Abstract
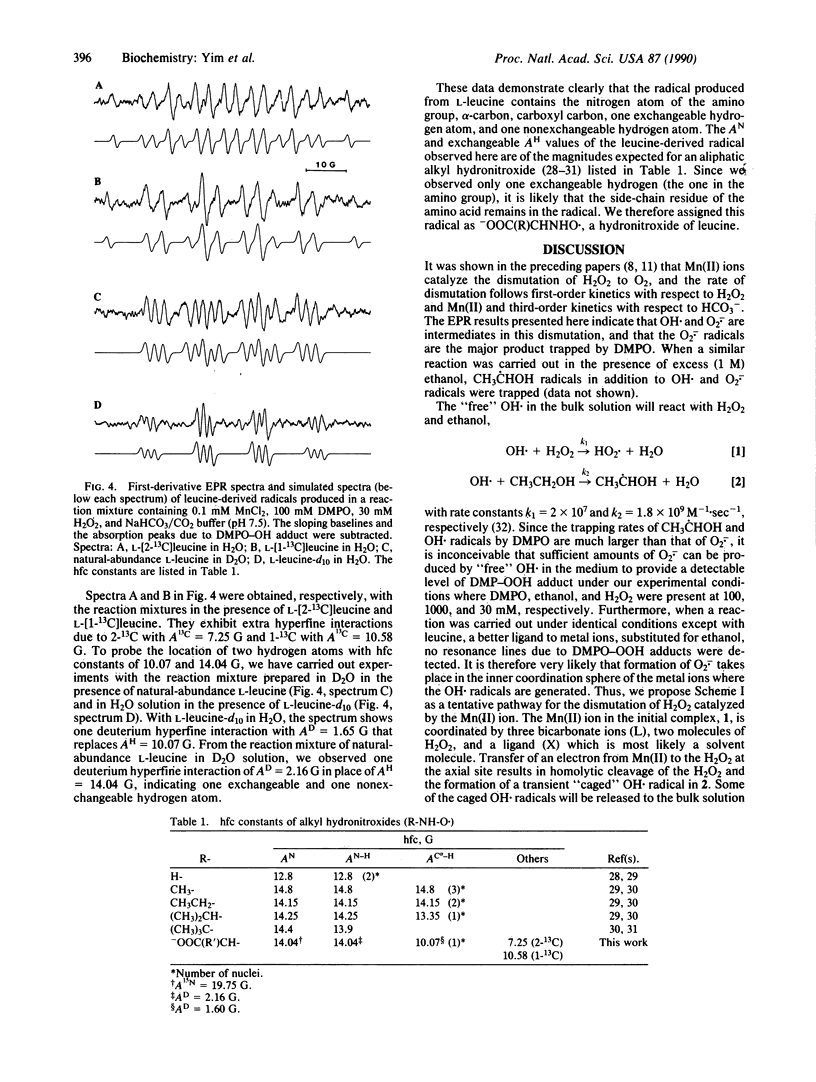
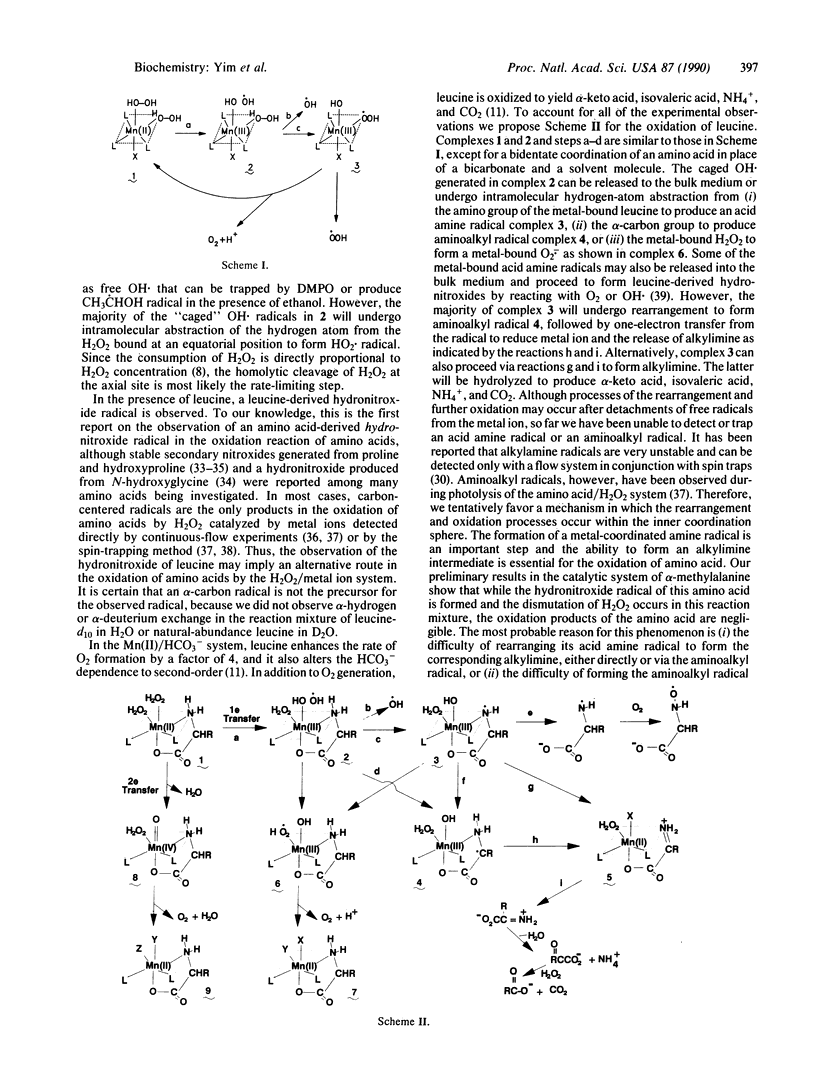
To examine the structural identities of reactive free radicals and the mechanism of the oxidative modification of proteins, we used EPR and spin-trapping methods to investigate the oxidation of amino acids by H2O2 as well as the decomposition of H2O2 itself catalyzed by Mn(II) ions. Superoxide and hydroxyl radicals (O2-. and OH.) were trapped by a spin trap, 5,5-dimethyl-1-pyrroline-1-oxide (DMPO), in a reaction mixture containing Mn(II) and H2O2 in bicarbonate/CO2 buffer. When Hepes was used in place of bicarbonate buffer, superoxide radical was not observed, indicating the importance of bicarbonate buffer. With addition of L-leucine to a similar reaction mixture, a leucine-derived radical that replaced the DMPO-superoxide adduct was detected in the absence and presence of DMPO. Using various isotope-enriched L-leucines, we successfully identified this radical as a hydronitroxide, -OOC(R)CHNHO.. The data are consistent with the formation of a transient "caged" OH. in the inner coordination sphere of Mn(II). This caged OH. is likely to undergo an intramolecular hydrogen-atom abstraction from the Mn-bound H2O2 or amino acid. Two reaction schemes are proposed to account for the experimental results shown here and in the preceding papers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgood G. S., Perry J. J. Characterization of a manganese-containing catalase from the obligate thermophile Thermoleophilum album. J Bacteriol. 1986 Nov;168(2):563–567. doi: 10.1128/jb.168.2.563-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Berlett B. S., Chock P. B., Yim M. B., Stadtman E. R. Manganese(II) catalyzes the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jan;87(1):389–393. doi: 10.1073/pnas.87.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Davies M. J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem J. 1989 Jan 15;257(2):603–606. doi: 10.1042/bj2570603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J., Paxton J. Spin trapping of superoxide. Mol Pharmacol. 1979 Sep;16(2):676–685. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol. 1982 Mar;21(2):262–265. [PubMed] [Google Scholar]
- Floyd R. A., Lewis C. A., Wong P. K. High-pressure liquid chromatography--electrochemical detection of oxygen free radicals. Methods Enzymol. 1984;105:231–237. doi: 10.1016/s0076-6879(84)05030-8. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Nagy I. Formation of long-lived hydroxyl free radical adducts of proline and hydroxyproline in a Fenton reaction. Biochim Biophys Acta. 1984 Oct 9;790(1):94–97. doi: 10.1016/0167-4838(84)90337-6. [DOI] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J. R., Bolton J. R. Superoxide formation in spinach chloroplasts: electron spin resonance detection by spin trapping. Biochem Biophys Res Commun. 1975 Jan 2;64(3):803–807. doi: 10.1016/0006-291x(75)90118-7. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Mottley C., Mason R. P. A direct electron spin resonance and spin-trapping investigation of peroxyl free radical formation by hematin/hydroperoxide systems. J Biol Chem. 1983 Mar 25;258(6):3855–3858. [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Kennedy C. H., Pryor W. A., Winston G. W., Church D. F. Hydroperoxide-induced radical production in liver mitochondria. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1123–1129. doi: 10.1016/s0006-291x(86)80160-7. [DOI] [PubMed] [Google Scholar]
- Kono Y., Takahashi M. A., Asada K. Oxidation of manganous pyrophosphate by superoxide radicals and illuminated spinach chloroplasts. Arch Biochem Biophys. 1976 Jun;174(2):454–462. doi: 10.1016/0003-9861(76)90373-8. [DOI] [PubMed] [Google Scholar]
- Mason R. P. Assay of in situ radicals by electron spin resonance. Methods Enzymol. 1984;105:416–422. doi: 10.1016/s0076-6879(84)05058-8. [DOI] [PubMed] [Google Scholar]
- Morehouse K. M., Mason R. P. The transition metal-mediated formation of the hydroxyl free radical during the reduction of molecular oxygen by ferredoxin-ferredoxin:NADP+ oxidoreductase. J Biol Chem. 1988 Jan 25;263(3):1204–1211. [PubMed] [Google Scholar]
- Nagy I., Floyd R. A. Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim Biophys Acta. 1984 Nov 9;790(3):238–250. doi: 10.1016/0167-4838(84)90028-1. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Rauckman E. J. Spin trapping of the primary radical involved in the activation of the carcinogen N-hydroxy-2-acetylaminofluorene by cumene hydroperoxide-hematin. Mol Pharmacol. 1980 Mar;17(2):233–238. [PubMed] [Google Scholar]
- Rosenthal I., Krishna C. M., Yang G. C., Kondo T., Riesz P. A new approach for EPR detection of hydroxyl radicals by reaction with sterically hindered cyclic amines and oxygen. FEBS Lett. 1987 Sep 28;222(1):75–78. doi: 10.1016/0014-5793(87)80194-1. [DOI] [PubMed] [Google Scholar]
- Rustgi S., Joshi A., Moss H., Riesz P. E.s.r. of spin-trapped radicals in aqueous solutions of amino acids. Reactions of the hydroxyl radical. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 May;31(5):415–440. doi: 10.1080/09553007714550521. [DOI] [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Riesz P., Finkelstein E., Russo A. Superoxide reaction with nitroxide spin-adducts. Free Radic Biol Med. 1989;6(2):141–148. doi: 10.1016/0891-5849(89)90111-1. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Berlett B. S., Chock P. B. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc Natl Acad Sci U S A. 1990 Jan;87(1):384–388. doi: 10.1073/pnas.87.1.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings W. C., Pattridge K. A., Strong R. K., Ludwig M. L. Manganese and iron superoxide dismutases are structural homologs. J Biol Chem. 1984 Sep 10;259(17):10695–10699. [PubMed] [Google Scholar]
- Thornalley P. J., Trotta R. J., Stern A. Free radical involvement in the oxidative phenomena induced by tert-butyl hydroperoxide in erythrocytes. Biochim Biophys Acta. 1983 Aug 23;759(1-2):16–22. doi: 10.1016/0304-4165(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Van der Veen J., Weil J. T., Kennedy T. E., Olcott H. S. Aliphatic hydroxylamines as lipid antioxidants. Lipids. 1970 Jun;5(6):509–512. doi: 10.1007/BF02532737. [DOI] [PubMed] [Google Scholar]



