Abstract
Purpose: To investigate the prognosis of esophageal squamous cell carcinoma with a microscopically incomplete (R1) resection margin following an esophagectomy, as well as the impact of adjuvant treatment on survival.
Methods: Data obtained from 124 patients with R1-resected ESCC were reviewed. The impact of clinicopathological factors and adjuvant treatment on the overall survival, locoregional recurrence, and distant recurrence were explored.
Results: For a median follow-up time of 16.8 months, the median overall survival of 124 patients was 25.6 months. The 1, 3, and 5-year overall survival rates were 75.6%±4.0%, 35.9%±5.1%, and 23.2%±5.0%, respectively. Adjuvant therapy was administered in 78 patients. In the univariate analyses, patients with a pN0 stage (log rank, p=0.028) and adjuvant chemotherapy (log rank, p=0.032) exhibited more favorable overall survival. In the multivariate analyses, the pN stage (HR=2.192, p=0.004) and adjuvant chemotherapy (HR=0.032, p=0.004) were independent prognostic factors for overall survival. Locoregional recurrence was the main failure pattern after R1 resection. The pN stage (HR=2.567, p=0.009) and adjuvant radiotherapy (HR=0.278, p=0.000) were independent prognostic factors for locoregional recurrence.
Conclusion: In R1-resected esophageal squamous cell carcinoma, adjuvant radiotherapy reduced locoregional recurrence; however, it did not improve overall survival. Adjuvant chemotherapy demonstrated benefits for overall survival. The pN stage was an independent prognostic factor for locoregional recurrence and overall survival.
Keywords: Esophageal squamous cell carcinoma, R1 resection, adjuvant chemotherapy, adjuvant radiotherapy, prognosis.
Introduction
Esophageal cancer represents 7% of all gastrointestinal malignancies worldwide and affects more than 400,000 individuals annually 1. In China, the estimated number of new cases of esophageal cancer is 477,900 per year 2, most of which consist of squamous cell histology. Surgical resection is an essential component of definitive treatment for locoregionally confined esophageal squamous cell carcinoma (ESCC). The goal of surgery is to achieve a complete resection of the tumor; however, 8.5-36.5% of patients have a microscopic (R1) residual tumor at the surgical margin 3,4. The prognosis and optimal adjuvant treatment for R1-resected ESCC are unclear.
A recent systematic review of 19 studies indicated that a positive circumferential margin was associated with a poor prognosis, especially in patients who received neoadjuvant therapy and had T3 stage disease 5. This review grouped microscopically (R1) and macroscopically (R2) incomplete resections together and included both adenocarcinoma and ESCC. Another large, multicenter, European study compared 242 patients with an R1 resection with patients who received an R0 resection and suggested that an R1 resection margin was an indicator of poor long-term survival (median overall survival (OS): 17.4 vs. 28.0 months, respectively). ESCC accounted for approximately half of the cohort 2.
Recognizing the negative impact of an R1 resection margin on survival, clinicians have employed adjuvant approaches to improve survival. For individuals with R1-resected ESCC who have not received preoperative chemoradiation or chemotherapy (CT), the National Comprehensive Cancer Network (NCCN) recommends postoperative chemoradiation 6. However, due to the rarity of this situation, limited data exist, and definitive recommendations are lacking. Some studies have suggested a survival benefit of adjuvant chemo(radio)therapy for an R1 resection margin 3, whereas other studies have not supported this finding 7. Poor tolerance of adjuvant therapy after major surgery is a substantial concern. Moreover, whether and how to administer adjuvant treatment remain questionable.
Therefore, the aim of this study is to investigate the prognosis of patients with R1-resected ESCC as well as the role of adjuvant treatment on survival.
Methods
Patient selection and data collection
Data of patients who underwent curative surgery for esophageal cancer in our center from 2000 to 2015 were retrospectively reviewed. The inclusion criteria consisted of squamous cell histology, pathological stage I-III, and an R1 resection margin. Patients who received neoadjuvant radiation, hormone therapy, or immunotherapy were excluded. According to the criteria of the College of American Pathologists 8, an R1 resection margin was defined as the presence of microscopic residual tumor at the circumferential, vertical or lateral margins of the surgical specimen. The pathological stage was classified according to the TNM staging system proposed by the American Joint Committee on Cancer (7th edition). Following the standard practice in our institution, all pathology specimens were examined by two pathologists. The final cohort consisted of 124 patients. The data collected included demographic characteristics, surgical and perioperative treatments, pathological results, recurrence information, survival outcomes and toxicity. Adjuvant treatment was defined as radiotherapy and/or chemotherapy that initiated within two months of surgery. The sufficiency of adjuvant chemotherapy was defined as three-weekly regimen of more than 2 cycles, or weekly regimen of more than 4 cycles. The Institutional Review Board approved the database and study design. Informed consent for data collection was obtained prior to treatment.
Follow-up
The beginning of the follow-up period was defined as the date of surgery. Clinical examinations, thoracoabdominal computed tomography, and upper gastrointestinal endoscopy were performed every 3-6 months until the disease progressed. Bone scans or PET scans were administered when distant metastases were suspected. The OS, locoregional recurrence (LR), and distant recurrence (DR) were recorded. Histological, cytological, or unequivocal radiological proof was required before a diagnosis of disease recurrence. LR was defined as recurrences within the area of resection, anastomotic sites or locoregional lymph nodes, including the mediastinum, supraclavicular fossa, and coeliac trunk region. DR included metastases to solid organs, pleura, peritoneum, and non-regional lymph nodes. OS was defined as the period from the date of surgery to the date of death from any cause or the last visit prior to December 31, 2015. The locoregional recurrence-free survival (LRFS) and distant recurrence-free survival (DRFS) were calculated as the time from the date of surgery to the date of LR and DR, respectively, or to the last visit prior to December 31, 2015. Adverse events were classified according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
Continuous variables are presented as medians (range) or means±standard deviation. Categorical variables are presented as percentages. The Kaplan-Meier method was used to estimate the OS, LRFS, and DRFS. The survival variables in the different subgroups were compared using a two-sided log-rank test. The variables with a p-value <0.05 in the univariate analysis were included in a multivariate analysis using the Cox's proportional hazards regression model. The statistical analysis was performed using SPSS Statistics ver. 19 for Windows (IBM Corporation, Armonk, NY, USA), and differences were considered significant at a p-value <0.05.
Results
1. Patient characteristics
The clinical and pathological characteristics of 124 patients with R1-resected ESCC are listed in Table 1. The median age of the patients was 57 years (range: 33-83 years). Males predominated with a male to female ratio of 4.39. The Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0-1 in 112 patients (90.3%) and 2 in 12 patients (9.7%). Twenty patients (16.1%) had a weight loss ≥10% over a 6-month period prior to surgery. The middle thoracic esophagus (54.8%) was the most common location of primary tumors. The approach for surgery was Mckeown (26.6%) for upper and advanced middle thoracic tumor; Ivor Lewis (12.1%) or a laparotomy with a left thoracotomy (60.5%) for lower and middle thoracic tumors. The mean number of lymph nodes harvested during surgery was 19.3±5.0. Adjuvant therapy was administered at 27-42 days (median: 32 days) after surgery in 78 patients (62.9%) and included adjuvant CT alone in 28 patients, radiotherapy (RT) alone in 26 patients, concurrent chemoradiotherapy (CCRT) in 17 patients, and sequential chemoradiotherapy (SCRT) in 7 patients. The most common regimen for adjuvant CT was taxol plus cisplatin every three weeks (n=21). The median dose for adjuvant RT was 60 Gy (range: 44-70 Gy). The CT regimens administered concurrently with RT included weekly cisplatin (n=3), weekly docetaxel plus cisplatin (n=3), 5-fluorouracil plus cisplatin every three weeks (n=3), and other regimens.
Table 1.
Clinical and pathological characteristics of 124 patients with R1-resected esophageal squamous cell carcinoma.
| Characteristics | Values, n (%) |
|---|---|
| Age (years) | |
| < 60 | 78 (62.9) |
| ≥ 60 | 46 (37.1) |
| Sex | |
| Male | 101 (81.5) |
| Female | 23(18.5) |
| ECOG PS | |
| 0-1 | 112 (90.3) |
| 2 | 12 (9.7) |
| Weight loss † | |
| < 10% | 92 (74.2) |
| ≥ 10% | 20 (16.1) |
| Unknown | 12 (9.7) |
| Tumor Location | |
| Upper thoracic | 12 (9.7) |
| Middle thoracic | 68 (54.8) |
| Lower thoracic | 36 (29.0) |
| Multiple locations | 8 (6.5) |
| pT | |
| pT1, 2 | 30 (24.2) |
| pT3, 4 | 94 (75.8) |
| pN | |
| pN0 | 42 (33.9) |
| pN1-3 | 82 (66.1) |
| pTNM stage | |
| Ia, b | 14 (11.3) |
| IIa, b | 35 (28.2) |
| IIIa, b, c | 75 (60.5) |
| Surgical technique | |
| Ivor Lewis | 15 (12.1) |
| McKeown | 33 (26.6) |
| Laparotomy + left thoracotomy | 75 (60.5) |
| Unknown | 1 (0.8) |
| Lymph node harvest ‡ | 19.3 ± 5.0 |
| Adjuvant therapy | |
| No | 46(37.1) |
| CT | 28 (22.6) |
| RT | 26 (21.0) |
| CCRT | 17 (13.7) |
| SCRT | 7 (5.6) |
† Weight loss was assessed over a 6-month period prior to surgery, and information regarding weight loss was obtained in 112 patients;
‡ mean±SD;
CT: chemotherapy; RT: radiotherapy; CRT: chemoradiotherapy; CCRT: concurrent chemoradiotherapy; SCRT: sequential chemoradiotherapy.
2. Univariate and multivariate analyses of OS
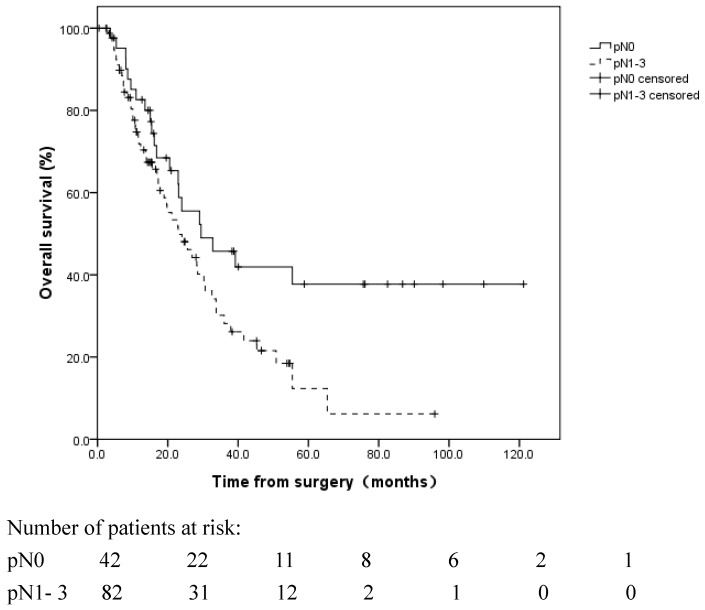
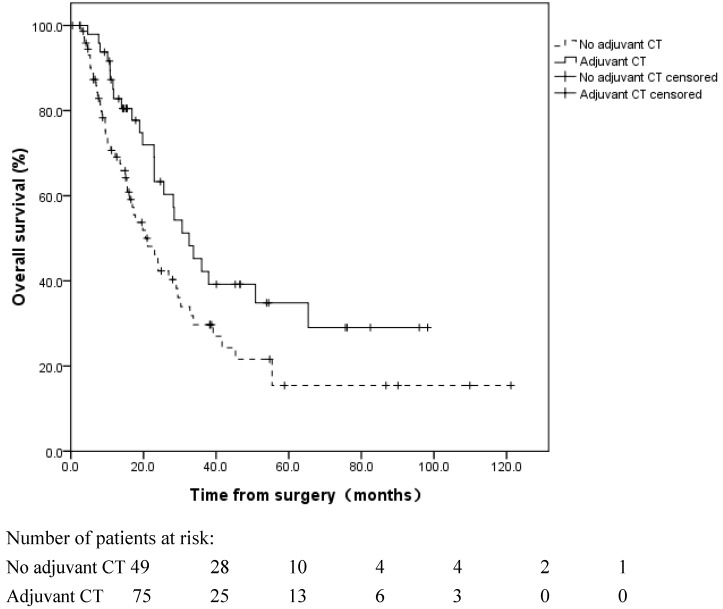
For a median follow-up time of 16.8 months (range: 0.4-121.2 months), until December 31, 2015, the median OS of the 124 patients was 25.6 months (95% CI: 20.5 to 30.7 months). The 1, 3, and 5-year OS rates were 75.6%±4.0%, 35.9%±5.1%, and 23.2%±5.0%, respectively. The influences of the clinicopathological factors and adjuvant treatment on OS are listed in Table 2. Patients with pN0 disease exhibited a more favorable OS than patients with pN1-3 disease (median OS: 29.5 vs. 23.0 months, respectively, log rank, p=0.028) (Figure 1). Adjuvant CT improved the median OS from 20.5 to 32.5 months (log rank, p=0.032) (Figure 2). Adjuvant RT had no significant survival benefit (log rank, p=0.439). In the multivariate analyses, the pN stage (HR=2.192, 95% CI 1.284-3.742, p=0.004) and adjuvant CT (HR=0.481, 95% CI 0.291-0.794, p=0.004) were independent prognostic factors for OS.
Table 2.
Univariate and multivariate analyses for overall survival in R1-resected esophageal squamous cell carcinoma (n=124).
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OS (median, CI) | P value | Hazard Ratio (CI) | P value | |
| Age (years) | 0.495 | |||
| < 60 | 24.0 (18.1-29.9) | |||
| ≥ 60 | 25.6 (15.4-35.8) | |||
| Sex | 0.444 | |||
| Male | 26.9 (21.0-32.9) | |||
| Female | 24.0 (7.5-40.4) | |||
| ECOG PS | 0.068 | |||
| 0-1 | 26.9 (19.7-34.2) | |||
| 2 | 17.8 (6.6-28.9) | |||
| Weight loss † | ||||
| < 10% | 28.2 (22.6-33.8) | 0.222 | ||
| ≥ 10% | 18.9 (15.5-22.4) | |||
| Tumor Location | 0.877 | |||
| Upper | 22.9 (14.1-31.7) | |||
| Middle | 28.5 (20.1-36.9) | |||
| Lower | 26.9 (18.0-35.6) | |||
| Multiple | 45.3 | |||
| pT stage | 0.515 | |||
| T1,2 | 32.8 (2.7-62.9) | |||
| T3,4 | 24.0 (18.8-29.3) | |||
| pN stage | 0.028 | 0.004 | ||
| N0 | 29.5 (10.5-48.5) | 1 | ||
| N1-3 | 23.0 (15.0-31.0) | 2.192 (1.284-3.742) | ||
| pTNM stage | 0.091 | |||
| Ia, b+IIa, b | 29.5(11.6-47.3) | |||
| IIIa, b, c | 24.0 (20.4-30.8) | |||
| Surgical technique | 0.365 | |||
| Ivor Lewis | 17.7 (0-36.3) | |||
| McKeown | 28.2 (19.4-37.1) | |||
| Laparotomy + left thoracotomy | 25.6 (19.4-31.8) | |||
| Adjuvant CT | 0.032 | 0.004 | ||
| No | 20.5 (13.6-27.5) | 1 | ||
| Yes | 32.5 (22.2-42.9) | 0.481 (0.291-0.794) | ||
| Adjuvant RT | 0.439 | |||
| No | 23.0 (15.2-30.8) | |||
| Yes | 29.5 (21.0-38.0) | |||
† Weight loss was assessed over a 6-month period prior to surgery;
CT: chemotherapy; RT: radiotherapy.
Figure 1.
Overall survival of R1-resected patients with different pN stages. Patients with pN0 disease exhibited a more favorable OS than patients with pN1-3 disease (median OS: 29.5 vs. 23.0 months, respectively, log rank, p=0.028).
Figure 2.
Overall survival of R1-resected patients with and without adjuvant chemotherapy (CT). Adjuvant CT improved the median OS from 20.5 to 32.5 months (log rank, p=0.032).
3. OS in different adjuvant treatment subgroups
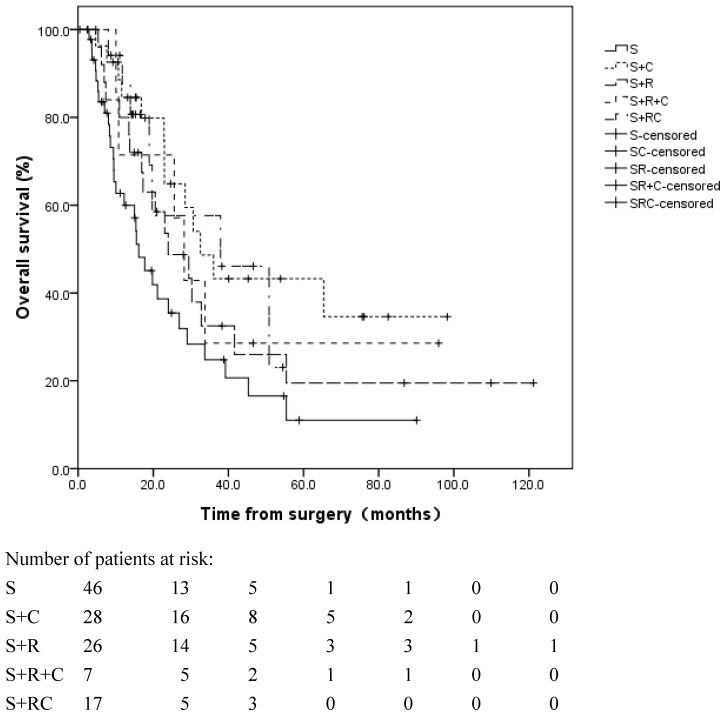
A significant difference was observed in the OS in the different adjuvant treatment groups (log rank, p=0.050, Figure 3). The median OS was 16.1 months in patients without adjuvant therapy, 32.6 months in patients with adjuvant CT alone, 24.0 months in patients with adjuvant RT alone, 28.2 months in patients with adjuvant SCRT and 37.2 months in patients with adjuvant CCRT.
Figure 3.
Overall survival for patients in different adjuvant treatment groups. The median OS was 16.1 months in group S, 32.6 months in group S+C, 24.0 months in group S+R, 28.2 months in group S+R+C and 37.2 months in group S+RC. S: surgery; S+R: surgery + adjuvant radiotherapy; S+C: surgery + adjuvant chemotherapy; S+R+C: surgery + adjuvant sequential chemoradiation; S+RC: surgery + adjuvant concurrent chemoradiation.
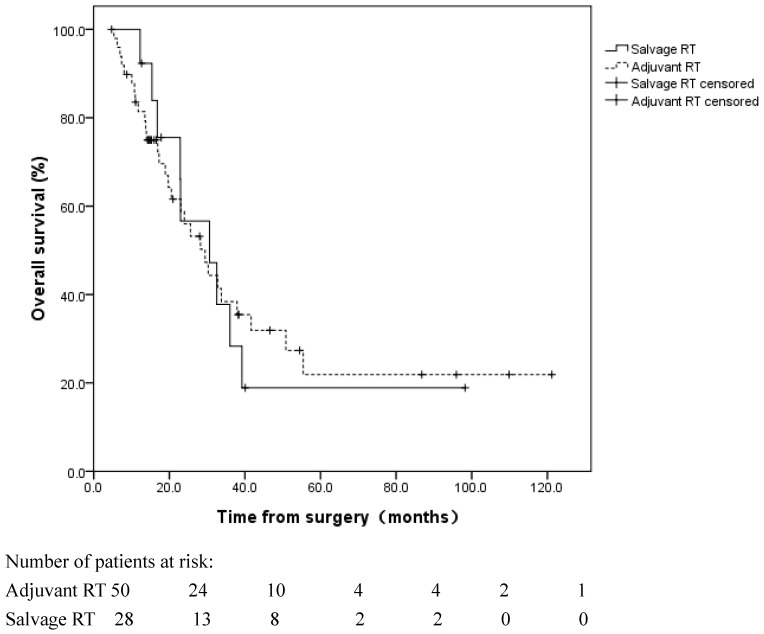
Fifty patients received adjuvant RT±CT. An additional 13 patients did not receive adjuvant RT; however, they received salvage RT following a locoregional recurrence (Table 3). No significant difference was observed in the OS between these two subgroups (log rank, p=0.908), with a median OS of 29.5 and 30.6 months in the patients who received adjuvant RT and salvage RT, respectively (Figure 4).
Table 3.
Initial failure patterns and salvage treatment in 93 patients with R1-resected esophageal squamous cell carcinoma.
| Initial failure patterns | Number | Salvage treatment (number) | ||||
|---|---|---|---|---|---|---|
| CT | RT | CRT | S | None | ||
| Distant recurrence alone | 8 | 2 | 0 | 1 | 1 | 4 |
| Locoregional recurrence alone | 33 | 3 | 10 | 3 | 4 | 13 |
| Combined recurrences | 8 | 4 | -- | -- | -- | 4 |
CT: chemotherapy; RT: radiotherapy. CRT: chemoradiation; S: surgery.
Figure 4.
Overall survival for patients with adjuvant radiotherapy (RT) following esophagectomy and patients with salvage RT following locoregional recurrence. No significant difference was observed in the OS between these two groups (log rank, p=0.908).
4. Univariate and multivariate analyses of LRFS and DRFS
Information regarding disease recurrence was obtained for 93 patients. During a median follow up time of 18.0 months, the disease recurred in 49 patients (52.7%). The initial failure patterns and the subsequent salvage treatments are listed in Table 3. Initial failures consisted of LR alone in 33 patients (35.5%), DR alone in 8 patients (8.6%), and combined recurrences in 8 patients (8.6%).
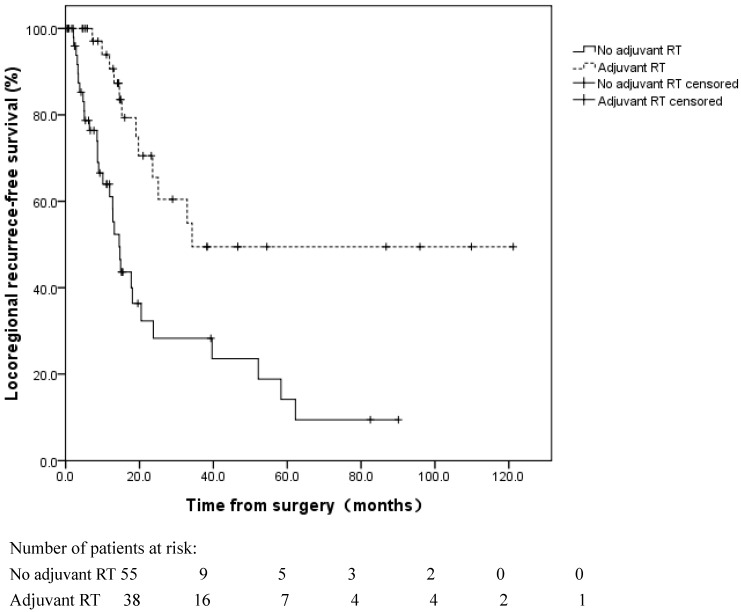
The 1 and 3-year LR rates were 21.1%±6.0% and 63.1%±8.5%, respectively. In the univariate analyses of the LRFS, LR was more common in node-positive (pN1-3) patients (log rank, p=0.029) and patients without adjuvant RT (log rank, p=0.000) (Figure 5). In the multivariate analyses, the pN stage (HR=2.567, 95% CI 1.270-5.189, p=0.009) and adjuvant RT (HR=0.278, 95% CI 0.141-0.548, p=0.000) were independent prognostic factors for LRFS (Table 4).
Figure 5.
Locoregional recurrence-free survival for patients with and without adjuvant radiotherapy (RT) following esophagectomy. Locoregional recurrence was more common in patients without adjuvant RT (log rank, p=0.000).
Table 4.
Univariate and multivariate analyses for locoregional recurrence-free survival in R1-resected esophageal squamous cell carcinoma (n=93).
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| 1-year LRFS (%) | P value | Hazard Ratio (95% CI) | P value | |
| Age (years) | 0.826 | |||
| <60 | 68.2 | |||
| ≥60 | 85.0 | |||
| Sex | 0.930 | |||
| Male | 73.7 | |||
| Female | 76.6 | |||
| ECOG PS | 0.059 | |||
| 0-1 | 76.6 | |||
| 2 | 41.7 | |||
| Weight loss † | 0.104 | |||
| <10% | 77.6 | |||
| ≥10% | 66.7 | |||
| Tumor Location | 0.712 | |||
| Upper | 77.8 | |||
| Middle | 73.9 | |||
| Lower | 74.0 | |||
| Multiple | 75.0 | |||
| pT stage | 0.228 | |||
| T1,2 | 65.4 | |||
| T3,4 | 73.5 | |||
| pN stage | 0.029 | 0.009 | ||
| N0 | 80.5 | 1 | ||
| N1-3 | 71.3 | 2.567 (1.270-5.189) | ||
| pTNM stage | 0.103 | |||
| Ia, b+IIa, b | 77.2 | |||
| IIIa, b, c | 72.1 | |||
| Surgical technique | 0.179 | |||
| Ivor Lewis | 72.9 | |||
| McKeown | 78.4 | |||
| Laparotomy + left thoracotomy | 72.1 | |||
| Adjuvant CT | 0.685 | |||
| No | 69.4 | |||
| Yes | 78.3 | |||
| Adjuvant RT | 0.000 | 0.000 | ||
| No | 61.1 | 1 | ||
| Yes | 90.7 | 0.278 (0.141-0.548) | ||
† Weight loss was assessed over a 6-month period prior to surgery;
CT: chemotherapy; RT: radiotherapy.
The 1 and 3-year DR rates were 12.8%±4.0% and 35.1%±7.9%, respectively. In the univariate analyses, pN stage (log rank, p=0.008) and pTNM stage (log rank, p=0.003) were factors prognostic of the DRFS. Neither factor was prognostic of DRFS in multivariate analyses. (Table 5).
Table 5.
Univariate and multivariate analyses for distant recurrence-free survival in R1-resected esophageal squamous cell carcinoma (n=93).
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| 1-year DRFS (%) | P value | Hazard Ratio (95% CI) | P value | |
| Age (years) | 0.219 | |||
| < 60 | 86.9 | |||
| ≥ 60 | 88.3 | |||
| Sex | 0.601 | |||
| Male | 88.3 | |||
| Female | 81.8 | |||
| ECOG PS | 0.104 | |||
| 0-1 | 89.6 | |||
| 2 | 60.0 | |||
| Weight loss † | 0.116 | |||
| <10% | 88.4 | |||
| ≥10% | 77.8 | |||
| Tumor Location | 0.987 | |||
| Upper | 100 | |||
| Middle | 78.3 | |||
| Lower | 96.4 | |||
| Multiple | --- | |||
| pT stage | 0.166 | |||
| T1,2 | 94.1 | |||
| T3,4 | 84.8 | |||
| pN stage | 0.008 | 0.829 | ||
| N0 | 91.1 | 1 | ||
| N1-3 | 85.3 | 1.317(0.108-16.032) | ||
| pTNM stage | 0.003 | 0.179 | ||
| Ia, b+IIa, b | 95.8 | 1 | ||
| IIIa, b, c | 85.7 | 5.573(0.455-68.212) | ||
| Surgical technique | 0.440 | |||
| Ivor Lewis | 88.9 | |||
| McKeown | 85.7 | |||
| Laparotomy + left thoracotomy | 87.6 | |||
| Adjuvant CT | 0.565 | |||
| No | 88.2 | |||
| Yes | 86.7 | |||
| Adjuvant RT | 0.402 | |||
| No | 83.8 | |||
| Yes | 90.8 | |||
† Weight loss was assessed over a 6-month period prior to surgery;
CT: chemotherapy; RT: radiotherapy.
5. Toxicity profiles in patients receiving adjuvant therapy
In patients receiving adjuvant CT alone (n=28), >=grade 3 toxicities were observed in two patients, one with grade 3 vomiting and the other with grade 4 myelosuppression. In patients receiving adjuvant RT alone (n=26), no toxicity >=grade 3 was observed. In patients receiving adjuvant RT+CT (n=24), one developed grade 3 myelosuppression, and one died of pneumonitis one week after RT.
Discussion
The R0 resection rate following esophagectomy has increased in recent years due to improved surgical techniques and standard surgical practices 9; however, R1 resection remains a problem, especially in patients with an upper third esophageal primary tumor, locally advanced disease, and previous malnutrition 3. R1 resection has been associated with a poor prognosis in many cancer types, including pancreatic, rectal, and lung cancers 10-12. With respect to ESCC, controversy exists regarding the prognosis and optimal adjuvant treatment for an R1 resection margin.
An analysis of the clinicopathological prognostic factors following esophagectomy in esophageal cancer indicated that resection margin involvement was a negative prognostic factor of survival 13. Adjuvant therapy was often provided to address the surgical insufficiency in R1-resected ESCC. In a study of esophageal cancer by Markar SR, an R1 resection margin was confirmed to be associated with reduced survival and increased LR in a propensity matched analysis 3. This study involved 242 R1-resected patients, including 112 patients with ESCC. Adjuvant therapy was provided to 86 of the 242 R1-resected patients (35.5%), which reduced distant metastases and improved the OS. In our study population, adjuvant therapy was offered to 78 patients (62.9%). Adjuvant CT significantly improved the median OS from 20.5 to 32.5 months (p=0.032). The increased percentage of patients who received adjuvant therapy in our cohort may explain, in part, the longer median survival time in our study compared to the study by Markar SR (25.6 vs. 17.4 months, respectively).
LR is the main cause of failure in patients undergoing radical esophagectomy 14. The LR rate has been reported to be 30% in R0 resection and 60% in R1 or R2 resection 15. Adjuvant RT following radical surgery has been reported to reduce the incidence of LR. However, findings regarding the role of adjuvant RT on survival are conflicting 16-20. In our cohort of R1-resected patients, LR remained the primary cause of failure. Adjuvant RT significantly decreased the incidence of LR; however, it failed to improve the OS. This finding highlights the importance of salvage therapy following LR. After LR, definitive CRT remains feasible for patients who have not previously received adjuvant RT. Our previous study demonstrated that CCRT was effective for recurrent ESCC following esophagectomy. Patients with recurrent disease who responded well to CCRT achieved long-term OS 21. In the current study, 13 patients who received salvage RT for LR achieved an OS of 30.6 months, which was comparable to patients who received adjuvant RT. Therefore, the benefit of adjuvant RT for OS might be diluted by the salvage therapy provided after recurrence. The timing of RT in R1-resected patients remains debatable.
Phase III trials that have compared surgery plus adjuvant CT to surgery alone for localized ESCC have indicated no benefit in OS 22,23. In the R1-resected subgroup, the role of adjuvant CT must be investigated. Our study demonstrated that adjuvant CT was an independent prognostic factor for OS, which supports the use of adjuvant CT after an R1 resection in ESCC patients.
Neoadjuvant CCRT has been established as a standard of care for locally advanced ESCC 24,25. Compared with surgery alone, neoadjuvant CCRT plus surgery decreased the rate of incomplete surgery and improved the locoregional control and OS. For R1-resected patients without previous neoadjuvant RT, CCRT may be offered in adjuvant settings to achieve better disease control. However, in this study, the multivariate analyses did not support the use of adjuvant RT. This finding was consistent with the findings of Markar SR 3. The insufficiency of adjuvant CCRT in R1 patients, combined with the fact that neoadjuvant CCRT increases the R0 resection rate, highlights the importance of the optimal administration of multidisciplinary approaches to avoid incomplete surgery.
To the best of our knowledge, this investigation is the first study to address the role of adjuvant therapy in R1-resected ESCC in detail. However, the current study has several limitations that should be considered. As a retrospective and observational study, the surgical technique, dose of adjuvant RT, and regimen of adjuvant CT were not uniform among the patients. Despite the inclusion of potential prognostic variables in the univariate and multivariate analyses of survival, other confounding factors may exist that were not considered. A prospective study with controlled regimens is warranted to determine the optimal therapy for R1 resection following an esophagectomy.
This retrospective study of 124 patients provided information regarding the management of R1-resected ESCC following an esophagectomy. According to our results, adjuvant RT reduced the LR; however, it did not improve the OS. Adjuvant CT provided survival benefits. A prospective study is required to confirm these findings.
Abbreviations
- CCRT
concurrent chemoradiotherapy
- CRT
chemoradiotherapy
- CT
chemotherapy
- DR
distant recurrence
- DRFS
distant recurrence free survival
- ESCC
esophageal squamous cell carcinoma
- LR
locoregional recurrence
- LRFS
locoregional recurrence free survival
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- R1
microscopically incomplete resection margin
- RT
radiotherapy
- SCRT
sequential chemoradiotherapy.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Markar SR, Gronnier C, Duhamel A. et al. Significance of microscopically incomplete resection margin after esophagectomy for esophageal cancer. Ann. Surg. 2015;263:712–718. doi: 10.1097/SLA.0000000000001325. [DOI] [PubMed] [Google Scholar]
- 4.Zafirellis K, Dolan K, Fountoulakis A. et al. Multivariate analysis of clinical, operative and pathologic features of esophageal cancer: who needs adjuvant therapy? Dis. Esophagus. 2002;15:155–159. doi: 10.1046/j.1442-2050.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Chen QX, Teng LS, Krasna MJ. Prognostic significance of positive circumferential resection margin in esophageal cancer: a systematic review and meta-analysis. Ann. Thorac. Surg. 2014;97:446–453. doi: 10.1016/j.athoracsur.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. http://www.NCCN.org.
- 7.Gilbert S, Martel AB, Seely AJ. Prognostic significance of a positive radial margin after esophageal cancer resection. J. Thorac. Cardiovasc. Surg. 2015;149:548–555. doi: 10.1016/j.jtcvs.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Deeter M, Dorer R, Kuppusamy M K. et al. Assessment of criteria and clinical significance of circumferential resection margins in esophageal cancer. Arch. Surg. 2009;144:618–624. doi: 10.1001/archsurg.2009.115. [DOI] [PubMed] [Google Scholar]
- 9.Mariette C, Taillier G, Van Seuningen I. et al. Factors affecting postoperative course and survival after en bloc resection for esophageal carcinoma. Ann. Thorac. Surg. 2004;78:1177–1183. doi: 10.1016/j.athoracsur.2004.02.068. [DOI] [PubMed] [Google Scholar]
- 10.Lad NL, Squires MH, Maithel SK. et al. Is it time to stop checking frozen section neck margins during pancreaticoduodenectomy? Ann. Surg. Oncol. 2013;20:3626–3633. doi: 10.1245/s10434-013-3080-9. [DOI] [PubMed] [Google Scholar]
- 11.Debove C, Maggiori L, Chau A. et al. What happens after R1 resection in patients undergoing laparoscopic total mesorectal excision for rectal cancer? A study in 333 consecutive patients. Colorectal Dis. 2015;17:197–204. doi: 10.1111/codi.12849. [DOI] [PubMed] [Google Scholar]
- 12.Riquet M, Achour K, Foucault C. et al. Microscopic residual disease after resection for lung cancer: a multifaceted but poor factor of prognosis. Ann. Thorac. Surg. 2010;89:870–875. doi: 10.1016/j.athoracsur.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 13.Mirnezami R, Rohatgi A, Sutcliffe RP. et al. Multivariate analysis of clinicopathological factors influencing survival following esophagectomy for cancer. Int. J. Surg. 2010;8:58–63. doi: 10.1016/j.ijsu.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa S, Kanda T, Kosugi S. et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J. Am. Coll. Surg. 2004;198:205–211. doi: 10.1016/j.jamcollsurg.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Kelsen D, Ginsberg R, Pajak T. et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N. Engl. J. Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 16.Ténière P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg. Gynecol. Obstet. 1991;173:123–130. [PubMed] [Google Scholar]
- 17.Xiao ZF, Yang ZY, Liang J. et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann. Thorac. Surg. 2003;75:331–336. doi: 10.1016/s0003-4975(02)04401-6. [DOI] [PubMed] [Google Scholar]
- 18.Xiao ZF, Yang ZY, Miao YJ. et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:82–90. doi: 10.1016/j.ijrobp.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber D, Rineer J, Vongtama D. et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J. Thorac. Oncol. 2010;5:244–250. doi: 10.1097/JTO.0b013e3181c5e34f. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Wang Z, Liu XY, Liu FY. Adjuvant radiotherapy after modified Ivor-Lewis esophagectomy: can it prevent lymph node recurrence of the mid-thoracic esophageal carcinoma? Ann. Thorac. Surg. 2009;87:1697–1702. doi: 10.1016/j.athoracsur.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 21.Bao Y, Liu S, Liu H. et al. Three-dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: clinical efficacy and failure pattern. Radiat. Oncol. 2013;8:241. doi: 10.1186/1748-717X-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando N, Iizuka T, Kakegawa T. et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J. Thorac. Cardiovasc. Surg. 1997;114:205–209. doi: 10.1016/S0022-5223(97)70146-6. [DOI] [PubMed] [Google Scholar]
- 23.Ando N, Iizuka T, Ide H. et al. Japan Clinical Oncology Group. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J. Clin. Oncol. 2003;21:4592–4596. doi: 10.1200/JCO.2003.12.095. [DOI] [PubMed] [Google Scholar]
- 24.Van Hagen P, Hulshof MC, van Lanschot JJ. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro J, van Lanschot JJ, Hulshof MC. et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]