI] [PubMed] [Google Scholar]
- 39.Rubin B. S., Lenkowski J. R., Schaeberle C. M., Vandenberg L. N., Ronsheim P. M., Soto A. M.2006. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology 147: 3681–3691. doi: 10.1210/en.2006-0189 [DOI] [PubMed] [Google Scholar]
- 40.Ryan B. C., Vandenbergh J. G.2006. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm. Behav. 50: 85–93. doi: 10.1016/j.yhbeh.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 41.Salian S., Doshi T., Vanage G.2009. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 85: 11–18. doi: 10.1016/j.lfs.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 42.Schönfelder G., Wittfoht W., Hopp H., Talsness C. E., Paul M., Chahoud I.2002. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 110: A703–A707. doi: 10.1289/ehp.021100703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Susiarjo M., Sasson I., Mesaros C., Bartolomei M. S.2013. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 9: e1003401. doi: 10.1371/journal.pgen.1003401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi O., Oishi S.2000. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ. Health Perspect. 108: 931–935. doi: 10.1289/ehp.00108931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timms B. G., Howdeshell K. L., Barton L., Bradley S., Richter C. A., vom Saal F. S.2005. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. U.S.A. 102: 7014–7019. doi: 10.1073/pnas.0502544102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyl R. W., Myers C. B., Marr M. C., Thomas B. F., Keimowitz A. R., Brine D. R., Veselica M. M., Fail P. A., Chang T. Y., Seely J. C., Joiner R. L., Butala J. H., Dimond S. S., Cagen S. Z., Shiotsuka R. N., Stropp G. D., Waechter J. M.2002. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 68: 121–146. doi: 10.1093/toxsci/68.1.121 [DOI] [PubMed] [Google Scholar]
- 47.vom Saal F. S., Hughes C.2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113: 926–933. doi: 10.1289/ehp.7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner C. K., Nakayama A. Y., De Vries G. J.1998. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology 139: 3658–3661. doi: 10.1210/endo.139.8.6223 [DOI] [PubMed] [Google Scholar]
- 49.Wolstenholme J. T., Rissman E. F., Connelly J. J.2011. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 59: 296–305. doi: 10.1016/j.yhbeh.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolstenholme J. T., Taylor J. A., Shetty S. R. J., Edwards M., Connelly J. J., Rissman E. F.2011. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE 6: e25448. doi: 10.1371/journal.pone.0025448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xi W., Lee C. K. F., Yeung W. S. B., Giesy J. P., Wong M. H., Zhang X., Hecker M., Wong C. K. C.2011. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod. Toxicol. 31: 409–417. doi: 10.1016/j.reprotox.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 52.Yabusaki R., Iwano H., Tsushima S., Koike N., Ohtani N., Tanemura K., Inoue H., Yokota H.2015. Weak activity of UDP-glucuronosyltransferase toward Bisphenol analogs in mouse perinatal development. J. Vet. Med. Sci. 77: 1479–1484. doi: 10.1292/jvms.15-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.