Abstract
Background
Rapastinel (GLYX-13) is a NMDA receptor modulator with glycine-site partial agonist properties. It is a robust cognitive enhancer and shows rapid and long-lasting antidepressant properties in both animal models and in humans.
Methods
Rapastinel was derived from a monoclonal antibody, B6B21, is a tetrapeptide (threonine-proline-proline-threonine-amide) obtained from amino acid sequence information obtained from sequencing one of the hypervariable regions of the light chain of B6B21. The in-vivo and in-vitro pharmacology of rapastinel was examined.
Results
Rapastinel was found to be a robust cognitive enhancer in a variety of learning and memory paradigms and shows marked antidepressant-like properties in multiple models including the forced swim (Porsolt), learned helplessness and chronic unpredictable stress. Rapastinel’s rapid-acting antidepressant properties appear to be mediated by its ability to activate NMDA receptors leading to enhancement in synaptic plasticity processes associated with learning and memory. This is further substantiated by the increase in mature dendritic spines found 24 hrs after rapastinel treatment in both the rat dentate gyrus and layer five of the medial prefrontal cortex. Moreover, ex vivo LTP studies showed that the effects of rapastinel persisted at least two weeks post-dosing.
Conclusion
These data suggest that rapastinel has significant effects on metaplasticity processes that may help explain the long lasting antidepressant effects of rapastinel seen in the human clinical trial results.
Keywords: Antidepressant, glycine site, GLYX-13, major depressive disorder, NMDA receptor, rapastinel, rapid acting
The discovery of B6B21
Rapastinel (formerly GLYX-13) is an amidated tetrapeptide, (threonine-proline-proline-threonine-amide) derived from cloning and sequencing the hypervariable regions of the heavy and light chains of a monoclonal antibody, B6B21. Fig. 1 shows part of the amino acid sequence of one of the hypervariable regions of the light chain of B6B21 that contains the sequence TPPT. Based on NMR analysis, Fig. 1 also shows that this molecule exists in a fairly rigid β-1 type turn, which is a common structural feature of many protein-protein interactions. Nuclear Overhauser effect analysis showed that rapastinel actually exists as a 3-ringed structure stabilized by hydrogen bonds. Thus the 3-dimensional structure of GLYX-13 is quite stable and complex for so simple a seeming small peptide [1, 2].
Fig. (1).
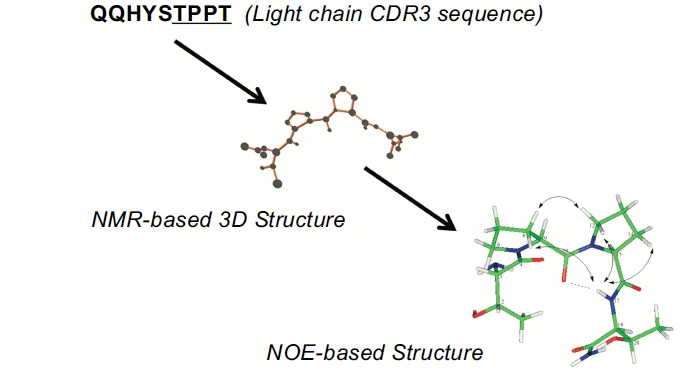
Three ways of looking at rapastinel. Based on combining NMR studies of rapastinel in solution with a nuclear Overhauser analysis, it appears that rapastinel exists as a rigid β-1 type structure that also has a triple ring structure held by hydrogen bonds. Thus a simple seeming tetrapeptide actually has a quite rich 3-dimensional structure under physiological conditions.
The monoclonal antibody (mAB), B6B21, was created in an attempt to generate a panel of monoclonal antibodies that could be used as tools to further dissect the molecular mechanisms that underlie synaptic plasticity associated with learning and memory. It was hypothesized that a judicious choice of immunogen and screening protocol could lead to the identification of a monoclonal AB that would facilitate learning and because of the exquisite specificity typically found for mABs, be useful to identify the molecular substrates underlying its learning and memory enhancing properties.
The immunogen chosen for these studies was dentate gyri from five-day old postnatal rats. Fresh, unfixed dentate gyri were immediately removed by the micropunch method of Palkovits and Brownstein [3], rapidly homogenized in PBS at 4°C, and injected intraperitoneally into Balb/c mice. An average of 50 mg wet weight of tissue was injected per mouse. This procedure was repeated 4 times over 2 months. Monoclonal antibodies were generated by conventional techniques using NS-1 as the parent myeloma cell line [4, 5].
The screening protocol chosen involved a sequence of steps based on the idea that the ideal mAB would recognize cell surface antigens expressed on living hippocampal neurons and that could be used to study synaptic plasticity in vitro before attempting to examine their learning and memory facilitation properties in vivo. Given that function (i.e., learning and memory enhancement in vivo) was the ultimate goal and not simply identification of novel ligands that modulate already known receptors, the key hurdle was to minimize the number of screens necessary without losing a key functional mAB along the way. In short, it was typical to obtain several hundred mABs from each immunization protocol but it was impossible to go directly to assaying each of them in an in vivo animal model of learning. The following is a brief summary of the screening protocol.
First, the mABs obtained from the dentate gyri immunogen were cloned at limiting dilution twice to insure monoclonality of each hybridoma obtained. Then individual hybridomas were grown in large enough quantities to be frozen for future use. Hybridomas growing in tissue culture were then combined into groups of 10; what was dubbed poly-hybridomas. This was a useful step because it greatly reduced the first screening step which was to evaluate histochemically the binding patterns of what would now be 10-20 poly-mABs to adult unfixed frozen sections of adult hippocampi. This reduced screening from several hundred immunohistochemical screens to approximately 15 followed by an additional 10 since each of the combined “poly-mABs” had been frozen as individual mABS (i.e., the hybridomas that secrete an individual mAB) and could readily be thawed and grown up for individual sub-screening.
From this step, mABs were chosen for binding to cell surfaces of living hippocampal neurons in primary cultures [6]. This was the key next step for it would insure that mABs that successfully passed this screen would recognize both hippocampal antigens expressed on living neurons. Historically, mAB technology had been used as an immunocytochemical tool to identify staining patterns. Two excellent examples of this being McKay and Zipser [7] who identified distinct neuronal cell surface binding patterns in the leech nervous system and Trisler et al., [8] who identified a cell surface antigen expressed in a 40-fold gradient across the chick retina [9].
The next step was to screen these mABs in rat hippocampal slice preparations and look for the enhancement of long-term potentiation (LTP). This was chosen as the next critical screen among many other possibilities because it made it possible to get synaptic plasticity data from a fully functional model system. The hippocampal slice and LTP had emerged as an excellent model of learning and memory. Moreover, the daunting task of having to screen a large number of mABs was still present. To this end, a strategy had to be developed in which a single hippocampal slice could be used as both the experimental and control tissue. These studies led to the identification of a unique LTP inhibitory mAB [10] and later to the identification of B6B21 the mAB that was used for in vivo studies.
At this point, because of the robust enhancement of LTP found and the role of NMDA receptors, pharmacological studies were undertaken to directly assess B6B21 for possible NMDA receptor modulatory properties. Subsequently, B6B21, was found to be a glycine-site partial agonist at the NMDA receptor [11].
B6B21 was then evaluated in vivo using trace eyeblink conditioning, an NMDAR- and hippocampus dependent test of associative learning and memory. Given that mABs are too large to cross the blood brain barrier (BBB) but the third ventricle literally bathes the hippocampal formation, a hippocampus-dependent trace eyeblink paradigm was ideally suited since this was a true associative learning paradigm both well characterized in animal models as well as in humans [12]. Remarkably it was reported in 1991 [11] that B6B21 did significantly enhance trace eyeblink conditioning, approximately 8 years after the first mABs were generated [13]. And it was these results that led to the idea that B6B21 could be used as a template to create small molecules with therapeutic potential which led to the creation of rapastinel [14].
The development of rapastinel
The amino acids that comprise the hypervariable regions or CDRs of an antibody molecule determine its binding specificity. Synthetic peptides, derived from these amino acid sequences have been demonstrated to possess biological activity similar to that of the intact antibody [15-20]. To design B6B21 antibody mimetics, the hypervariable regions of the light chain of B6B21 were cloned using reverse transcriptase-polymerase chain reaction (RT-PCR) technology [21]. Upon cloning of both the heavy and light chains of the monoclonal antibody, B6B21, only one sequence, QQHYSTPPT (glutamine, glutamine, histidine, tyrosine, serine, threonine, proline, proline, threonine), found in the light chain, (see Fig. 1 for the complete light chain sequence) showed NMDA receptor binding activity. From this sequence a panel of peptides was synthesized and assessed as previously described [22]. Of these, GLYX-13, the TPPT-amide, was found to have the most robust binding activity [22]. GLYX-13 stimulated [3H]MK-801 binding to approximately 130% of control at 1 µM (Fig. 2). TPPT-amide was later named GLYX-13 as it was the thirteenth peptide in the series showing the most activity in the MK-801 assay described above. Recently GLYX-13 has been renamed as rapastinel.
Fig. (2).
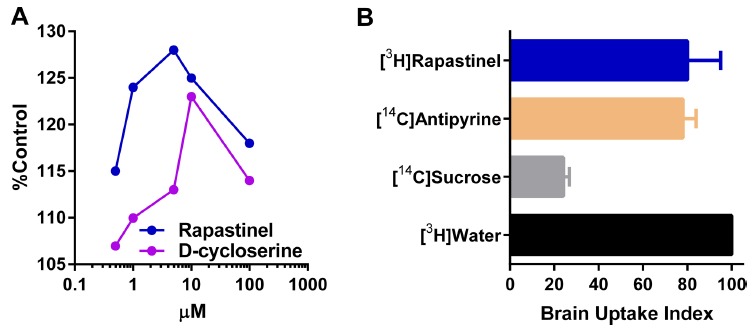
Rapastinel activates NMDA receptor activity and readily crosses the blood–brain barrier. (A) Comparison of Rapastinel NMDA receptor modulatory activity compared with D-cycloserine. NMDA receptor modulatory activity was measured by monitoring [3H]MK-801 binding in well washed rat forebrain membrane preps in the presence of glutamate but not glycine. Both Rapastinel and D-cycloserine showed an optimal increase in [3H]MK-801 binding in the range of 1-10 µM. Each data point represents the average of two experiments each done in triplicate (n=6) with the values for each data point varying by no more than 3-5%. Baseline MK-801 binding was 0.6 ± 0.06 pmol/mg protein. (B) Blood–brain-permeability studies were performed using [3H]-Rapastinel, along with the appropriate controls, was injected into rats IV and binding was measured in brain homogenates using liquid scintillation spectrometry. Using these methods a brain uptake index (Oldendorf, 1970) of 80 ± 15 was obtained suggesting that Rapastinel readily crossed the blood–brain barrier. Data were adapted with permission from [14].
These results suggest that rapastinel was acting as a glycine-site partial agonist since it overrode the effects of a competitive glycine site antagonist, 7-chloro-kyneurinic acid and gave dose response curves similar to another partial agonist, DCS. The next study chosen was to establish if rapastinel crossed the blood brain barrier. BBB permeability was determined using methods previously described [23]. Using a brain uptake index of 100% for water, it can be seen that GLYX-13 is nearly as permeable as water with an index of approximately 80% (Fig. 2). Interestingly, GLYX-13 crosses the BBB more efficiently than both heroin and codeine under similar conditions [14, 24].
From here a variety of behavioral pharmacology studies were undertaken (Fig. 3). In the first series, rapastinel was tested in a variety of hippocampus learning and memory paradigms using young rats and cognitively impaired aging rats. The paradigms used were trace eyeblink conditioning, alternating T-maze, Morris water maze and positive emotional learning [14, 25, 26]. Robust cognitive enhancement in each paradigm was observed but noteworthy was the fact that rapastinel was able to reverse the cognitive deficits found in the learning impaired aging animals in each paradigm as well. The positive emotional learning paradigm was included in part because it has been established to be a medial prefrontal cortex-dependent process.
Fig. (3).
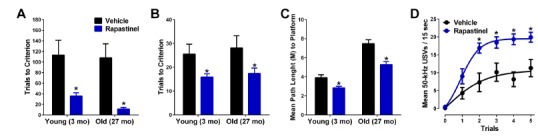
Rapastinel is a cognitive enhancer. (A-C) The effects of an optimal cognitive enhancing dose of rapastinel (1 mg/kg, IV, 15 min post-dosing) in young adult (3 months) or learning-impaired aged (27 month old) rats in hippocampal dependent trace eyeblink conditioning (A), alternating T-maze (B), and Morris water maze (C) tests. (D) GLYX-13 (1 mg/kg, IV, 15 min post-dosing) in young adult (3 months) rats facilitated positive emotional learning as measured by rates of hedonic 50-kHz USVs in response to a conditioned stimulus that predicts heterospecific rough-and-tumble play. Data are expressed as Mean ± SEM. * P<0.05 Fisher's PLSD post hoc test vs. vehicle. Data adapted with permission from [14, 25, 26].
In a second series of studies, rapastinel was assessed for its antidepressant properties. As shown in Figs. 4-5, rapastinel displayed marked antidepressant-like properties in a variety of models including forced swim, learned helplessness, novelty-induced hypophagia. Further studies were performed using a mild chronic unpredictable stress model (CUS) to induce a depressive-like state. This model has been well characterized and used to study many of the neurobiological underpinnings of depression [28]. Rapastinel was found to rapidly and markedly reverse the prodepressive effects of CUS in the same models above as well as the sucrose preference test. Likewise (Fig. 6) the cognitive defects in positive emotional learning, induced by CUS, were also reversed by rapastinel.
Fig. (4).
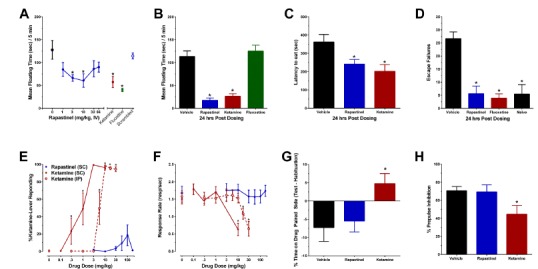
Rapastinel has antidepressant-like properties without psychotomimetic side effects in rats. (A-D) The antidepressant-like effects of an optimal antidepressant-like dose of rapastinel (3 mg/kg, IV) in the rat Porsolt test measured at 1 hr (A) or 24 hrs (B) post-dosing, the novelty induced hypophagia test 1 hr post-dosing (C), or the learned helplessness test 24 hrs post dosing (D). Rapastinel does not show ketamine-like discriminative stimulus effects (E) or sedative effects (F) in rats trained to discriminate 10 mg/kg ketamine from saline. (G-H) Rapastinel (10 mg/kg, IV) does not show ketamine-like rewarding effects in the conditioned place preference (G), or sensory-motor gating deficits in the pre-pulse inhibition (H) tests in rats. Data are expressed as Mean ± SEM. * P<0.05 Fisher's PLSD post hoc test vs. vehicle. Data adapted with permission from [27].
Fig. (5).
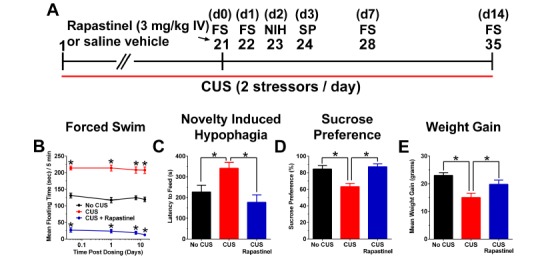
Rapastinel produces a rapid-acting and long-lasting antidepressant-like effect in a Chronic Unpredictable Stress (CUS) paradigm. (A) Schematic demonstrating the timeline for CUS exposure, drug administration, and behavioral testing. Numbers in parentheses represent days after drug administration. Rats were exposed to CUS and administered a single dose of Rapastinel (3 mg/kg, IV) or 0.9% sterile saline vehicle (1 ml/kg, IV) on day 21. Mean ± SEM floating time in the (B) Porsolt forced swim test (FS) in CUS treated rats treated with a single dose of Rapastinel (3 mg/kg, IV; n = 10), saline vehicle (n = 10), or No CUS exposed control rats (n = 9) tested 1 hr, 1 day, 1 week, and 2 weeks post-dosing. Mean ± SEM (C) latency to feed in the novelty induced hypophagia (NIH) test (2 days post-dosing), (D) sucrose preference (SP) in the sucrose preference test, 3 days post-dosing, and (E) change in body weight (2 weeks post-dosing compared to CUS day 0). * P < .05 Fisher’s PLSD post hoc test vs. No CUS group or CUS vehicle group. Data adapted with permission from [29].
Recently, based in part on the fact that rapastinel showed significant cognitive enhancing properties as well as antidepressant properties, studies were undertaken to evaluate it as a possible therapeutic candidate for post-traumatic stress disorder. The same CUS treatment used above was also used as part of a contextual fear extinction paradigm. As shown in Fig. 6, rapastinel facilitated contextual fear extinction and prevented fear extinction and re-consolidation in rats exposed to CUS.
Fig. (6).
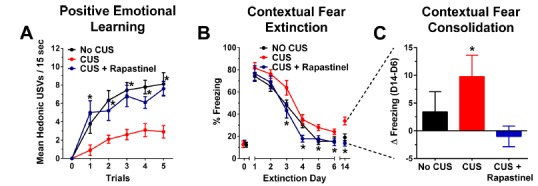
Rapastinel facilitates MPFC-dependent positive emotional learning and fear extinction in CUS-treated rats. (A) Mean ± SEM hedonic USVs in response to a conditioned stimuli that predicts heterospecific play 3 hrs post-dosing with Rapastinel (3 mg/kg, IV) or 0.9% sterile saline vehicle (1 ml/kg, IV) in 2-3 month old adult male SD rats exposed to a depressogenic regimen of chronic unpredictable stress (CUS; 2 stressors / day for 21 consecutive days) or non-stressed control rats (No CUS). A similar increase in PEL was seen in animals tested 2 weeks post-dosing. (B-C) Mean ± SEM % time freezing in CUS treated animals dosed with Rapastinel (3 mg/kg IV) or sterile saline vehicle (1 ml/kg IV) 1 hr before extinction day 1. Three shocks (0.5 mA, 1 s) were administered 90, 210, and 330 sec after animals were placed in the conditioning chamber on D0. During extinction, rats were submitted to a 5 min non-reinforced test trial every 24 hrs for 6 consecutive days (D1-6), and on day 14 post-conditioning (consolidation trial). Freezing was quantified via FreezeView software and was measured at baseline (30-60 sec) on D0, and during the last 3 min of each extinction trial. N = 9 - 14 rats per group. * P < .05 Fisher’s PLSD post hoc test vs. No CUS group (A-B), * P < .05 within subjects t-test, 2 tailed (C). Data adapted with permission from [29].
And finally a series of studies were performed to evaluate the long-term antidepressant and cognitive enhancing effects of rapastinel. A single dose of rapastinel produced marked antidepressant-like and cognitive enhancing effects one week post-dosing (Fig. 7). Rapastinel exerted its cognitive enhancing antidepressant-like, CUS reversal, and CFE effects at the same dose. CPP, an NMDA receptor antagonist, blocked the antidepressant-like effects of rapastinel demonstrating that receptor activation is required for its effects. Rapastinel, 24 hrs post-dosing was also found to significantly enhance the formation of LTP in both hippocampal and MPFC slices in vitro and led to a significant increase in mature dendritic spine formation in the dentate gyrus and layer 5 of the MPFC in rats (Fig. 8). Thus it appears that rapastinel exerts its cognitive-enhancing and antidepressant properties by activating NMDA receptors leading to a form of NMDA receptor dependent plasticity akin to LTP.
Fig. (7).
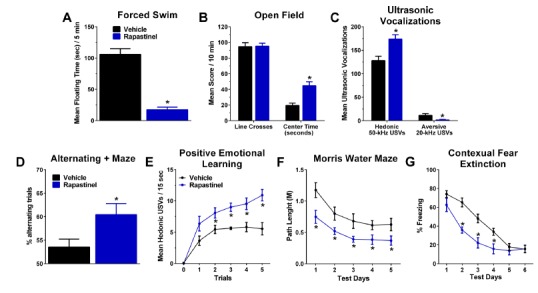
A single dose of rapastinel produces antidepressant-like (A-C) or cognitive enhancing (D-G) effects in multiple models 1 week post-dosing. Male 2-3 month old Sprague Dawley rats were pretreated with a single dose of rapastinel (3 mg/kg, IV) or sterile saline vehicle (0.9%, 1 ml/kg) and were tested 1 week post-dosing in the following behavioral model of depression: (A) Porsolt forced swim test with floating time (sec) quantified during the 5 min test; (B) open field test with total number of line crosses and time (sec) spent in the center compartment of the open field being measured during the 10 min test; (C) Ultrasonic vocalization (USVs) test with total hedonic and aversive USVs measured during the 3 min test session. Alternatively, animals received behavioral tests of learning and memory, 1 week post dosing (D-E) or were dosed 24 hrs before the first of 5 (F) or 6 (G) daily test sessions: (D) % of alternating trials in the spontaneous alternating closed arm plus maze test; (E) hedonic ultrasonic vocalizations in response to a conditioned stimuli that predicts heterospecific play in the USVs test; (F) Path length to find the hidden platform in the movable platform version of the Morris water maze test; (G) % freezing during contextual fear extinction. N = 8-21 rats per group. Mean ± SEM, * P < .05 ANOVA or Fisher’s PLSD post hoc test Rapastinel vs. vehicle. Data adapted with permission from [30].
Fig. (8).
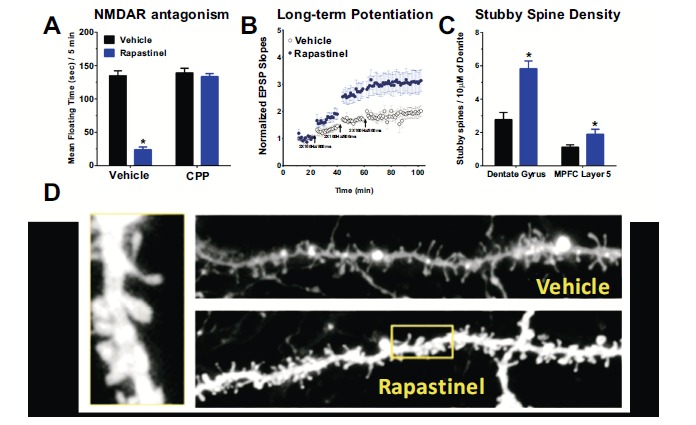
The therapeutic-like effects of Rapastinel are due to activation of NMDA receptor dependent synaptic plasticity. (A) The induction of the antidepressant-like effect of rapastinel is blocked by the NMDAR antagonist CPP. Mean (±SEM) floating time in the Porsolt forced swim test in 2-3 month old male SD rats pretreated with the NMDAR receptor antagonist CPP (10 mg/kg ip; or saline vehicle ip) 1 hr before rapastinel (3 mg/kg, IV) dosing and tested 1 hr post dosing with rapastinel. (B) A single in vivo dose of rapastinel (3 mg/kg iv; filled blue circles) in 2-3 month old male SD rats significantly enhanced the magnitude of long-term potentiation (LTP) of synaptic transmission compared to vehicle treated controls (open black circles), tested in vitro 24 hrs post-dosing at Schaffer collateral-CA1 synapses after 1, 2 and 3 sub-maximal high-frequency stimulus trains (2x100Hz/800ms). (C) Rapastinel (3 mg/kg, IV; 24 hrs post-dosing) increased the density (spines / 10 µM of dendrite) of the most electrophysiologically active spine type (stubby spines) in the dentate gyrus (primary apical, 100-150 µM from the dendrite) or MPFC layer 5 tufts. (D) Representative laser-scanning confocal micrographs of layer 5 MPFC dendrites from rapastinel and vehicle treated animals. Data was adapted with permission from [30].
Mechanism of action
Rapastinel was created from the amino acid sequence information derived from a hypervariable region of a mAB that was shown to act as a NMDA receptor glycine site partial agonist, suggesting that rapastinel would act in a similar fashion. As shown in Fig. 9, several lines of evidence supported this in fact was the case: (1) rapastinel was able to compete with the glycine site competitive antagonist, 7-CK, in a dose dependent fashion to activate NMDA receptors in membrane preparations derived from rat hippocampal synaptosomes; (2) Electrophysiological studies using murine NMDA receptors expressed in oocytes showed rapastinel to have glycine site partial agonist properties; (3) electrophysiology studies using rat hippocampus slices showed rapastinel to be a robust NMDA receptor-dependent LTP-enhancer; (4) partial agonist studies using the Stephenson method [31] using either NMDA receptor-specific current readout or D-serine-induced [3H]MK-801 binding as the readout also showed that rapastinel displayed
Fig. (9).
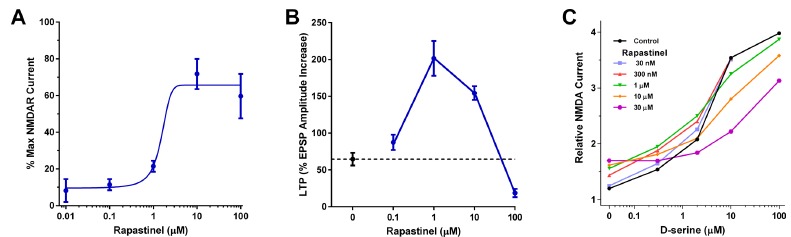
Rapastinel is an NMDAR functional glycine site partial agonist. (A) The effects of rapastinel on NMDA currents in oocytes. Cells were injected with e1/z1 cRNA, voltage-clamped at-80 mV in the presence of 100 μM NMDA, varying concentrations of rapastinel, and no exogenous glycine. Data are expressed as a percentage of the current elicited by 10 μM glycine in the same cell, usually in the same trial. Results from nine cells injected with e1/z1 cRNA; error bars indicate SEM. (B) The effects of rapastinel (0.1–100 μM) on LTP induced by a high frequency stimulus train (3 × 100 Hz/500 ms) at Schaffer collateral–CA1 synapses. Each point represents mean ± SEM of normalized field e.p.s.p. slope. (C) Pharmacologically isolated NMDAR currents were recorded from whole cell patch clamp recordings of CA1 pyramidal neurons in slices. Rapastinel by itself (left-most symbols) elicited increased NMDA receptor channel current to 20% the maximum current elicited by the full agonist D-serine. Rapastinel added in increasing concentrations shifted the dose-response of D-serine to the right. Kp = 1.3 µM was calculated using the Stephenson method [31]. Data was adapted with permission from [14, 32].
partial agonist properties. Taken together these data make a compelling case for rapastinel as a glycine site partial agonist. However each of the assays employed measured a functional readout and as such rapastinel has been described as a functional glycine site partial agonist since no direct ligand binding studies or competition studies with radiolabeled glycine or an analog have been reported. Thus, at face value it could be argued that rapastinel works by another mechanism which could include, among others, binding to an allosteric site, interfering with NMDA receptor protein-protein interactions necessary for optimal function such as those of the post-synaptic density complex, or binding to a NMDA receptor modulating protein directly. And one cannot rule out that an effective direct ligand binding assay simply has yet to be developed. In lieu of such an assay, studies are in progress using in silico methods of recently published NMDA receptor X-ray crystallographic data [33] to guide a program of creating point mutations aimed directly at inhibiting rapastinel’s ability to effect NMDA receptor function when these receptors are expressed in model systems such as human embryonic kidney (HEK) cells.
Summary and Conclusions
Rapastinel has been shown in two Phase II clinical trials to have significant rapid acting and long lasting antidepressant effects [34, 35]. Rapastinel was created using a monoclonal antibody that enhanced learning and acted as a NMDA receptor glycine site partial agonist as a template by cloning and sequencing its heavy and light chains, creating peptides from this information and screening them for glycine site modulation properties using an in vitro membrane assay. The therapeutic potential of rapastinel was assessed by establishing that it readily crossed the BBB, showed no obvious side effects, was a robust cognitive enhancer and displayed rapid and long lasting antidepressant-like effects in a variety of models. Mechanism studies have shown that rapastinel modulates the glycine site of the NMDA receptor but are based on functional and thus indirect readouts. As such it remains unclear as to the exact mechanism of rapastinel’s action although it does seem clear that it happens via glycine site modulation. Finally, it is proposed that rapastinel exerts its cognitive-enhancing and antidepressant effects via an NMDA receptor triggered LTP-like synaptic plasticity mechanism associated with learning and memory (Fig. 10).
Fig. (10).
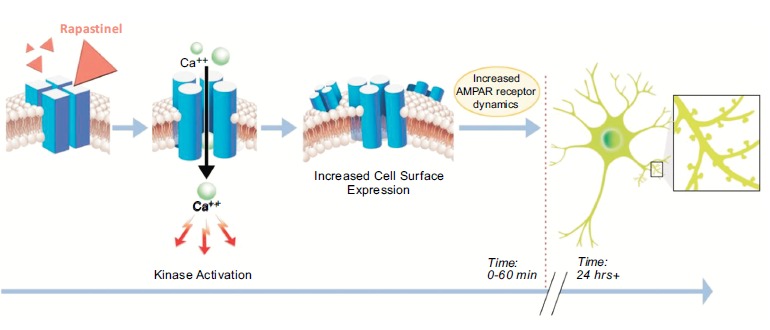
Proposed model of rapastinel’s mechanism of action. Rapastinel has a very short (approximately 7 min) half-life in plasma. It binds directly to NMDA receptor subtypes triggering a conformational change that reads out as if rapastinel is a glycine site partial agonist leading directly to increases in intracellular calcium and hypothesized changes in intracellular kinases/phosphatases that alter both the phosphorylation state of the NMDA receptor as well as the phosphoproteome. These processes in turn alter the cell-surface expression of NMDA receptor subtypes, in particular there is an increase in NR2B expression which in turn interacts with AMPA receptors that leads to LTP-like synaptic plasticity processes associated with learning and memory formation. By 24 hrs, significant alterations in mature dendritic spine formation can also be observed.
ACKNOWLEDGEMENTS
J.R. Moskal is further supported by grants from The Ralph and Marian Falk Medical Research Trust (Chicago, IL), J.S. Burgdorf is supported by NIH grant MH094835, P.K. Stanton with NS044421, and J.F. Disterhoft with MH47340.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Marion D. An introduction to biological NMR spectroscopy. Mol. Cell. Proteomics. 2013;12(11):3006–3025. doi: 10.1074/mcp.O113.030239. [http://dx.doi.org/ 10.1074/mcp.O113.030239]. [PMID: 23831612]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts G.C. NMR spectroscopy in structure-based drug design. Curr. Opin. Biotechnol. 1999;10(1):42–47. doi: 10.1016/s0958-1669(99)80008-1. [http://dx.doi.org/10. 1016/S0958-1669(99)80008-1]. [PMID: 10047507]. [DOI] [PubMed] [Google Scholar]
- 3.Palkovits M., Brownstein M.J. Microdissection of brain areas by punch techniqu. In: Cuello A.C., editor. Methods in Neuroscience, Microdissection Techniques. Chichester, UK: Wiley; 1983. pp. 1–36. [Google Scholar]
- 4.Galfre G., Howe S.C., Milstein C., Butcher G.W., Howard J.C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977;266(5602):550–552. doi: 10.1038/266550a0. [http://dx.doi. org/10.1038/266550a0]. [PMID: 558524]. [DOI] [PubMed] [Google Scholar]
- 5.Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur. J. Immunol. 1976;6(7):511–519. doi: 10.1002/eji.1830060713. [http://dx.doi.org/10.1002/eji.1830060713]. [PMID: 825377]. [DOI] [PubMed] [Google Scholar]
- 6.Moskal J.R., Schaffner A.E. Monoclonal antibodies to the dentate gyrus: immunocytochemical characterization and flow cytometric analysis of hippocampal neurons bearing a unique cell-surface antigen. J. Neurosci. 1986;6(7):2045–2053. doi: 10.1523/JNEUROSCI.06-07-02045.1986. [PMID: 3734875]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipser B., McKay R. Monoclonal antibodies distinguish identifiable neurones in the leech. Nature. 1981;289(5798):549–554. doi: 10.1038/289549a0. [http://dx.doi.org/10.1038/289549a0]. [PMID: 6162105]. [DOI] [PubMed] [Google Scholar]
- 8.Trisler G.D., Schneider M.D., Nirenberg M. A topographic gradient of molecules in retina can be used to identify neuron position. Proc. Natl. Acad. Sci. USA. 1981;78(4):2145–2149. doi: 10.1073/pnas.78.4.2145. [http://dx.doi.org/10.1073/pnas.78.4.2145]. [PMID: 6166002]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskal J.R., Trisler D., Schneider M.D., Nirenberg M. Purification of a membrane protein distributed in a topographic gradient in chicken retina. Proc. Natl. Acad. Sci. USA. 1986;83(13):4730–4733. doi: 10.1073/pnas.83.13.4730. [http://dx.doi.org/10.1073/pnas.83.13.4730]. [PMID: 3460067]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanton P.K., Sarvey J.M., Moskal J.R. Inhibition of the production and maintenance of long-term potentiation in rat hippocampal slices by a monoclonal antibody. Proc. Natl. Acad. Sci. USA. 1987;84(6):1684–1688. doi: 10.1073/pnas.84.6.1684. [http://dx.doi.org/10.1073/pnas. 84.6.1684]. [PMID: 3470749]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haring R., Stanton P.K., Scheideler M.A., Moskal J.R. Glycine-like modulation of N-methyl-D-aspartate receptors by a monoclonal antibody that enhances long-term potentiation. J. Neurochem. 1991;57(1):323–332. doi: 10.1111/j.1471-4159.1991.tb02131.x. [http://dx.doi.org/10.1111/j.1471-4159.1991. tb02131.x]. [PMID: 1828831]. [DOI] [PubMed] [Google Scholar]
- 12.Weiss C., Disterhoft J.F. Exploring prefrontal cortical memory mechanisms with eyeblink conditioning. Behav. Neurosci. 2011;125(3):318–326. doi: 10.1037/a0023520. [http://dx.doi.org/10.1037/a0023520]. [PMID: 21517143]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson L.T., Moskal J.R., Disterhoft J.F. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359(6396):638–641. doi: 10.1038/359638a0. [http://dx.doi.org/ 10.1038/359638a0]. [PMID: 1406995]. [DOI] [PubMed] [Google Scholar]
- 14.Moskal J.R., Kuo A.G., Weiss C., Wood P.L. OConnor Hanson, A.; Kelso, S.; Harris, R.B.; Disterhoft, J.F. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49(7):1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [http://dx.doi.org/10.1016/j.neuropharm.2005.06.006]. [PMID: 16051282]. [DOI] [PubMed] [Google Scholar]
- 15.Saragovi H.U., Fitzpatrick D., Raktabutr A., Nakanishi H., Kahn M., Greene M.I. Design and synthesis of a mimetic from an antibody complementarity-determining region. Science. 1991;253(5021):792–795. doi: 10.1126/science.1876837. [http://dx.doi.org/10.1126/science.1876837]. [PMID: 1876837]. [DOI] [PubMed] [Google Scholar]
- 16.Williams W.V., Kieber-Emmons T., VonFeldt J., Greene M.I., Weiner D.B. Design of bioactive peptides based on antibody hypervariable region structures. Development of conformationally constrained and dimeric peptides with enhanced affinity. J. Biol. Chem. 1991;266(8):5182–5190. [PMID: 2002053]. [PubMed] [Google Scholar]
- 17.Williams W.V., Moss D.A., Kieber-Emmons T., Cohen J.A., Myers J.N., Weiner D.B., Greene M.I. Development of biologically active peptides based on antibody structure. Proc. Natl. Acad. Sci. USA. 1989;86(14):5537–5541. doi: 10.1073/pnas.86.14.5537. [http://dx.doi.org/ 10.1073/pnas.86.14.5537]. [PMID: 2501789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams W.V., Guy H.R., Rubin D.H., Robey F., Myers J.N., Kieber-Emmons T., Weiner D.B., Greene M.I. Sequences of the cell-attachment sites of reovirus type 3 and its anti-idiotypic/ antireceptor antibody: modeling of their three-dimensional structures. Proc. Natl. Acad. Sci. USA. 1988;85(17):6488–6492. doi: 10.1073/pnas.85.17.6488. [http://dx.doi.org/10.1073/pnas.85.17.6488]. [PMID: 2457914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang C.Y., Brunck T.K., Kieber-Emmons T., Blalock J.E., Kohler H. Inhibition of self-binding antibodies (autobodies) by a VH-derived peptide. Science. 1988;240(4855):1034–1036. doi: 10.1126/science.3368787. [http:// dx.doi.org/10.1126/science.3368787]. [PMID: 3368787]. [DOI] [PubMed] [Google Scholar]
- 20.Novotný J., Handschumacher M., Haber E. Location of antigenic epitopes on antibody molecules. J. Mol. Biol. 1986;189(4):715–721. doi: 10.1016/0022-2836(86)90502-4. [http://dx.doi.org/10.1016/0022-2836(86)90502-4]. [PMID: 2431154]. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H., Gurney M.E. Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 1990;10(11):3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [PMID: 2230938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskal J.R., Yamamoto H., Colley P.A. The use of antibody engineering to create novel drugs that target N-methyl-D-aspartate receptors. Curr. Drug Targets. 2001;2(3):331–345. doi: 10.2174/1389450013348399. [http://dx.doi. org/10.2174/1389450013348399]. [PMID: 11554557]. [DOI] [PubMed] [Google Scholar]
- 23.Oldendorf W.H. Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res. 1970;24(2):372–376. doi: 10.1016/0006-8993(70)90123-x. [http://dx.doi.org/10.1016/0006-8993(70) 90123-X]. [PMID: 5490302]. [DOI] [PubMed] [Google Scholar]
- 24.Bradbury M.W., Patlak C.S., Oldendorf W.H. Analysis of brain uptake and loss or radiotracers after intracarotid injection. Am. J. Physiol. 1975;229(4):1110–1115. doi: 10.1152/ajplegacy.1975.229.4.1110. [PMID: 1190324]. [DOI] [PubMed] [Google Scholar]
- 25.Burgdorf J., Kroes R.A., Weiss C., Oh M.M., Disterhoft J.F., Brudzynski S.M., Panksepp J., Moskal J.R. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–523. doi: 10.1016/j.neuroscience.2011.05.001. [http://dx.doi.org/10.1016/j.neuroscience.2011.05.001]. [PMID: 21645591]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgdorf J., Zhang X.L., Weiss C., Matthews E., Disterhoft J.F., Stanton P.K., Moskal J.R. The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol. Aging. 2011;32(4):698–706. doi: 10.1016/j.neurobiolaging.2009.04.012. [http://dx.doi.org/10.1016/j.neurobiolaging. 2009.04.012]. [PMID: 19446371]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgdorf J., Zhang X.L., Nicholson K.L., Balster R.L., Leander J.D., Stanton P.K., Gross A.L., Kroes R.A., Moskal J.R. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38(5):729–742. doi: 10.1038/npp.2012.246. [http://dx.doi. org/10.1038/npp.2012.246]. [PMID: 23303054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill M.N., Hellemans K.G., Verma P., Gorzalka B.B., Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci. Biobehav. Rev. 2012;36(9):2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [http://dx.doi. org/10.1016/j.neubiorev.2012.07.001]. [PMID: 22776763]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgdorf J., Kroes R.A., Zhang X.L., Gross A.L., Schmidt M., Weiss C., Disterhoft J.F., Burch R.M., Stanton P.K., Moskal J.R. Rapastinel (GLYX-13) has therapeutic potential for the treatment of post-traumatic stress disorder: Characterization of a NMDA receptor-mediated metaplasticity process in the medial prefrontal cortex of rats. Behav. Brain Res. 2015;294:177–185. doi: 10.1016/j.bbr.2015.07.039. [http://dx.doi.org/10.1016/j.bbr.2015.07.039]. [PMID: 26210936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgdorf J., Zhang X.L., Weiss C., Gross A., Boikess S.R., Kroes R.A., Khan M.A., Burch R.M., Rex C.S., Disterhoft J.F., Stanton P.K., Moskal J.R. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience. 2015;308:202–211. doi: 10.1016/j.neuroscience.2015.09.004. [http://dx.doi.org/10.1016/j. neuroscience.2015.09.004]. [PMID: 26343295]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson R.P. A modification of receptor theory. Br. Pharmacol. Chemother. 1956;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [http://dx.doi.org/ 10.1111/j.1476-5381.1956.tb00006.x]. [PMID: 13383117]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X.L., Sullivan J.A., Moskal J.R., Stanton P.K. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55(7):1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [http://dx.doi.org/10.1016/j.neuropharm.2008.08.018]. [PMID: 18796308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karakas E., Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344(6187):992–997. doi: 10.1126/science.1251915. [http://dx.doi.org/10.1126/science.1251915]. [PMID: 24876489]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preskorn S., Macaluso M., Mehra D.O., Zammit G., Moskal J.R., Burch R.M. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 2015;21(2):140–149. doi: 10.1097/01.pra.0000462606.17725.93. [http://dx.doi.org/10.1097/01.pra.0000462606.17725.93]. [PMID: 25782764]. [DOI] [PubMed] [Google Scholar]
- 35.Moskal J.R., Burch R., Burgdorf J.S., Kroes R.A., Stanton P.K., Disterhoft J.F., Leander J.D. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin. Investig. Drugs. 2014;23(2):243–254. doi: 10.1517/13543784.2014.852536. [http://dx.doi.org/10.1517/13543784. 2014.852536]. [PMID: 24251380]. [DOI] [PMC free article] [PubMed] [Google Scholar]


