Abstract
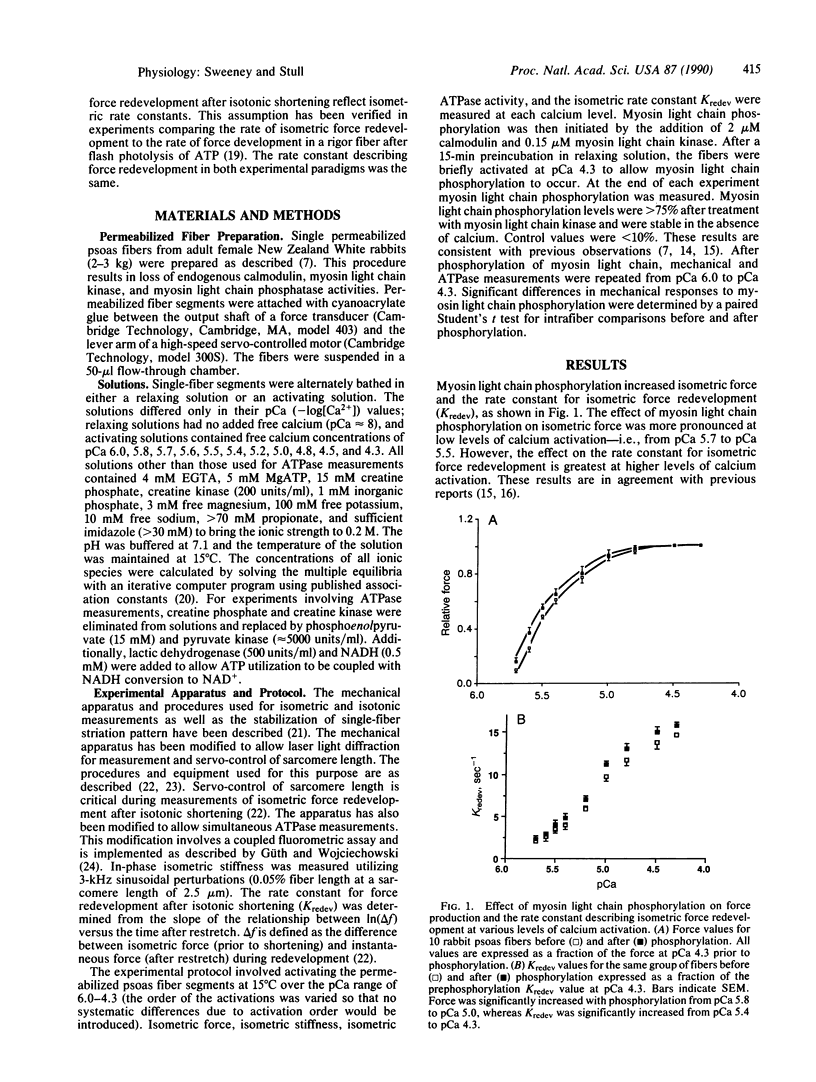
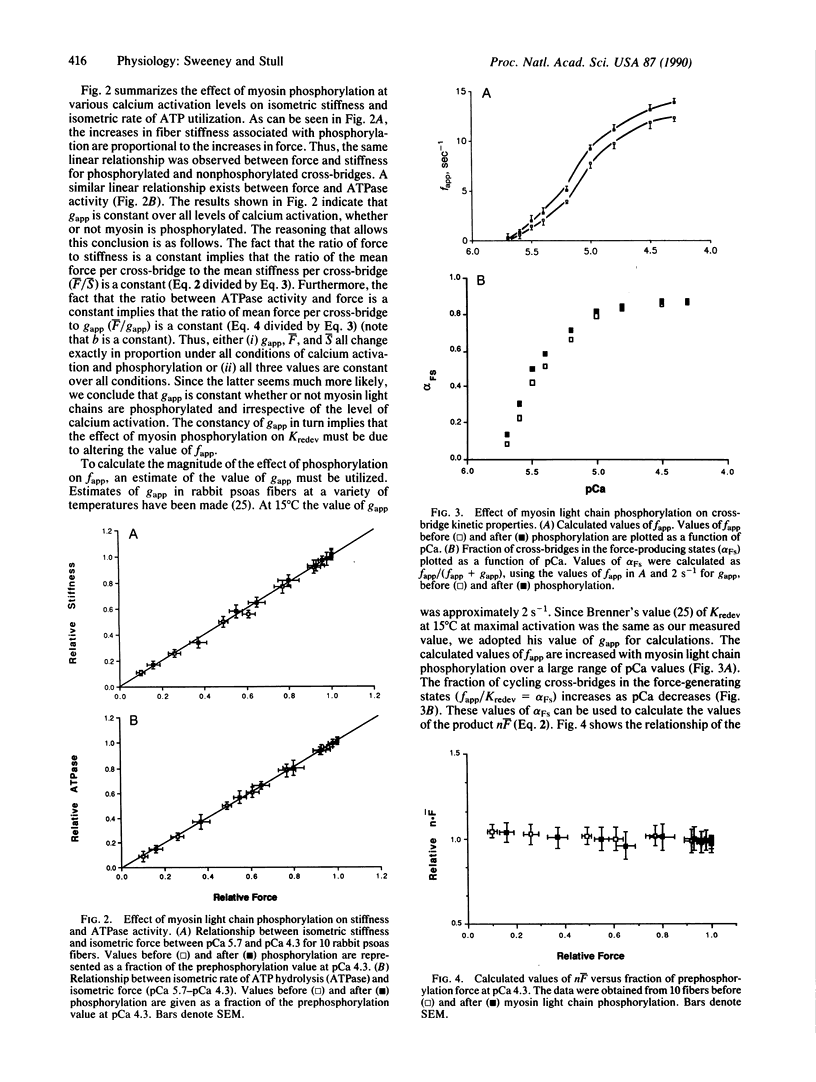
Myosin light chain phosphorylation in permeable skeletal muscle fibers increases isometric force and the rate of force production at submaximal levels of calcium activation; myosin light chain phosphorylation may underlie the increased rate and extent of force production associated with isometric twitch potentiation in intact fibers. To understand the mechanism by which myosin light chain phosphorylation manifests these effects, we have measured isometric force, isometric stiffness, rate of isometric force redevelopment after isotonic shortening, and isometric ATPase activity in permeabilized rabbit psoas muscle fibers. These measurements were made in the presence and absence of myosin light chain phosphorylation over a range of calcium concentrations that caused various levels of activation. The results were analyzed with a two-state cross-bridge cycle model as suggested by Brenner [Brenner, B. (1988) Proc. Natl. Acad. Sci. USA 85, 3265-3269]. The results indicate that myosin light chain phosphorylation exerts its effect on force generation and the isometric rate of force redevelopment in striated muscle through a single mechanism, namely, by increasing the rate constant describing the transition from non-force-generating cross-bridges to force-generating states (fapp). gapp, the reverse rate constant, is unaffected by phosphorylation as are the number of cycling cross-bridges. Since both calcium and myosin light chain phosphorylation increase fapp, the possibility is considered that modulation of fapp may represent a general mechanism for regulating force in actin-myosin systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsotti R. J., Butler T. M. Chemical energy usage and myosin light chain phosphorylation in mammalian skeletal muscle. J Muscle Res Cell Motil. 1984 Feb;5(1):45–64. doi: 10.1007/BF00713151. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986 May;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-ATPase in solution. Basic Res Cardiol. 1986;81 (Suppl 1):1–15. doi: 10.1007/978-3-662-11374-5_1. [DOI] [PubMed] [Google Scholar]
- Butler T. M., Siegman M. J., Mooers S. U., Barsotti R. J. Myosin light chain phosphorylation does not modulate cross-bridge cycling rate in mouse skeletal muscle. Science. 1983 Jun 10;220(4602):1167–1169. doi: 10.1126/science.6857239. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Myosin light chain phosphorylation is associated with a decrease in the energy cost for contraction in fast twitch mouse muscle. J Biol Chem. 1982 Mar 10;257(5):2121–2124. [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Phosphorylation of myosin light chains in mouse fast-twitch muscle associated with reduced actomyosin turnover rate. Science. 1982 Aug 27;217(4562):835–837. doi: 10.1126/science.6285472. [DOI] [PubMed] [Google Scholar]
- Güth K., Wojciechowski R. Perfusion cuvette for the simultaneous measurement of mechanical, optical and energetic parameters of skinned muscle fibres. Pflugers Arch. 1986 Nov;407(5):552–557. doi: 10.1007/BF00657515. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Klug G. A., Botterman B. R., Stull J. T. The effect of low frequency stimulation on myosin light chain phosphorylation in skeletal muscle. J Biol Chem. 1982 May 10;257(9):4688–4690. [PubMed] [Google Scholar]
- Krarup C. Evoked responses in normal and diseased muscle with particular reference to twitch potentiation. Acta Neurol Scand. 1983 Nov;68(5):269–315. doi: 10.1111/j.1600-0404.1983.tb04838.x. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Dillon P. F., Meyer R. A., Brown T. R., Krisanda J. M., Sweeney H. L. 31P NMR spectroscopy, chemical analysis, and free Mg2+ of rabbit bladder and uterine smooth muscle. J Biol Chem. 1986 Nov 5;261(31):14420–14429. [PubMed] [Google Scholar]
- Manning D. R., Stull J. T. Myosin light chain phosphorylation and phosphorylase A activity in rat extensor digitorum longus muscle. Biochem Biophys Res Commun. 1979 Sep 12;90(1):164–170. doi: 10.1016/0006-291x(79)91604-8. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Stull J. T. Myosin light chain phosphorylation-dephosphorylation in mammalian skeletal muscle. Am J Physiol. 1982 Mar;242(3):C234–C241. doi: 10.1152/ajpcell.1982.242.3.C234. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Greaser M. L., Moss R. L. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989 May;93(5):855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., Houston M. E., Iwamoto G. A., Stull J. T. Phosphorylation of rabbit skeletal muscle myosin in situ. J Cell Physiol. 1985 Nov;125(2):301–305. doi: 10.1002/jcp.1041250219. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A., Stull J. T., Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem. 1985 Jul 5;260(13):7951–7954. [PubMed] [Google Scholar]
- Sweeney H. L., Corteselli S. A., Kushmerick M. J. Measurements on permeabilized skeletal muscle fibers during continuous activation. Am J Physiol. 1987 May;252(5 Pt 1):C575–C580. doi: 10.1152/ajpcell.1987.252.5.C575. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J. Myosin phosphorylation in permeabilized rabbit psoas fibers. Am J Physiol. 1985 Sep;249(3 Pt 1):C362–C365. doi: 10.1152/ajpcell.1985.249.3.C362. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Stull J. T. Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. Am J Physiol. 1986 Apr;250(4 Pt 1):C657–C660. doi: 10.1152/ajpcell.1986.250.4.C657. [DOI] [PubMed] [Google Scholar]



