Abstract
Introduction: Reducing costs by improving storage efficiency has been a focus of the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen Repository (Biorepository) and Biologic Specimen and Data Repositories Information Coordinating Center (BioLINCC) programs for several years.
Methods: Study specimen profiles were compiled using the BioLINCC collection catalog. Cost assessments and calculations on the return on investments to consolidate or reduce a collection, were developed and implemented.
Results: Over the course of 8 months, the NHLBI Biorepository evaluated 35 collections that consisted of 1.8 million biospecimens. A total of 23 collections were selected for consolidation, with a total of 1.2 million specimens located in 21,355 storage boxes. The consolidation resulted in a savings of 4055 boxes of various sizes and 10.2 mechanical freezers (∼275 cubic feet) worth of space.
Conclusion: As storage costs in a biorepository increase over time, the development and use of information technology tools to assess the potential advantage and feasiblity of vial consolidation can reduce maintenance expenses.
Keywords: : sustainability, return on investment, consolidation, biorepository
Background
The National Heart, Lung, and Blood Institute (NHLBI) within the United States' National Institutes of Health (NIH) established a Biologic Specimen Repository (Biorepository) in 1975 to support the need for archived collections to address emerging blood-safety concerns. These early collections made several seminal contributions to public health by contributing to the identification of the viral origin of the agent responsible for non-A, non-B hepatitis, and the transfusion transmissibility of HIV.1,2 Toward the end of the 1990s, the scientific and public health value, and the cost-effectiveness of centrally maintaining collections, was recognized and the Biorepository mission was expanded to acquire biospecimens from NHLBI studies with unique patient populations that had high potential scientific utility. Currently, just over four million vials from 46 historical and contemporary NHLBI clinical studies that are linked to their phenotypic data are available to qualified investigators at no cost other than the cost of shipping the biospecimens. Access to the collections is provided online through the Biologic Specimen and Data Repositories Information Coordinating Center (BioLINCC) website at www.biolincc.nhlbi.nih.gov. BioLINCC was established in 2008 to coordinate the activities necessary to increase the scientific use of the stored biospecimens and associated data, acquire new high-quality collections, and maintain collections in an efficient and cost-effective manner.3,4
Reducing infrastructure costs by improving storage efficiency has been a focus of the Biorepository and BioLINCC programs for several years. Biospecimen collections had been received as bulk transfers at the end of a clinical study and freezer boxes had not been consolidated before transfer. In addition, nonconsecutive unoccupied spaces occurred as vials were requested and distributed. The result was that 30% of available freezer space was not being utilized. In a collaborative effort, the Biorepository and BioLINCC developed an IT visualization tool to provide a freezer-wide view of vial locations.5 This enabled cost-effective reduction strategies to be developed and available freezer space to be assessed at a freezer and box level, thus enabling more accurate projections of future freezer needs and potential areas for vial consolidation. The methods used and the results of the consolidation efforts are discussed below.
Methods
Study specimen profiles that were compiled for the BioLINCC study catalog were assessed.6 The material types available, vial type and known discrepancy types, and rates were evaluated along with request history to assess future research potential. Study evaluations were prioritized based on the activity level for each study collection with higher activity collections prioritized over lower activity collections.
The configuration for each storage container was mapped within the biological specimen inventory (BSI) system, the tool used to manage the inventory. This included defining the number, dimensions and configuration of space within each storage box, rack, and freezer within the repository. Each storage box was reserved in the BSI system for a particular study so that directional placement of vials would group vials from a single study and material type. The number and location of freezers, including their proximity to one another, were also taken into account in the placement determination to minimize the number of locations that would need to be accessed to fulfill future requests and maximize retrieval efficiency.7,8
Reports were generated in the BSI for each selected study to determine if any specimens were marked for discard due to inability to link to clinical data, no consent for broad future uses, or questionable material integrity. The size and number of storage boxes with available usable specimen occupancy rate brackets of <25%, <50%, and <75% were determined.
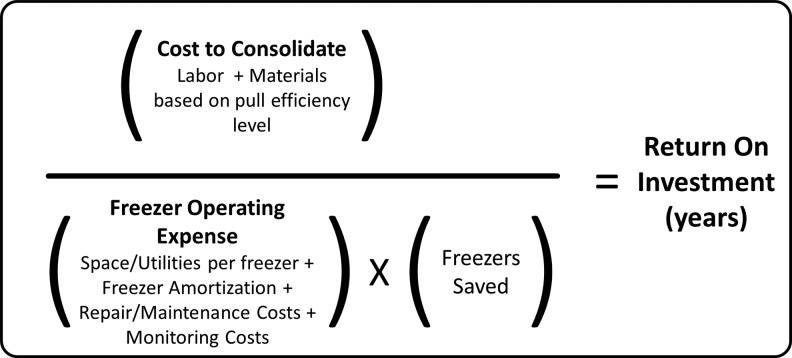
The cost to consolidate specimens at each occupancy rate was then compared with the cost of storage to calculate the return on investment (ROI). See Figure 1 for the calculation parameters that should be considered.
FIG. 1.
Return on investment calculation.
The cost to consolidate took into account the number of vials to be transferred and the QC parameters that would be checked. The occupancy rate of boxes was taken into account, as low-occupancy boxes would require more effort per vial to retrieve. The freezer operating expense took into account the total ownership cost of each unit (space, electrical consumption, amortization, monitoring, and routine maintenance) and the estimated repair and unit replacement expenses based on a life expectancy of 12 years. The number of freezers saved was determined by the number of boxes that could be conserved by consolidation.
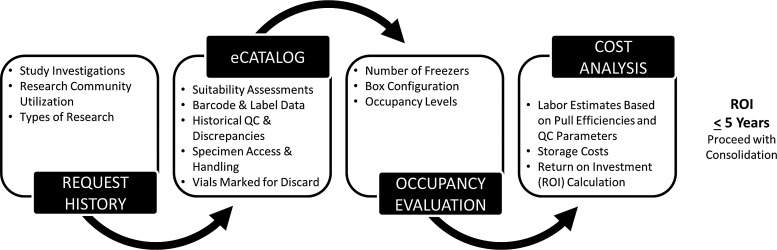
The ROI was calculated by comparing these costs and calculating the time that it took to recoup the expenses associated with the consolidation. This ROI was conservative as it did not take the future pull efficiency savings into account. Collections that had a ROI of ≤5 years and/or those with most significant space savings were added to the queue for consolidation. Figure 2 illustrates the process that was used for the consolidation evaluation.
FIG. 2.
Consolidation evaluation process.
Vials from boxes targeted for consolidation were requisitioned in the BSI. Laboratory technicians removed boxes from freezers according to the controlled specimen handling procedures used for retrieval of biospecimens for investigators. A quality control check was performed on each sample to ensure that the correct vial was identified. Consolidation was completed on a vial-by-vial basis, with the new position loaded into the system. Vials that were previously marked for discard were destroyed and all other vials were consolidated into a smaller number of boxes and then relocated into freezers that were less than 10 years old. Specimens were consolidated into a minimum of two study freezers for risk mitigation purposes.
Results/Discussion
Over the course of 8 months, the NHLBI Biorepository evaluated 35 collections that consisted of 1.8 million biospecimens. A total of 21 collections with an ROI of ≤5 years at the 75% occupancy threshold and 2 additional collections where specimens were intermixed with other studies that had <5 year ROI or where consolidation provided a significant amount of space savings (ARDS10 and WLM) were consolidated (Table 1). The 12 studies that were not selected for consolidations were left in place within existing freezers and are listed in Table 2.
Table 1.
Reduction of Storage Space by Study
| Study | Total vials in collection | Boxes saved | Freezers saved | ROI (years) |
|---|---|---|---|---|
| ACCESS | 43,657 | 207 | 0.5 | 4.1 |
| ACTG | 73,735 | 72 | 0.2 | 3.8 |
| ARDS 1 | 6435 | 95 | 0.2 | 1.8 |
| ARDS 2 | 4122 | 78 | 0.2 | 0.8 |
| ARDS 3 | 3689 | 46 | 0.1 | 2.4 |
| ARDS 4 | 17,022 | 49 | 0.1 | 2.7 |
| ARDS 5 | 31,235 | 258 | 0.6 | 3.5 |
| ARDS 7 | 4546 | 21 | 0.1 | 1.1 |
| ARDS 10 | 12,781 | 34 | 0.1 | 6.2 |
| BMT 0201 | 6821 | 81 | 0.2 | 1.7 |
| BMT 0302 | 435 | 18 | 0.0 | 1.0 |
| DASH | 17,358 | 65 | 0.2 | 3.5 |
| DASH Sodium | 21,341 | 130 | 0.3 | 1.4 |
| DISC/NGHS | 45,365 | 1042 | 2.6 | 2.1 |
| HHS | 94,018 | 154 | 0.4 | 2.7 |
| LAP | 60,411 | 102 | 0.3 | 4.7 |
| P2C2 | 7356 | 48 | 0.1 | 1.7 |
| PREMIER | 41,799 | 152 | 0.4 | 1.6 |
| TRAP | 59,489 | 298 | 0.7 | 2.6 |
| TSS | 348,283 | 135 | 0.3 | 2.4 |
| TTVS | 245,363 | 280 | 0.7 | 2.8 |
| VATS | 66,113 | 548 | 1.4 | 3.6 |
| WLM | 34,511 | 142 | 0.4 | 5.3 |
| Total | 1,245,885 | 4055 | 10.2 | |
| Median | 31,235 | 102 | 0.3 | 2.6 |
ROI, return on investment.
Table 2.
Studies Not Selected for Consolidation
| Study | Calculated ROI (years) | Estimated freezer savings |
|---|---|---|
| BMT0402 | 5.5 | 0.06 |
| NANB | 1.95 | 0.06 |
| RADAR | 4.29 | 0.08 |
| REDS II MS | 4.39 | 0.03 |
| RISE | 3.67 | 0.02 |
| TOPCAT | 4.1 | 0.08 |
| ARDS6 | 6.57 | 0.01 |
| ARDS8 | N/A | 0 |
| ARDS9 | 2.71 | 0.01 |
| ARDS11 | N/A | 0 |
| MHCS | 3.4 | 0.09 |
| WNV | 0.56 | 0.07 |
The 23 selected collections had a total of 1.2 million specimens located in 21,355 storage boxes. The consolidation resulted in a savings of 4055 boxes of various sizes and ∼10.2 freezers' worth of space. The DISC/NGHS studies generated the greatest space savings in large part because much of the inventory was stored in larger vials, and because it had been well used over the years resulting in a large number of boxes with a low occupancy rates.
Conclusion
The cost of storage of a biorepository increases over time, in part, due to decreasing occupancy level of storage containers as vials are withdrawn. Information technology tools that assess storage efficiency can be combined with ROI tools that allow for the evaluation of the cost labor to consolidate versus the cost to store inefficiently packed boxes. Together they provide an effective set of management tools for determining when to consolidate collections.
Acknowledgments
This work was funded by Contract No. HHSN268201400035C, entitled “NHLBI Biological Specimen Repository” and HHSN 26201400014C entitled “The Biologic Specimen and Data Repositories Information Coordinating Center.”
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mosley JW, Aach RD, Hollinger FB, et al. . Non-A, non-B hepatitis and antibody to hepatitis C virus. JAMA 1990;263:77–78 [PubMed] [Google Scholar]
- 2.Kleinman SH, Niland JC, Azen SP, et al. . Prevalence of antibodies to human immunodeficiency virus type 1 among blood donors prior to screening. The Transfusion Safety Study/NHLBI Donor Repository. Transfusion 1989;29:572–580 [DOI] [PubMed] [Google Scholar]
- 3.Giffen CA, Carroll LE, Adams JT, Brennan SP, Coady SA, Wagner EL. Providing contemporary access to historical biospecimen collections: Development of the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). Biopreserv Biobank 2015;13:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner E, Shea K, Carroll L, et al. . Sustaining and maximizing the scientific value of historical collections: The NHLBI biorepository business plan, contributed poster session, International Society of Biological and Environmental Science Annual Meeting 2014
- 5.Wagner EL, Carroll LE, Shea K, et al. . Maintaining an open scientific resource of archival collections without cost recovery: The NHLBI BioLINCC and biorepository tool chest. Biopreserv Biobank 2016;14:A-1-A-63. [Google Scholar]
- 6.Adams J, Giffen C, Krzystan J, Shea K, Carroll L, Wagner E. Building an online catalog to standardize and centralize the knowledge base of a diverse group of unique historical biospecimen collections: The NHLBI BioLINCC program approach. Biopreserv Biobank 2015;13:A-44 [Google Scholar]
- 7.Shea K, Krzystan J, Marchesani L, et al. . Make each touch count: Leveraging IT tools to maximize space and operational efficiency in the NHLBI biorepository. Biopreserv Biobank 2015;13:A-11 [Google Scholar]
- 8.Shea K, Marchesani L, Meagher K, Wagner EL. Maximizing storage efficiency in the NHLBI biorepository: Using technology to drive continuous improvement. Biopreserv Biobank 2016;14:A-1-A-63 [Google Scholar]