Abstract
Abstract: Background: Diffuse intrinsic pontine gliomas represent a unique subtype of primary brain tumors occuring in a specific location and age. Their growth demonstrates early invasion and, following diagnosis, rapid growth not responsive to common therapies. Until recently, the genetic and cellular basis of these tumors was unknown. Genetic evidence implicates mutations in the histone genes in the origin of these tumors.
Methods: Surgical biopsies performed on selected patients have resulted in the establishment of anatomically accurate mouse models that have been used to examine patterns of growth and response to new therapeutic agents.
Results: Human derived pontine glioma models recapitulate the invasive patterns of growth. The grade of the original tumor affects the latency of tumor growth after implantation.
Conclusion: The use of human-derived xenograft models allows for improved pre-clinical testing of new therapeutic targets in a tumor- and organ-specific manner.
Keywords: Bioluminescent imaging, brainstem glioma, DIPG, H3K27M mutation, infiltrating astrocytoma, xenograft model
Introduction
Pediatric brain tumors are rare, occuring in only several thousand children in the United States each year. Although hematologic malignancies are more common, brain tumors have poorer responses to therapy and survivors often have substantial morbidity and longterm cognitive side effects. The genetic characterization of some tumor types, such as medulloblastoma, has progressed dramatically in the past ten years [1]. However, other tumor types are less well defined.
Tumors arising in the brainstem represent approximately 10% of all brain tumors in children. Although there is a range in the malignancy of these tumors, the majority occur in a restricted anatomic area, that of the ventral pons. These tumors, known as diffuse intrinsic pontine gliomas (DIPG), are typically malignant in nature and virtually all patients die within two years after diagnosis [2]. The factors that contribute to the dismal prognosis include extensive infiltration of the brainstem at the time of diagnosis, an anatomic location which precludes surgical resection, and resistance to most types of conventional cancer therapeutics. A large number of Phase I studies conducted over the past twenty years have failed to demonstrate any improvement in survival, or even any response, in the majority of these patients. Indeed, the only modality of therapy that prolongs survival, fractionated radiotherapy, has not changed in several decades.
Because of the typical location of DIPGs, surgical biopsy was felt to be redundant, and diagnosis was made on the basis of features identified on imaging studies [3]. A consequence of this change in practice was that cell and/or tissue resources that could be used for preclinical testing were rarely available. In contrast to the progress in the understanding of the genetics of adult malignant glioma [4], it could be argued that the lack of DIPG tissue samples has hindered progress in identifying underlying oncogenic steps. Recently, genetic analyses of tissues obtained at autopsy have identified a number of DIPG gene and chromosomal alterations, including mutation of the gene encoding histone H3, which is rare among adult malignant gliomas [5]. These results support the existence of key genetic differences between adult gliomas and DIPGs, thereby underscoring the need for thorough molecular profiling of the brainstem tumors, for facilitating improved understanding of associated molecular biology, and the related development of rational therapeutic approaches for treating DIPG.
Relevant Anatomic Location
The use of animal models in cancer therapeutics should facilitate pre-clinical translation and testing of new agents, prior to entry into human clinical trials. For this important reason, it is essential that models recapitulate the human disease as faithfully as possible. Obviously, there are limitations in terms of organ size and animal physiology that cannot be avoided. However, for many in vivo screening assays, widely divergent tumor cell lines are used and the results extrapolated to tumor types far removed from the cell line being tested.
Historically, pre-clinical testing of new therapeutic agents was performed in a variety of standard in vivo models, starting with flank, subcutaneous tumors, and then moving to standard intracranial tumor models. It is clear that subcutaneous models may permit an evaluation of general pharmacokinetics, but they do not represent an ideal tumor model to test organ-specific therapeutic response [6]. Furthermore, standard tumor models often used well-established cell lines (e.g. U87, U251) that often grew in a pattern that did not resemble a primary brain tumor in any way. A central feature of DIPGs are their extensive degree of infiltration; a feature that may result from absence of specific types of proteoglycan molecules in the tumor micro-environment [7]. Although valuable information can be gained from broad cellular effects, it is also clear that biologically-targeted therapeutics need to be tested in genetically faithful systems.
Most intracranial tumors are grown in a location corresponding to the telencephalon, or the deep frontal lobe. The first challenge to examining the biology of DIPGs was to grow tumors in the appropriate anatomic location. Previously, there were few attempts using the brainstem as a target for cell deposition because of the small size of the rodent pons, and perceived side effects from tumor growth in that location. The initial steps were the development of targets and tools that would allow deposition of cells into the rodent brainstem. Because of the increased size of the rat brainstem, this animal type was better suited to the initial technical refinement [8]. This work was subsequently extended to use mice which were less expensive and allowed the use of genetically modified animals if necessary [9]. Using modified entry measurements (a burr hole was placed 1.0 mm behind the lambda, and 9.6 mm deep from the inner surface of the skull), it was possible to obtain reliable tumor growth in the rodent brainstem. The consistency of the implanted tumor cell method facilitates pre-clinical testing.
Imaging of Xenografted Tumors
Tumor growth in the brainstem, as in other locations, usually leads to symptoms and animal demise. The availability of non-invasive tumor imaging greatly facilitates measurement of tumor growth and improves the ability to perform therapeutic testing. There are different techniques that can be used for animal in vivo imaging including positron emission tomography (PET) and single-photon emission tomography (SPECT) [10]. These are based on the use of radionuclides which has substantial technical requirements. Magnetic resonance imaging (MRI) provides excellent anatomic resolution but can be expensive, particularly when longitudinal experiments are planned [11].
Bioluminescence imaging (BLI) relies on the detection of the emission of photons based on energy-dependent reactions catalyzed by cells or organisms that have been genetically- modified to express luciferase. Transfection of brain tumor cell lines with a lentiviral vector containing firefly luciferase (Fluc) allowed reproducible imaging using the Xenogen IVIS Lumina system coupled. Testing with agents such as temozolomide showed that luminescence in rodents receiving TMZ treatment was substantially reduced when the treatment was completed [12]. BLI measurements are a good surrogate of tumor volume, particularly when luciferase expression is consistent throughout the tumor. From a practical perspective, BLI allows a reduction in the sizes of animal cohorts when planning pre-clinical therapeutic testing.
Patient-Derived Tumor Models
Patient-derived cell lines are derived from many primary tumor types. However, because of the rarity of fresh tissue available from DIPGs, the first true cell lines were obtained from autopsy specimens [13]. These cells were grown as neurospheres in serum-free media supplemented with epidermal growth factor (EGF) and fibroblast growth factor (FGF); subsequent growth was improved with the addition of platelet-derived growth factor (PDGF). Examination of these cells supported a role for hedgehog (Hh) signalling. From a conceptual perspective, cells obtained at autopsy are by definition obtained from patients well after recurrence after substantial therapeutic intervention. It would be ideal to evaluate tissues obtained directly from initial surgical biopsy. A technical limitation of the surgical biopsy obtained from DIPGs was the very small size with individual biopsy specimens measuring only 3-4 mm in length, and less than 1 mm in diameter. Nevertheless, starting from small fragments of tissue, it is possible to establish a primary cell lines that are tumorigenic in animal models [14].
One difficulty of this approach was highlighted in one case where the diagnostic histopathology of the tissue obtained from a surgical biopsy was consistent with an infiltrative astrocytoma, WHO grade II [14]. Based on previous experience with establishing primary cell cultures from low grade glial tumors, we anticipated this tissue would have a low likelihood of engraftment, and would likely become senescent during extended cell culture. Therefore, we transduced SF7761 cells with a retrovirus vector containing hTERT (human telomerase reverse transcriptase) for cell immortalization. This step avoided senescence and facilitated growth of the cells both in vivo and in vitro (Fig. 1). A limitation of this approach is that a genetic variant is introduced that was not present in the original tumor.
Fig. (1).
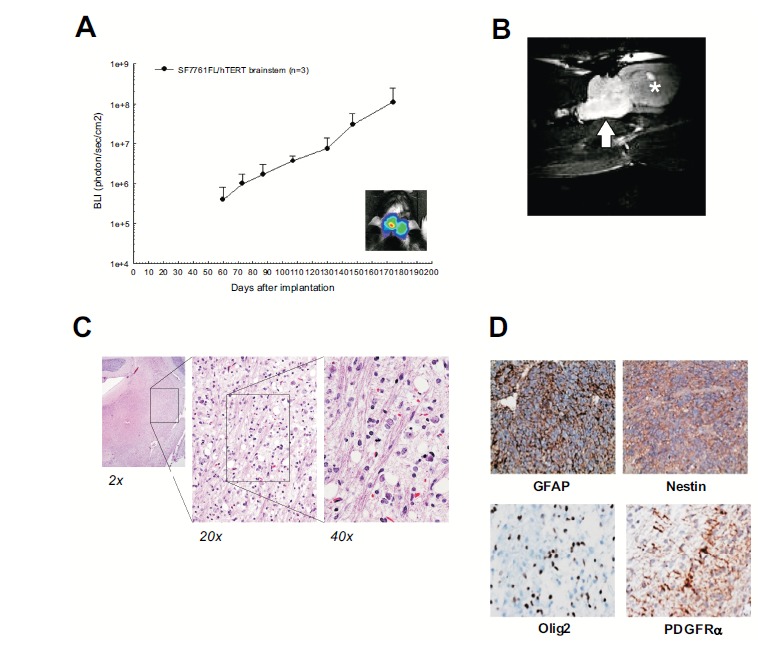
Association between bioluminescence signal and corresponding tumor volume in rats receiving brainstem injection of patient-derived DIPG cells. A) Implantation of tumor cells in the rodent pons is detectable with BLI. B) MR imaging shows high signal within the pons, similar to the human imaging study. C) The histopathology shows a diffusely infiltrating tumor occupying the pons; once again similar to the human tissue sample. D) Immunohistochemistry demonstrates that the tumor cells are positive for GFAP, Nestin, Olig2 (scattered), and PDGFRI (reprinted by permission).
Examination of corresponding xenografts in rat brainstems demonstrated a diffusely infiltrating tumor, similar to that observed in the surgical biopsy specimen. Xenograft tumor cells appeared to extend through the pons, and in some cases to expand the structure, also similar to that observed in patients with DIPG.
Molecular Features
Recent papers have demonstrated that there are unique alterations in pediatric gliomas as compared to their adult counterparts [15]. Similarly, there are regional differences between tumors that arise in the hindbrain as compared to the cerebral hemispheres, such as has been described for ependymomas arising in different CNS locations [16]. The relative percentage of self-renewing cells also appears to be increased in most pediatric CNS tumors as compared to tumors obtained from adult patients [17].
Until very recently, none of the signaling pathways involved in DIPG formation were known. Two recent studies of pediatric high-grade gliomas noted that PDGFRA amplification occurred in 29-36% of DIPGs [15a, 18]. Up-regulation of PDGFR signaling in Nestin+ cells of the IVth ventricle subventricular zone [19], or cells of the dorsolateral pons [20] results in formation of glioblastoma in mouse models. Our analysis reveal that SF7761 cells have elevated PDGFRIα and Rb, but lack expression of the tumor suppressor p16INK4a. These cells also express Nestin and Olig2 [14]. Isolation of primary cell cultures obtained at the time of biopsy also may play a role in testing tumor response to therapy. Using cell cultures obtained from surgical biopsies, the PDGF receptor inhibitor dasatinib was tested and found to inhibit cell proliferation and invasion [21].
Gliomas arising in children and young adults in midline structures such as the brainstem and diencephalon have a high frequency of mutations in the H3F3 gene [5b, 15b, c, 22]. These mutations result in modification of epigenetic marks that ultimately affect gene transcription. The consistency of the H3K27M mutation in DIPG is striking, as is the profound effect on the regulation of many gene targets [23]. A substantial percentage of DIPG tumors are also known to carry mutations in the ACVR1 gene, when mutated in the germ line causes a pathologic predispostion towards ossification of soft tissues [24]. The direct relationship between this gene mutation and DIPG oncogenesis is unclear.
Cell lines isolated from DIPG also carry the H3K27M mutation which facilitates both in vitro and in vivo examination of the effects of this mutation. The specific feature associated with the H3K27M mutation is an alteration in the level of histone di- and tri-methylation; a modification which can be targeted by agents that regulate histone methylation [25]. One of these agents, GSKJ2, is an active inhibitor of the histone demethylases, and in part, can reverse the transcriptional, and tumorigenic effects of the K27M mutation.
Limitations
A fundamental limitation with all human-based xenograft model systems is the use of immunodeficient animals. The absence of an attenuated host response clearly modifies tumor growth both in terms of tumor morphology, and efficiency of engraftment. While certain assumptions are acceptable in the interpretation of results from xenografted models, achieving relevant conclusions in the area of therapeutic assessment is a challenge. Certain fundamental biologic features are likely to be similar in immunodeficient and immunocompetent animals, but others such as tumor associated inflammation, tissue invasion, response to injury are probably very different.
An important conceptual difference between xenografted tumors and de novo tumors is the process of tumor initiation. Presumably, there are early genetic changes that trigger deregulated tumor growth in the host tissue. In other tumor types such as medulloblastoma, genetic changes can be constructed in transgenic mice that result spontaneous tumor formation indistinguishable from the human disease [26]. The onset of tumor formation makes pre-clinical testing more difficult but from a biologic perspective, transgenic models should be considered more accurate in terms of tumor and host biology.
Summary
Human-derived animal models provide a mechanism to study biologically relevant tumors in an in vivo setting. Coupled with anatomically relevant sites and tumors that maintain some aspects of the original tumor biology, appropriate results can be derived. These models are particularly valuable for pre-clinical screening of therapeutic agents, although their limitations should be clearly recognized.
ACKNOWLEDGEMENTS
This work was supported by the Pediatric Brain Tumor Foundation Institute Award to the University of California San Francisco.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.a) Jones D.T., Jager N., Kool M., Zichner T., Hutter B., Sultan M., Cho Y.J., Pugh T.J., Hovestadt V., Stutz A.M., Rausch T., Warnatz H.J., Ryzhova M., Bender S., Sturm D., Pleier S., Cin H., Pfaff E., Sieber L., Wittmann A., Remke M., Witt H., Hutter S., Tzaridis T., Weischenfeldt J., Raeder B., Avci M., Amstislavskiy V., Zapatka M., Weber U.D., Wang Q., Lasitschka B., Bartholomae C.C., Schmidt M., von Kalle C., Ast V., Lawerenz C., Eils J., Kabbe R., Benes V., van Sluis P., Koster J., Volckmann R., Shih D., Betts M.J., Russell R.B., Coco S., Tonini G.P., Schuller U., Hans V., Graf N., Kim Y.J., Monoranu C., Roggendorf W., Unterberg A., Herold-Mende C., Milde T., Kulozik A.E., von Deimling A., Witt O., Maass E., Rossler J., Ebinger M., Schuhmann M.U., Fruhwald M.C., Hasselblatt M., Jabado N., Rutkowski S., von Bueren A.O., Williamson D., Clifford S.C., McCabe M.G., Collins V.P., Wolf S., Wiemann S., Lehrach H., Brors B., Scheurlen W., Felsberg J., Reifenberger G., Northcott P.A., Taylor M.D., Meyerson M., Pomeroy S.L., Yaspo M.L., Korbel J.O., Korshunov A., Eils R., Pfister S.M., Lichter P. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. doi: 10.1038/nature11284. [http://dx.doi.org/10.1038/nature11284]. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Northcott P.A., Shih D.J., Peacock J., Garzia L., Morrissy A.S., Zichner T. StA1/4tz, A.M.; Korshunov, A.; Reimand, J.; Schumacher, S.E.; Beroukhim, R.; Ellison, D.W.; Marshall, C.R.; Lionel, A.C.; Mack, S.; Dubuc, A.; Yao, Y.; Ramaswamy, V.; Luu, B.; Rolider, A.; Cavalli, F.M.; Wang, X.; Remke, M.; Wu, X.; Chiu, R.Y.; Chu, A.; Chuah, E.; Corbett, R.D.; Hoad, G.R.; Jackman, S.D.; Li, Y.; Lo, A.; Mungall, K.L.; Nip, K.M.; Qian, J.Q.; Raymond, A.G.; Thiessen, N.T.; Varhol, R.J.; Birol, I.; Moore, R.A.; Mungall, A.J.; Holt, R.; Kawauchi, D.; Roussel, M.F.; Kool, M.; Jones, D.T.; Witt, H.; Fernandez-L, A.; Kenney, A.M.; Wechsler-Reya, R.J.; Dirks, P.; Aviv, T.; Grajkowska, W.A.; Perek-Polnik, M.; Haberler, C.C.; Delattre, O.; Reynaud, S.S.; Doz, F.F.; Pernet-Fattet, S.S.; Cho, B.K.; Kim, S.K.; Wang, K.C.; Scheurlen, W.; Eberhart, C.G.; Fevre-Montange, M.; Jouvet, A.; Pollack, I.F.; Fan, X.; Muraszko, K.M.; Gillespie, G.Y.; Di Rocco, C.; Massimi, L.; Michiels, E.M.; Kloosterhof, N.K.; French, P.J.; Kros, J.M.; Olson, J.M.; Ellenbogen, R.G.; Zitterbart, K.; Kren, L.; Thompson, R.C.; Cooper, M.K.; Lach, B.; McLendon, R.E.; Bigner, D.D.; Fontebasso, A.; Albrecht, S.; Jabado, N.; Lindsey, J.C.; Bailey, S.; Gupta, N.; Weiss, W.A.; Bognar, L.; Klekner, A.; Van Meter, T.E.; Kumabe, T.; Tominaga, T.; Elbabaa, S.K.; Leonard, J.R.; Rubin, J.B.; Liau, L.M.; Van Meir, E.G.; Fouladi, M.; Nakamura, H.; Cinalli, G.; Garami, M.; Hauser, P.; Saad, A.G.; Iolascon, A.; Jung, S.; Carlotti, C.G.; Vibhakar, R.; Ra, Y.S.; Robinson, S.; Zollo, M.; Faria, C.C.; Chan, J.A.; Levy, M.L.; Sorensen, P.H.; Meyerson, M.; Pomeroy, S.L.; Cho, Y.J.; Bader, G.D.; Tabori, U.; Hawkins, C.E.; Bouffet, E.; Scherer, S.W.; Rutka, J.T.; Malkin, D.; Clifford, S.C.; Jones, S.J.; Korbel, J.O.; Pfister, S.M.; Marra, M.A.; Taylor, M.D. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. PMID: 22832583[http://dx.doi.org/10.1038/nature11327]. [PMID: 22832581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson S.S., Laningham F., Fisher P.G. Advances toward an understanding of brainstem gliomas. J. Clin. Oncol. 2006;24(8):1266–1272. doi: 10.1200/JCO.2005.04.6599. [http://dx.doi.org/10.1200/JCO.2005.04.6599]. [PMID: 16525181]. [DOI] [PubMed] [Google Scholar]
- 3.Barkovich A.J., Krischer J., Kun L.E., Packer R., Zimmerman R.A., Freeman C.R., Wara W.M., Albright L., Allen J.C., Hoffman H.J. Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr. Neurosurg. 1990-1991;16(2):73–83. doi: 10.1159/000120511. [http://dx.doi.org/10.1159/000120511]. [PMID: 2132928]. [DOI] [PubMed] [Google Scholar]
- 4.Phillips H.S., Kharbanda S., Chen R., Forrest W.F., Soriano R.H., Wu T.D., Misra A., Nigro J.M., Colman H., Soroceanu L., Williams P.M., Modrusan Z., Feuerstein B.G., Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [http://dx.doi.org/10.1016/j.ccr.2006.02.019]. [PMID: 16530701]. [DOI] [PubMed] [Google Scholar]
- 5.a) Khuong-Quang D.A., Buczkowicz P., Rakopoulos P., Liu X.Y., Fontebasso A.M., Bouffet E., Bartels U., Albrecht S., Schwartzentruber J., Letourneau L., Bourgey M., Bourque G., Montpetit A., Bourret G., Lepage P., Fleming A., Lichter P., Kool M., von Deimling A., Sturm D., Korshunov A., Faury D., Jones D.T., Majewski J., Pfister S.M., Jabado N., Hawkins C. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. doi: 10.1007/s00401-012-0998-0. [http://dx.doi.org/10.1007/s00401-012-0998-0]. [PMID: 22661320]. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wu G., Broniscer A., McEachron T.A., Lu C., Paugh B.S., Becksfort J., Qu C., Ding L., Huether R., Parker M., Zhang J., Gajjar A., Dyer M.A., Mullighan C.G., Gilbertson R.J., Mardis E.R., Wilson R.K., Downing J.R., Ellison D.W., Zhang J., Baker S.J. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. [http://dx.doi.org/10.1038/ng.1102]. [PMID: 22286216]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannini C., Sarkaria J.N., Saito A., Uhm J.H., Galanis E., Carlson B.L., Schroeder M.A., James C.D. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncol. 2005;7(2):164–176. doi: 10.1215/S1152851704000821. [http://dx.doi.org/10.1215/ S1152851704000821]. [PMID: 15831234]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silver D.J., Siebzehnrubl F.A., Schildts M.J., Yachnis A.T., Smith G.M., Smith A.A., Scheffler B., Reynolds B.A., Silver J., Steindler D.A. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J. Neurosci. 2013;33(39):15603–15617. doi: 10.1523/JNEUROSCI.3004-12.2013. [http://dx.doi.org/10.1523/JNEUROSCI.3004-12.2013]. [PMID: 24068827]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashizume R., Ozawa T., Dinca E.B., Banerjee A., Prados M.D., James C.D., Gupta N. A human brainstem glioma xenograft model enabled for bioluminescence imaging. J. Neurooncol. 2010;96(2):151–159. doi: 10.1007/s11060-009-9954-9. [http://dx.doi.org/10.1007/s11060-009-9954-9]. [PMID: 19585223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki Y., Hashizume R., Ozawa T., Banerjee A., Prados M., James C.D., Gupta N. An experimental xenograft mouse model of diffuse pontine glioma designed for therapeutic testing. J. Neurooncol. 2012;108(1):29–35. doi: 10.1007/s11060-011-0796-x. [http://dx.doi.org/10.1007/ s11060-011-0796-x]. [PMID: 22231932]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandhu G.S., Solorio L., Broome A.M., Salem N., Kolthammer J., Shah T., Flask C., Duerk J.L. Whole animal imaging. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010;2(4):398–421. doi: 10.1002/wsbm.71. [http://dx. doi.org/10.1002/wsbm.71]. [PMID: 20836038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito R., Bringas J.R., McKnight T.R., Wendland M.F., Mamot C., Drummond D.C., Kirpotin D.B., Park J.W., Berger M.S., Bankiewicz K.S. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [http://dx.doi.org/10.1158/0008-5472.CAN-03-3631]. [PMID: 15059914]. [DOI] [PubMed] [Google Scholar]
- 12.Dinca E.B., Sarkaria J.N., Schroeder M.A., Carlson B.L., Voicu R., Gupta N., Berger M.S., James C.D. Bioluminescence monitoring of intracranial glioblastoma xenograft: response to primary and salvage temozolomide therapy. J. Neurosurg. 2007;107(3):610–616. doi: 10.3171/JNS-07/09/0610. [http://dx.doi.org/10.3171/JNS-07/09/0610]. [PMID: 17886562]. [DOI] [PubMed] [Google Scholar]
- 13.Monje M., Mitra S.S., Freret M.E., Raveh T.B., Kim J., Masek M., Attema J.L., Li G., Haddix T., Edwards M.S., Fisher P.G., Weissman I.L., Rowitch D.H., Vogel H., Wong A.J., Beachy P.A. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl. Acad. Sci. USA. 2011;108(11):4453–4458. doi: 10.1073/pnas.1101657108. [http://dx.doi.org/10.1073/pnas.1101657108]. [PMID: 21368213]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume R., Smirnov I., Liu S., Phillips J.J., Hyer J., McKnight T.R., Wendland M., Prados M., Banerjee A., Nicolaides T., Mueller S., James C.D., Gupta N. Characterization of a diffuse intrinsic pontine glioma cell line: implications for future investigations and treatment. J. Neurooncol. 2012;110(3):305–313. doi: 10.1007/s11060-012-0973-6. [http://dx.doi.org/10.1007/ s11060-012-0973-6]. [PMID: 22983601]. [DOI] [PubMed] [Google Scholar]
- 15.a) Paugh B.S., Qu C., Jones C., Liu Z., Adamowicz-Brice M., Zhang J., Bax D.A., Coyle B., Barrow J., Hargrave D., Lowe J., Gajjar A., Zhao W., Broniscer A., Ellison D.W., Grundy R.G., Baker S.J. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 2010;28(18):3061–3068. doi: 10.1200/JCO.2009.26.7252. [http://dx.doi.org/10.1200/ JCO.2009.26.7252]. [PMID: 20479398]. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schwartzentruber J., Korshunov A., Liu X.Y., Jones D.T., Pfaff E., Jacob K., Sturm D., Fontebasso A.M., Quang D.A., Tonjes M., Hovestadt V., Albrecht S., Kool M., Nantel A., Konermann C., Lindroth A., Jager N., Rausch T., Ryzhova M., Korbel J.O., Hielscher T., Hauser P., Garami M., Klekner A., Bognar L., Ebinger M., Schuhmann M.U., Scheurlen W., Pekrun A., Fruhwald M.C., Roggendorf W., Kramm C., Durken M., Atkinson J., Lepage P., Montpetit A., Zakrzewska M., Zakrzewski K., Liberski P.P., Dong Z., Siegel P., Kulozik A.E., Zapatka M., Guha A., Malkin D., Felsberg J., Reifenberger G., von Deimling A., Ichimura K., Collins V.P., Witt H., Milde T., Witt O., Zhang C., Castelo-Branco P., Lichter P., Faury D., Tabori U., Plass C., Majewski J., Pfister S.M., Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [http://dx.doi.org/10.1038/ nature10833]. [DOI] [PubMed] [Google Scholar]; c) Sturm D., Witt H., Hovestadt V., Khuong-Quang D.A., Jones D.T., Konermann C., Pfaff E., Tonjes M., Sill M., Bender S., Kool M., Zapatka M., Becker N., Zucknick M., Hielscher T., Liu X.Y., Fontebasso A.M., Ryzhova M., Albrecht S., Jacob K., Wolter M., Ebinger M., Schuhmann M.U., van Meter T., Fruhwald M.C., Hauch H., Pekrun A., Radlwimmer B., Niehues T., von Komorowski G., Durken M., Kulozik A.E., Madden J., Donson A., Foreman N.K., Drissi R., Fouladi M., Scheurlen W., von Deimling A., Monoranu C., Roggendorf W., Herold-Mende C., Unterberg A., Kramm C.M., Felsberg J., Hartmann C., Wiestler B., Wick W., Milde T., Witt O., Lindroth A.M., Schwartzentruber J., Faury D., Fleming A., Zakrzewska M., Liberski P.P., Zakrzewski K., Hauser P., Garami M., Klekner A., Bognar L., Morrissy S., Cavalli F., Taylor M.D., van Sluis P., Koster J., Versteeg R., Volckmann R., Mikkelsen T., Aldape K., Reifenberger G., Collins V.P., Majewski J., Korshunov A., Lichter P., Plass C., Jabado N., Pfister S.M. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. PMID: 22286061[http://dx.doi.org/10.1016/j.ccr. 2012.08.024]. [PMID: 23079654]. [DOI] [PubMed] [Google Scholar]
- 16.Witt H., Mack S.C., Ryzhova M., Bender S., Sill M., Isserlin R., Benner A., Hielscher T., Milde T., Remke M., Jones D.T., Northcott P.A., Garzia L., Bertrand K.C., Wittmann A., Yao Y., Roberts S.S., Massimi L., Van Meter T., Weiss W.A., Gupta N., Grajkowska W., Lach B., Cho Y.J., von Deimling A., Kulozik A.E., Witt O., Bader G.D., Hawkins C.E., Tabori U., Guha A., Rutka J.T., Lichter P., Korshunov A., Taylor M.D., Pfister S.M. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20(2):143–157. doi: 10.1016/j.ccr.2011.07.007. [http://dx.doi.org/10.1016/j.ccr.2011.07.007]. [PMID: 21840481]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thirant C., Bessette B., Varlet P., Puget S., Cadusseau J. Tavares, Sdos.R.; Studler, J.M.; Silvestre, D.C.; Susini, A.; Villa, C.; Miquel, C.; Bogeas, A.; Surena, A.L.; Dias-Morais, A.; Leonard, N.; Pflumio, F.; Bieche, I.; Boussin, F.D.; Sainte-Rose, C.; Grill, J.; Daumas-Duport, C.; Chneiweiss, H.; Junier, M.P. Clinical relevance of tumor cells with stem-like properties in pediatric brain tumors. PLoS One. 2011;6(1):e16375. doi: 10.1371/journal.pone.0016375. [http://dx. doi.org/10.1371/journal.pone.0016375]. [PMID: 21297991]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarghooni M., Bartels U., Lee E., Buczkowicz P., Morrison A., Huang A., Bouffet E., Hawkins C. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J. Clin. Oncol. 2010;28(8):1337–1344. doi: 10.1200/JCO.2009.25.5463. [http://dx.doi.org/10.1200/JCO.2009.25.5463]. [PMID: 20142589]. [DOI] [PubMed] [Google Scholar]
- 19.Becher O.J., Hambardzumyan D., Walker T.R., Helmy K., Nazarian J., Albrecht S., Hiner R.L., Gall S., Huse J.T., Jabado N., MacDonald T.J., Holland E.C. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70(6):2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [http://dx.doi.org/10.1158/0008-5472.CAN-09-2503]. [PMID: 20197468]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masui K., Suzuki S.O., Torisu R., Goldman J.E., Canoll P., Iwaki T. Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. Glia. 2010;58(9):1050–1065. doi: 10.1002/glia.20986. [http://dx.doi.org/10.1002/glia.20986]. [PMID: 20468047]. [DOI] [PubMed] [Google Scholar]
- 21.Truffaux N., Philippe C., Paulsson J., Andreiuolo F., Guerrini-Rousseau L., Cornilleau G., Le Dret L., Richon C., Lacroix L., Puget S., Geoerger B., Vassal G., Ostman A., Grill J. Preclinical evaluation of dasatinib alone and in combination with cabozantinib for the treatment of diffuse intrinsic pontine glioma. Neuro-oncol. 2014 doi: 10.1093/neuonc/nou330. [PMID: 25534822]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sturm D., Bender S., Jones D.T., Lichter P., Grill J., Becher O., Hawkins C., Majewski J., Jones C., Costello J.F., Iavarone A., Aldape K., Brennan C.W., Jabado N., Pfister S.M. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat. Rev. Cancer. 2014;14(2):92–107. doi: 10.1038/nrc3655. [http://dx.doi.org/10.1038/ nrc3655]. [PMID: 24457416]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan K.M., Fang D., Gan H., Hashizume R., Yu C., Schroeder M., Gupta N., Mueller S., James C.D., Jenkins R., Sarkaria J., Zhang Z. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. doi: 10.1101/gad.217778.113. [http://dx.doi.org/10.1101/gad.217778.113]. [PMID: 23603901]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a) Buczkowicz P., Hoeman C., Rakopoulos P., Pajovic S., Letourneau L., Dzamba M., Morrison A., Lewis P., Bouffet E., Bartels U., Zuccaro J., Agnihotri S., Ryall S., Barszczyk M., Chornenkyy Y., Bourgey M., Bourque G., Montpetit A., Cordero F., Castelo-Branco P., Mangerel J., Tabori U., Ho K.C., Huang A., Taylor K.R., Mackay A., Bendel A.E., Nazarian J., Fangusaro J.R., Karajannis M.A., Zagzag D., Foreman N.K., Donson A., Hegert J.V., Smith A., Chan J., Lafay-Cousin L., Dunn S., Hukin J., Dunham C., Scheinemann K., Michaud J., Zelcer S., Ramsay D., Cain J., Brennan C., Souweidane M.M., Jones C., Allis C.D., Brudno M., Becher O., Hawkins C. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014;46(5):451–456. doi: 10.1038/ng.2936. [http://dx. doi.org/10.1038/ng.2936]. [PMID: 24705254]. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fontebasso A.M., Papillon-Cavanagh S., Schwartzentruber J., Nikbakht H., Gerges N., Fiset P.O., Bechet D., Faury D., De Jay N., Ramkissoon L.A., Corcoran A., Jones D.T., Sturm D., Johann P., Tomita T., Goldman S., Nagib M., Bendel A., Goumnerova L., Bowers D.C., Leonard J.R., Rubin J.B., Alden T., Browd S., Geyer J.R., Leary S., Jallo G., Cohen K., Gupta N., Prados M.D., Carret A.S., Ellezam B., Crevier L., Klekner A., Bognar L., Hauser P., Garami M., Myseros J., Dong Z., Siegel P.M., Malkin H., Ligon A.H., Albrecht S., Pfister S.M., Ligon K.L., Majewski J., Jabado N., Kieran M.W. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat. Genet. 2014;46(5):462–466. doi: 10.1038/ng.2950. [http://dx.doi.org/10.1038/ ng.2950]. [PMID: 24705250]. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Taylor K.R., Mackay A., Truffaux N., Butterfield Y.S., Morozova O., Philippe C., Castel D., Grasso C.S., Vinci M., Carvalho D., Carcaboso A.M., de Torres C., Cruz O., Mora J., Entz-Werle N., Ingram W.J., Monje M., Hargrave D., Bullock A.N., Puget S., Yip S., Jones C., Grill J. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat. Genet. 2014;46(5):457–461. doi: 10.1038/ng.2925. [http://dx.doi.org/10.1038/ng.2925]. [PMID: 24705252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashizume R., Andor N., Ihara Y., Lerner R., Gan H., Chen X., Fang D., Huang X., Tom M.W., Ngo V., Solomon D., Mueller S., Paris P.L., Zhang Z., Petritsch C., Gupta N., Waldman T.A., James C.D. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat. Med. 2014;20(12):1394–1396. doi: 10.1038/nm.3716. [http://dx.doi.org/10.1038/nm.3716]. [PMID: 25401693]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lau J., Schmidt C., Markant S.L., Taylor M.D., Wechsler-Reya R.J., Weiss W.A. Matching mice to malignancy: molecular subgroups and models of medulloblastoma. Childs Nerv. Syst. 2012;28(4):521–532. doi: 10.1007/s00381-012-1704-1. [http://dx.doi.org/10.1007/s00381-012-1704-1]. [PMID: 22315164]. [DOI] [PMC free article] [PubMed] [Google Scholar]


